Firing properties of neurones in the laterodorsal hypothalamic area during sleep and wakefulness
Abstract
Abstract In undrugged, head-restrained rats, neuronal activity was recorded in and around the laterodorsal hypothalamic area where orexin neurones are distributed. Among 22 neurones observed across whole sleep–waking states, half (n = 11) were most active during paradoxical sleep and least active during waking. Others were equally more active during paradoxical sleep and waking than during slow-wave sleep (n = 6), or were most active during waking and least active during paradoxical sleep (n = 3). The majority of neurones started to increase firing activity prior to the transition of sleep–waking states. These results suggest that the area of the hypothalamus containing orexin neurones plays a role in sleep–waking regulation.
INTRODUCTION
In the laterodorsal hypothalamic area, which largely overlaps with the feeding centre,1 neurones containing recently identified peptides, orexins (orexin A and B), are distributed.2 It has been suggested that the peptides are involved in the regulation of sleep– waking cycles.3 This area has drawn a lot of interest, and future elucidation of the role of this area in regulating sleep–waking is anticipated. In the present study, we recorded the neuronal activity in the laterodorsal hypothalamic area of rats repeating sleep and wakefulness.
SUBJECTS AND METHOD
Experiments were performed on five undrugged, painlessly head-restrained Sprague–Dawley rats (Japan SLC Inc., Shizuoka, Japan), which were prepared for recording single neuronal activity in the lateral hypothalamic area simultaneously with a cortical electroencephologram (EEG) and electromyography (EMG) recordings from the neck to judge their sleep–wakefulness condition, as reported elsewhere.4 After allowing the rats to recover from surgery for more than 7 days, they were placed in a slowly rotating wheel, with food and water given ad libitum, to deprive them of sleep for 12 h prior to the recording session. Single neuronal activity was recorded extracellularly through a glass pipette microelectrode containing Pontamine Sky Blue to mark the positions of the neurones being recorded. The neuronal activity, EEG and EMG were fed to a computer through an Oxford CED 1410 data processor. After the experiment, histological sections of the hypothalamus were processed with anti-orexin antibodies to clarify the distribution of orexin neurones.
RESULTS
Neuronal activity from 22 neurones was recorded during the entire sleep–waking cycles including wakefulness (W), slow-wave sleep (SWS), and paradoxical sleep (PS). The neurones were located in the lateral hypothalamic area or in adjacent areas such as the dorsomedial hypothalamic nucleus, zona incerta or subincertal nucleus.
The neurones were classified according to their firing pattern during the sleep–waking states and named after the stage during which they were most active. The largest population (n = 11) was P neurones. As shown in Fig. 1, they were most active during paradoxical sleep, and least active or silent during wakefulness. A majority of neurones (n = 10) started to increase their firing rate 3–39 s in advance of the onset of PS; the firing rate reached maximum around PS onset which was maintained during PS. In the remaining one neurone the increase of firing started after the PS onset.
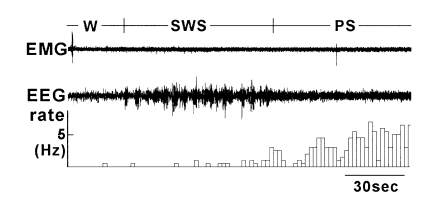
. An example of a change in firing rate of P neurone from waking (W) to slow-wave sleep (SWS) to paradoxical sleep (PS). EMG, electromyography recording of neck muscle activity; EEG, cortical electroencephalogram; rate, firing rate of each neurone calculated per 2 s bin. During PS, complete atonia occurred, but an artifact of cardiac pulsation was superimposed onto the EMG trace.
Approximately one-quarter of the recorded neurones (n = 6) were WP neurones, which were more active both during W and PS than during SWS. Another group was W neurones (n = 3), which were most active during W and least active during PS. All neurones of the WP and W groups began to increase their firing rate 1–30 s prior to the transition of sleep–waking states (from SWS to W or PS in WP neurones and from SWS or PS to W in W neurones). The remaining neurones (n = 2) did not show a clear change in the firing rate across the sleep–waking states.
Regardless of the groups, firing of neurones during the most active state was less than 15 Hz (mostly < 10 Hz) with the exception of only one in the WP group whose firing rate reached 40 Hz. For most neurones, firing during the active states was not fully constant but fluctuated considerably.
DISCUSSION
In and around the area where orexin neurones are distributed, we found many neurones that changed their activity across sleep–waking states. As the change started prior to the transition of the states, they may be concerned with sleep–waking regulation. It is not clear whether the neurones encountered in the present study were orexinergic. However, even if they themselves were not, they were in close vicinity to orexin neurones and, very possibly, had interactions with the latter. According to behavioural and EEG observations of mice lacking genes of orexin or its receptors,2,3 orexin neurones play an important role in sleep–waking regulation. The present study supports this concept at the level of neuronal activity.
The orexin- or receptor-knockout mice have frequent attacks that resemble narcolepsy, which is a condition of the direct transition from waking to a PS-like state.3 How this relates to the results of the present study, which found that the largest population of neurones were most active during PS, remains to be clarified.




