Early ontogeny and placentation of the grey short-tailed opossum, Monodelphis domestica (Didelphidae: Marsupialia): contribution to the reconstruction of the marsupial morphotype
Abstract
enThis study provides new findings on the placenta of Monodelphis domestica and a reconstruction of the marsupial morphotype. To achieve this, early ontogeny and placentation of the grey short-tailed opossum, M. domestica, from 3 h after copulation until birth (day 15), were studied and compared with other mammals. Both the ultrastructure and histochemistry of egg membranes, foetal membranes, oviduct and uterus were examined. The results of this study provide the first detailed ultrastructural description of a trophoblastic syncytium in a marsupial. In addition, this is the first original documentation of an invasive trophectoderm and an inflammatory reaction at parturition in M. domestica. These findings were compared with literature data and included into the reconstruction of the marsupial morphotype. Based on marsupial phylogeny as proposed by Luckett (J. Mammal. Evol. 2, 255–283, 1994), characters that are consistent at least within didelphids and dasyurids were determined to be characters of the marsupial morphotype. These characters are a central yolk separated from the peripheral yolk-poor cytoplasm in the unfertilized oocyte, the presence of a zona pellucida, a mucoid coat and a shell coat, the absence of a corona radiata, oviductal mucoid secretion, no shell secretion distal to the isthmus of the oviduct, uterine shell secretion, a short tubal passage (1 day at maximum), the apposition of blastomeres to the zona pellucida prior to intercellular association, the absence of a morula stage, the polarity of the zygotic yolk, the localized segmentation of deutoplasm (yolk) during the first cleavage and subsequent extrusion of yolk vesicles during the first two cleavage stages. With regard to the marsupial morphotype, the non-polarized yolk distribution in the zygote [Hartman (J. Morphol. 27, 1–84, 1916); McCrady (Am. Anat. Mem. 16, 1–233, 1938)] is a derived character of Didelphis virginiana. Didelphis virginiana [Hartman (J. Morphol. 27, 1–84, 1916); Hartman (J. Morphol. 32, 1–139, 1919); McCrady (Am. Anat. Mem. 16, 1–233, 1938)] and Didelphis marsupialis (Hill, Q. J. Micr. Sci. 63, 91–139, 1918) share the synapomorphous reduction of deutoplasmolysis to a generalized extrusion of vesicles. The absence of separated yolk and consequently a cleavage without yolk extrusion (Renfree and Lewis, Reprod. Fert. Dev. 8, 725–742, 1996) are apomorphies of macropodids. This is possibly correlated with the association of blastomeres in early cleavage stages (Renfree and Lewis, Reprod. Fert. Dev. 8, 725–742, 1996). A yolk sac placenta and a vascularized allantochorion can be assumed for part of the ontogeny in the marsupial morphotype, irrespective of the formation of an allantoic placenta at near term stages. The character polarization of the mode of placentation and parturition needs further investigation.
Abstract
deFrühe Ontogenie und Plazentation der grauen Hausspitzmausbeutelratte, Monodelphis domestica (Didelphidae: Marsupialia): Ein Beitrag zur Rekonstruktion des Grundplans der Marsupialia
Die vorliegende Arbeit beschreibt die frühe Ontogenese und Plazentation von 3 Stunden nach der Kopulation bis zur Geburt der Beutelratte Monodelphis domestica. Es wird die Ultrastruktur und Histochemie der Eihäute, der Fetalmembranen, des Oviductes und des Uterus beschrieben. Erstmalig wird die Ultrastruktur eines trophoblastischen Syncytiums bei einem Beuteltier beschrieben. Weiterhin wird ein invasives Trophektoderm und eine Entzündungsreaktion zum Zeitpunkt der Geburt bei M. domestica festgestellt. Die Befunde dieser Studie und Literaturdaten werden verglichen und in eine Grundplanrekonstruktion integriert. Merkmale, die mindestens zwischen Vertretern der Didelphidae und Dasyuridae übereinstimmen, werden basierend auf dem phylogenetischen System der Marsupialia nach Luckett, J. Mammal. Evol. 2, 255–283, 1994, für den Grundplan der Marsupialia angenommen. Diese Merkmale sind zentral separierter Dotter und peripheres dotterarmes Zytoplasma in der unbefruchteten Eizelle, das Vorhandensein von Zona pellucida, Mucoidschicht und Schalenhaut, das Fehlen einer Corona radiata, die Mucoidsekretion durch den Oviduct, die Schalensekretion durch den Uterus und nicht distal der Isthmusregion des Oviductes, eine kurze Tubenwanderung (maximal einen Tag), die Anlagerung der Blastomeren an die Zona pellucida vor der interzellulären Verbindung, das Fehlen eines Morulastadiums, die Dotterpolarität in der Zygote, die lokale Dotterabtrennung bei der ersten Teilung und die anschließende Dotterextrusion während der ersten beiden Teilungen. In Bezug auf den Grundplan der Marsupialia ist die unpolare Dotterverteilung in der Zygote ein abgeleitetes Merkmal von Didelphis virginiana. Didelphis virginiana und Didelphis marsupialis teilen als Synapomorphie die Reduktion der Deutoplasmolyse auf eine generelle Vesikelextrusion. Das Fehlen separierten Dotters in der Oocyte und die resultierende Furchung ohne Dotterextrusion [Renfree and Lewis, Reprod. Fert. Dev. 8, 725–742, 1996] ist eine Apomorphie der Macropodidae. Hiermit hängt möglicherweise die frühe Zusammenlagerung der Blastomeren zusammen [Renfree and Lewis, Reprod. Fert. Dev. 8, 725–742, 1996]. Ein vaskularisiertes Allantochorion und eine Dottersackplazenta können für einen Teil der Ontogenese im Grundplan der Marsupialia angenommen werden. Ob das Allantochorion neben der Respiration auch dem Stoffaustausch diente ist unklar. Die Lesrichtung für den Modus der Plazentation und der Geburt bedarf weiterer Untersuchungen.
-
- bc
-
- blastocyst cavity
-
- bm
-
- blastomeres
-
- CRL
-
- crown–rump length
-
- DAB
-
- diaminobenzidine
-
- dpc
-
- days post copulation
-
- em
-
- endometrium
-
- EM
-
- electron microscopy
-
- ER
-
- endoplasmatic reticulum
-
- ESI
-
- electron spectroscopic imaging
-
- fbc
-
- foetal blood cell
-
- fc
-
- formative cytoplasm
-
- HC
-
- histochemistry
-
- hpc
-
- hours post copulation
-
- ht
-
- histiotrophe
-
- ic
-
- intercellular capillary
-
- l
-
- lipid droplet
-
- LM
-
- light microscopy
-
- mc
-
- mucoid coat
-
- me
-
- mesenchyme
-
- mt
-
- mitochondrion
-
- n
-
- nucleus
-
- ne
-
- nucleated erythrocytes
-
- PAS
-
- Periodic Acid Schiff’s Reagent
-
- pp
-
- pseudopodia-like process
-
- pr
-
- propria
-
- r
-
- ribosomes
-
- rer, rER
-
- rough endoplasmatic reticulum
-
- S.E.M.
-
- Standard Error of Mean
-
- SEM
-
- scanning electron microscopy
-
- sl
-
- syncytial knot
-
- sm
-
- shell coat
-
- sp
-
- secretory process
-
- st
-
- sperm tail
-
- te
-
- tubal epithelium
-
- TEM
-
- transmission electron microscopy
-
- tr
-
- trophoblast
-
- trc
-
- trophoblast cell
-
- trs
-
- trophoblastic syncytium
-
- ue
-
- uterine epithelium
-
- ug
-
- uterine gland
-
- ul
-
- uterine lumen
-
- uv
-
- uterine vessel
-
- vv
-
- vitelline vessel
-
- y
-
- yolk
-
- yp
-
- yolky cytoplasm
-
- zp
-
- zona pellucida
Introduction
This study is a contribution to the reconstruction of the morphotype of recent marsupials by providing current findings, a character polarization and a phylogenetic interpretation.
Marsupialia (Metatheria) and Placentalia (Eutheria) form the monophyletic Theria, which are the sistergroup of the Monotremata (Novacek et al. 1988; Shoshani and McKenna 1998; Zeller 1999b). Although marsupials and placentals are both viviparous (unlike the egg-laying monotremes) their modes of reproduction differ considerably (see Table 1). The polarization of most of these characters, as well as their evolutionary significance are still unresolved. For the reconstruction of the evolution of reproductive strategies in both groups, the morphotypes, which are the sum of characters present in the last common ancestors (cf. Hennig 1950; Königsmann 1975), of Theria, Marsupialia, Placentalia and internal groups must be reconstructed.
The morphotype reconstruction is based on marsupial systematics as proposed by Luckett (1994). According to Luckett (1994), marsupials can be divided into the monophyletic sister-taxa Australidelphia and Ameridelphia. Ameridelphians comprise all American marsupials except Dromiciops australis, australidelphians consist of all Australasian marsupials including the South American Dromiciops australis. The monophyly of these groups is supported by the sperm pairing in the epididymis of ameridelphians (Temple-Smith 1987) and the fusion of two facets on the calcaneus (confluent lower ankle joint pattern, Szalay 1993) in australidelphians. This basal dichotomy of marsupials is essential for the morphotype reconstruction, based on character optimization and outgroup comparison. For character optimization and outgroup comparison the further division into syndactyls and diprotodonts is useful. The phylogeny of marsupials is represented by the cladogram in Fig. 1.
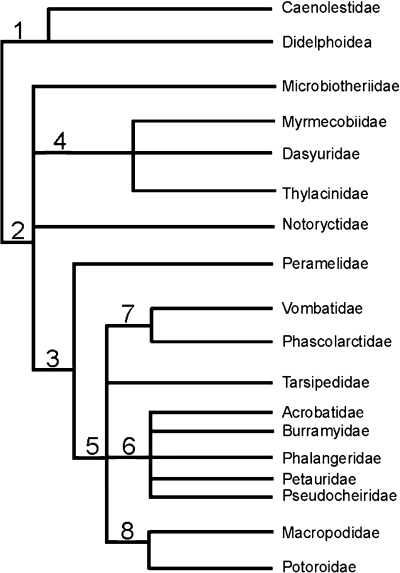
, Ameridelphia; 2, Australidelphia; 3, Syndactyla; 4, Dasyuroidea; 5, Diprotodontia; 6, most phalangeroids; 7, Vombatiformes; 8, Macropodoidea
The differences in reproductive biology could have had important influence on the differences in extinction rates, radiation and migration between marsupials and placentals at the Cretaceous–Tertiary boundary (Archibald 1982; Case and Woodburne 1986; Caroll 1997). We regard the reconstruction of the marsupial morphotype as a first step toward an evolutionary scenario that explains the mammalian evolution at the Cretaceous–Tertiary boundary.
Materials and methods
Monodelphis domestica was bred in a colony established at the Institute of Anatomy of the University of Göttingen. This colony was later moved to the Institute of Zoology of the University of Mainz and then on to the Institute of Systematic Zoology at the Museum of Natural History, Berlin, where it is presently located. Estrus of female Monodelphis is induced by the presence of male pheromones (Fadem 1985; Hinds et al. 1992). Copulation takes place within the estrus (Baggott et al. 1987; Fadem and Rayve 1985). Ovulation occurs 20 (Mate et al. 1994), 22.5 (n=1, own observation) to 24 h (Baggott and Moore 1990) after copulation. Fertilization occurs 22 (Mate et al. 1994), 22.5 (n=1, own observation) to 24 h after copulation (Mate et al. 1994). Mating was monitored by direct observation. All embryos and stages mentioned in this study are dated according to the time of copulation. Thus, the first day of pregnancy coincides with the day of fertilization, that is, about 24 h after mating. Parturition occurs between the 14th and 15th day after copulation (Fadem et al. 1982; personal observation) after a 13- to13.5-day period of gestation (Mate et al. 1994).
The consistency between our findings and descriptions of Mate et al. (1994) confirmed the normality of embryos described in this study. Abnormal stages were not included in the description. In addition, the chronology of development in M. domestica as described here matches the timetable given by Mate et al. (1994).
Twenty-seven adult female M. domestica were killed with chloroform at different times of pregnancy, ranging between a few hours and 15 days after copulation. They were fixed by perfusion through the vascular system immediately after their death. Gravid uteri, embryos and pieces of yolk-sac placenta were prepared for histochemistry, light and electron microscopy (EM) according to the following procedures.
Light microscopy
Specimens were either (1) fixed with Bouin′ s and embedded in paraplast or (2) fixed with 4% formol and embedded in methylmethacrylate (Kulzer). Specimens embedded in paraplast were sectioned at 7 μm and stained with hematoxylin and eosin (HE), azan or trichrome (Mason-Goldner). Specimens embedded in methylmethacrylate were sectioned at 2 μm and stained with HE.
Frozen sections were obtained from tissues shock frozen in liquid N2 and later sectioned at 4–6 μm with a Kryostat (LeicaFrigocut, Leica Instruments GmbH, Heidelbergerstr. 17–19, 69226 Nußloch, Germany) at −25 °C.
Histochemistry
Four methacrylate sections of specimen 82 [29 h post copulation (hpc)] and one frozen section of specimen 135 [7 days post copulation (dpc)] were stained with Kongo red (Romeis 1968). One frozen section of specimen 135 was also stained for keratin by Martinotti (Romeis 1968) to demonstrate the shell coat. Numerous frozen sections of specimen 135 were stained with Sudan red.
Twenty-four methacrylate sections of specimens 45 (22.5 hpc), four of specimen 85 (29 hpc) and eight of specimen 95 (4dpc) were stained with PAS. Six paraffin sections of specimen 45 (22.5 hpc) were stained with peroxidase-conjugated Ulex-europaeus-lectin (substrate: DAB).
Four frozen sections of specimen 135 were fixed in acetone (6 °C) for 10 min and treated with antibodies against keratin, laminin, collagen type IV and fibronectin (Table 2); the immunoreaction was visualized by the usage of biotinylated secondary antibodies and the biotin–avidin–peroxidase method according to Dako LSAB®-Kit (DAKO Diagnostika GmbH, Hamburg, Germany). In addition, sections were treated with collagenase (Sigma no. C-0773; Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany). Antibodies against transferrin were applied to two frozen sections of specimen 132 and to two paraffin sections of specimen 28 (12 dpc), followed by the application of Dako LSAB®-Kit.
Control: sections treated with all chemicals used in immunohistochemistry without the primary antibody did not stain.
Electron microscopy
For transmission electron microscopy (TEM), specimens were fixed by perfusion through the maternal vascular system with a mixture of 1.5% glutaraldehyde and 1.5% freshly prepared paraformaldehyde in 0.1 M cacodylate buffer (pH 7.35; total osmolality 658 mosmol/l). Specimens of yolk-sac placenta (endometrium with adjacent yolk-sac wall) prepared for TEM were cut into small pieces and further fixed for 1 h with glutaraldehyde/paraformaldehyde in cacodylate buffer. Tissue blocks were rinsed for 1 h in cacodylate buffer, post-fixed for 2 h with 1% osmium tetroxide by immersion (specimen 194 (14 dpc) with 1% tannic acid) and embedded in araldite after dehydration with ethanol. Ultrathin sections (60–80 nm) were stained with lead citrate and uranyl acetate and examined with a Zeiss CEM 902 electron microscope (Carl Zeiss Oberkochen, Germany) operating at 80 kV with an integrated imaging electron energy spectrometer and a Phillips CM 10 (Phillips Electron Optics, Chatswood, NSW, Australia) operating at 60 kV. Electron spectroscopic imaging (ESI) without elemental analysis was performed at energy losses of either 0 eV (‘elastic bright field’) or 250 eV (with the carbon signal at minimum). For scanning electron microscopy (SEM), embryos or pieces of endometrium were dried according to the critical point method, sputtered with gold palladium and examined with the Zeiss DSM 960 scanning electron microscope.
Photography
Whole embryos were photographed with a Wild macroscope M 400 (Leica), and a Zeiss Axioplan was used for microphotography.
The specimens and procedures adopted are summarized in Table 2.
Taxonomy
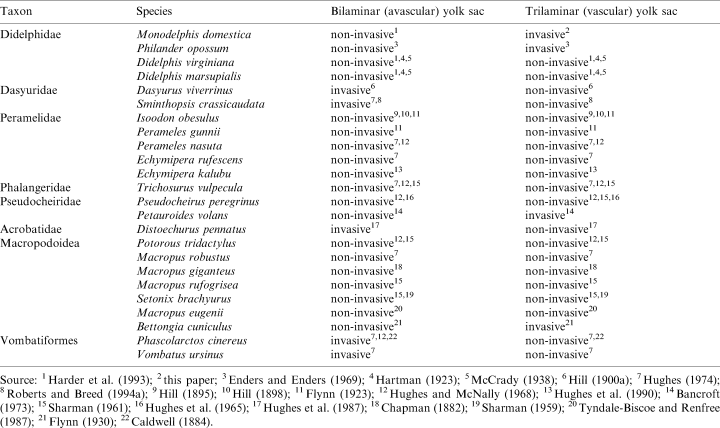
Species names are used according to the nomenclature of Wilson and Reeder (1993). If authors cited in this paper used synonyms, the correct name is given in square brackets. Classification of species into higher taxonomic units also follows the taxonomy of Wilson and Reeder (1993). The species included in both the discussion and the morphotype reconstruction belong to the higher marsupial taxa as summarized in Table 3.
Phylogenetic reconstruction
Reconstruction of morphotypes follows the algorithm for reconstructing ancestral states using parsimony as described by Cunningham et al. (1998). As pointed out by Cunningham et al. (1998), ‘the algorithm uses a “downpass” and “uppass” traversal to optimize ancestral states using two rules: Rule 1: if descendant nodes share any states in common, assign the set of shared states to the ancestor; Rule 2: if no states are shared in descendant nodes, assign the union of descendant’s states to ancestor.’ (p. 362, Box 1). According to the algorithm, the downpass optimization proceeds ‘down’ the tree towards the root, optimizing each ancestral node. The uppass optimization proceeds ‘up’ the tree away from the root, optimizing each ancestral node. In a final optimization, the sets of downpass and uppass reconstruction are optimized to the state that has the greatest number in both reconstructions. This procedure implies that there is no constant outgroup. Instead, the outgroup changes as does the respective ingroup during the process of optimizing within the group of investigation.
This procedure is conducted using the cladogram by Luckett (1994). We favour this hypothesis of marsupial phylogeny, because it is based on total evidence (molecular and morphological data). Since ontogenetic data are often restricted to didelphids and dasyurids, which are representatives of ameridelphians and australidelphians, respectively, consistent data within both groups are assumed to be characters of the marsupial morphotype. Certain of these morphotype hypotheses can be supported by findings among additional australidelphians. Inconsistent data within ameridelphians or australidelphians were polarized using the mentioned method for morphotype reconstruction.
Due to insufficient and inconsistent data concerning marsupial placentation, reconstruction of the allantochorion in the marsupial morphotype was separately conducted by optimizing with the sistergroup (Placentalia) of marsupials and the outgroup of the Theria (Monotremata). In some cases, the state of marsupial morphotype characters (e.g. zona pellucida, mucoid coat) is determined by outgroup comparison with monotremes.
Results
By 3 hpc, 11 tertiary follicles were present in the right ovary investigated. The oocytes contain large yolk vacuoles and are surrounded by follicular epithelial cells. Paired spermatozoa can be seen in the oviduct on SEM images. One hour later, the follicle epithelium degenerates.
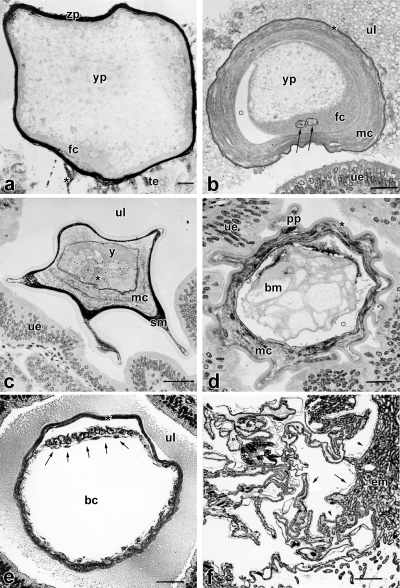
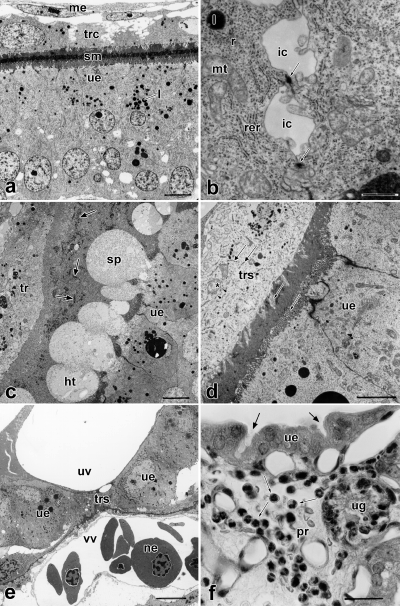
By 22.5 hpc, the corpora lutea have developed. The eight oocytes passing each oviduct lack a corona radiata and are merely surrounded by the zona pellucida, which is PAS-reactive. They measure 170 μm in diameter. The oocyte nucleus is located in the peripheral, formative cytoplasm, which contains minimal yolk (Fig. 2a). The formative, yolk-free cytoplasm encloses the yolky deuteroplasm (Fig. 2a), which in turn encloses a small central zone that contains little yolk. Paired spermatozoa are visible inside the oviduct by light microscopy (LM) and SEM (24 hpc). Fertilization occurs in the oviduct (Fig. 2a). At this time, the second polar body is separated off. The surface of the zygote is in close association with the tubal epithelium (Fig. 2a) and binds the lectin Ulex europaeus agglutinin (UEA) like oviduct cells, but unlike the uterine epithelium. Mucoproteid layers covering the surface of the conceptus are secreted by non-ciliated oviductal cells. Corpora lutea are already present in the ovary.
dpc. The yolk-sac placenta is greatly expanded with elaborate folds increasing the surface area. em, endometrium; arrows, yolk sac; *, embryo; scale bar=500 μm
By 29 hpc, the zygotes (n=5) have entered the uterus in the pronuclear stage. Thus, a maximum of 7 h is needed for the passage down the oviduct. Each zygote is covered by a concentric, lamellar mucoproteid layer (mucoid coat) (Fig. 2b), in which spermatozoa are trapped. A secondary yolk polarity is achieved by polar accumulation of the formative cytoplasm around the pronuclei (Fig. 2b), which fuse at 31 hpc. The uterine epithelium secretes the proteinaceous shell coat, which is Kongo red reactive but does not bind antibodies against keratin.
At the fourth day after copulation, the 16-cell stage (n=1) has been achieved. The yolk, which is not affected by cleavage, forms a compact mass opposite the blastomeres (Fig. 2c). This is due to deutoplasmolysis and apocrine yolk extrusion during the first and second cleavage at 42–50 hpc and 58–68 hpc, respectively (Fromm and Zeller 1997). The diameter of the conceptus measures 180 μm inside the zona pellucida and its entire diameter measures 440 μm. The mucoid coat surrounding the zona pellucida consists of two layers. The thinner outer one is strong PAS reactive, whereas the thicker inner one is less reactive to PAS. Pseudopodia-shaped processes of mucoid coat and shell coat project into the niches of the endometrium (Fig. 2d). The shell coat cannot be stained with PAS.
By 5 dpc, the blastomeres of the conceptus (n=7) contain large yolk vacuoles. One day later, the zona pellucida is partly broken down (n=9). The blastomeres containing yolky lipid droplets in vacuoles form the early unilaminar blastocyst. The mucoid coat is still visible.
By 7 dpc (bilaminar blastocyst, n=10), the mucoid coat and yolk have been incorporated into the blastocyst and the blastomeres are apposed to the peripheral shell coat (Fig. 2e). At this time, the diameter of the blastocyst measures 340 μm and almost doubles within one day (670 μm by 8 dpc). At this stage the pseudopodia-shaped projections are absent. The embryonic area partly consists of a thickened ectoderm with underlying endoderm, and it does not form an inner compact cell mass (Fig. 2e).
The shell coat surrounding the blastocyst cannot be stained with PAS or Alcian blue and does not bind antibodies against cytokeratin, laminin or fibronectin. It is resistant to collagenase, but can be stained for keratin by Martinotti, and Kongo red. Keratin was not detectable by immunohistochemistry.
By 10 dpc (embryo, five to six somites, n=6), the vesicle has formed a bulging sphere 5 mm in diameter. The embryo has a neural groove, neural crest with a developing mesenchyme, a primitive streak, a paraxial mesoderm with five to six somites, a lateral plate mesoderm with pericardium, extraembryonic mesoderm and a yolk sac cavity.
One-third of the yolk sac lying towards the embryonic pole forms the trilaminar omphalopleure, the abembryonic part is bilaminar. Ultrastructurally, the trophendoderm (yolk sac endoderm) consists of flat cells connected by cell projections and tight junctions. Wing-shaped processes of trophendoderm cells partly appose to the bases of trophectoderm cells in bilaminar omphalopleure and project with short processes into the basal folding, whereas extended parts of trophectoderm (ectoderm of the yolk sac) cells remain uncovered. The cells have abundant polyribosomes, mitochondria, endoplasmatic reticula (ER) and lipid droplets.
The trophectoderm (outer wall of the yolk sac) is formed of columnar to flattened distinct cells leaving small intercellular capillaries for transport in between. Apices are united by junctional complexes. Exocytotic vesicles secrete into wide intercellular gaps (about 15 μm in width) between trophendoderm and trophectoderm. The apical surface is densely covered with microvilli directed to the endometrium. The cells contain numerous mitochondria, smooth and rough ER, polyribosomes, vacuoles and lipid droplets. Endocytotic complexes and transport vesicles are present. The cell bases are folded. All this indicates active transepithelial transport of material.
The cells of the one-layered, high-columnar uterine epithelium are densely covered with microvilli on their luminal surface, their bases border a capillary net in the propria. A basal lamina could not be found. The propria contains only few cells. The endometrial glands consist of a thick pad of coiled tubular glands, which in some areas extend for 3 mm. They are devoid of glandular ducts and secrete histiotrophes directly into the uterine lumen. The myometrium does not exceed 0.2 mm. The shell coat is still intact and measures 1.8 μm.
By 11 dpc (4 mm total length), the differentiation of the frontal half of the embryo is highly advanced following rapid development. It shows a strong curvature of head and neck, a dorsally concave lumbar curvature, a foregut with visceral clefts and visceral arches, brain plate with eye anlagen, ear placodes, a heart anlage, and crest-shaped anlagen of upper extremities. The vitelline duct is still wide open. The neural tube, somites, notochord, aortae, coelom and nephrogenic crest can be seen on transverse sections through the embryo. The cranial and caudal ends of the neural tube are still open and form neuropores, respectively. Two-thirds of the anterior end of the embryo are sunk into the yolk sac and covered with the two-layered proamnion (ecto- and endoderm). The caudal third of the embryo is still part of the surface of the conceptus. A small extra-embryonic coelom can be seen between the trilaminar yolk sac and proamnion.
The yolk sac is trilaminar for over one-third of its extension. It measures 5–6 mm in diameter and is greatly expanded with elaborate folds increasing the surface area (cf. Fig. 2f). It is closely apposed to the endometrium (Fig. 3a). The endodermal cells of the bilaminar omphalopleure have long projections enclosing large intercellular spaces between trophendoderm and trophectoderm. In the trilaminar yolk sac wall the endodermal cells are flattened and closely connected to each other. Blood vessels with endothelium and haemocytoblasts are developed within the mesenchyme of the trilaminar yolk sac. The sinus terminalis appears at the border between the bi-and trilaminar omphalopleure.
0 μm
The trophectoderm mainly consists of distinct cells united by desmosomes with intercellular transport capillaries in between. In particular, the cells of the trophectoderm of the bilaminar omphalopleure are flat and contain large vacuoles exceeding the diameter of the cell nucleus. Ultrastructurally, the trophectoderm is formed in most parts by distinct cells, which are apically united by junctional complexes (Figs 3b, 4a) with intercellular transport capillaries in between (Fig. 3b). Some trophectodermal cells of the trilaminar omphalopleure have already fused and intercellular borders can not be seen. The nuclei of those cells are close to each other. The trophectoderm is densely covered with microvilli (Figs 3a, 4a), and pinocytotic vesicles are present on its surface. The cells contain numerous transport vesicles, mitochondria, lipid droplets, rough and smooth ER, free ribosomes, and big euchromatic nuclei with a maximum diameter of 21 μm (Figs 3b, 4a). This indicates the contribution of the trophectoderm to both transport and synthesis.
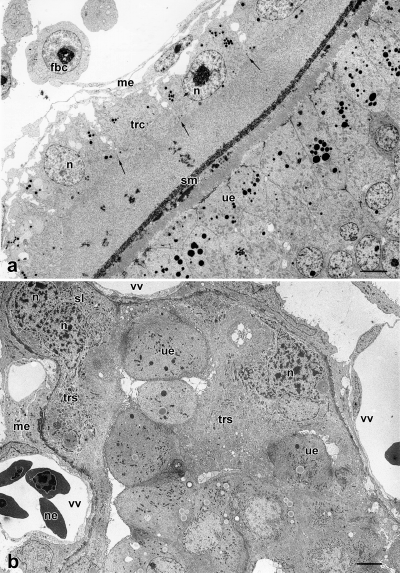
dpc, syncytiotrophoblast. The trophoblast cells fused to form a syncytium (trs). Nuclei (n) are located in syncytial knots (sl). Only thin lamellae of syncytial epithelium separate uterine epithelium and vitelline vessels (vv). The trophoblastic syncytium penetrates the uterine epithelium (ue). me, mesenchyme; ne, nucleated erythrocyte; scale bar=10 μm
The cells of the single-layered, high-columnar uterine epithelium contain often more than one large euchromatic nucleus (up to 15 μm in diameter) with prominent nucleoli (Fig. 3a). They are connected by apical junctional complexes, and are covered with a dense apical border of microvilli of 3 μm length (Fig. 3a). Secretory canaliculi are located between the cells of the apocrine secreting epithelium. The cells contain mitochondria, rER, polyribosomes, secretory granules and abundant lipid droplets (Fig. 4a). The uterine epithelium mainly serves as a pathway for materials from the maternal blood to the embryo. The basal plasmalemma is folded and borders on a capillary net in the propria, which is situated 0.5–2 μm beneath the epithelium. A basal lamina is lacking. The capillary endothelium is not fenestrated. Two layers of endometrial glands can be distinguished: The endometrial glands near the myometrium consist of a single-layered secreting epithelium with basal nuclei and cytoplasm that is comb shaped due to abundant inclusions. The endometrial glands near the uterine epithelium have mostly homogeneous cytoplasm and central nuclei, with inclusions and a comb-shaped cytoplasm that is restricted to their bases. The cells of the uterine glands contain ergastoplasm, dictyosomes, mitochondria, lipid droplets and secretory granules. The surface is covered partly with branching microvilli and partly with kinocilia. The uterine glands are the sites of synthesis and secretion of histiotrophes. The propria contains more free cells (histiocytes, plasmacytes, lymphocytes, polymorphonuclear granulocytes) on its subepithelial side than on its basal side.
The shell coat, which measures 2.3 μm, is unruptured at this stage (Figs 3a, 4a). It consists of narrow meshes, through which all material for the exchange between mother and embryo has to pass. Shell coats of adjacent embryos do not fuse.
By 12 dpc [5 mm crown–rump length (CRL)], the differentiation of the embryo is more advanced, especially in the facial and branchial regions as well as the upper extremities. The large heart supports the yolk sac circulation. The lumbar curvature is flattened out, the caudal amnion expanded and the embryo has completely sunk into the yolk sac. The exocoelom is expanded.
Only the yolk sac, which is now vascularized over two-thirds of its extension, contributes to the placenta (Fig. 2f). The endodermal cells of the vascular part are flat and in close contact with each other. The yolk sac vessels are the sites of blood formation by mitotic multiplication of proerythroblasts and myeloblasts. Immunohistochemistry demonstrates transferrin within the maternal epithelium as well as in the trophectoderm and the underlaying vascular layer of the omphalochorion, indicating transferrin-rich histiotrophe passing the meshes of the shell coat (Zeller 1999a).
The trophectoderm still consists in most parts of distinct, laterally interconnected cells, that are united by apical junctional complexes (compare Fig. 4a). The length of the microvilli is reduced. The cells contain large vacuoles and big nuclei mainly arranged in a regular pattern. At some areas of the trilaminar omphalopleure the nuclei of the trophectoderm are situated close to each other and between those areas the trophectoderm free of nuclei is flattened. The extent of folding of the endometrium is increased (Fig. 2f). The length of microvilli of the uterine epithelium is reduced (1.7 μm). Macro- and microapocrine secretion occurs. The endometrial glands secrete histiotrophes (Fig. 3c). The thickness of the glandular pad is reduced to about 1.5 mm. The former two-layered condition of the glandular pad has been lost. The shell coat, remnants of which can be seen between trophoblast and endometrium (Fig. 3c), is ruptured.
By 13 dpc (8.5 mm CRL), the neural tube is closed, peripheral nerves and ganglia, the eye cup, the labyrinth vesicle, an epithelial nasal sac, a muscular tongue, a chondrified endoskeleton with occipital pillar, an axial skeleton, Meckel’s cartilage and the anterior extremity, a gut canal, liver, heart, lung and mesonephros are differentiated. The frontal acropodium is pentarch, whereas the back one is still paddle-shaped.
The shell coat is completely broken down. Most of the trophectodermal cells of the trilaminar omphalopleure have fused to form a trophoblastic syncytium (see below).
By 14 dpc (10 mm CRL), the embryo has a two-layered head amnion lined by high microvilli on the side of the yolk sac. This indicates a transport of amniotic fluid between yolk sac and amnion. The exocoelom, which never covers the whole embryo, is located ventrolaterally to the embryo, from its navel to the caudal end. The allantois is small.
The trophendoderm of the trilaminar omphalopleure consists of flat, expanded cells united by junctional complexes. It is covered with short microvilli on the side of the yolk sac lumen. The yolk sac vessels around the navel are embedded in large, bulging cells. The trophendodermal cells of the bilaminar omphalopleure are also flattened and close to the base of the trophectodermal cells (Fig. 5d).
μm
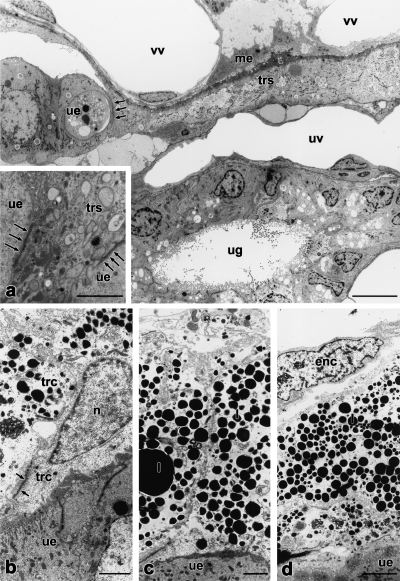
Most cells of the trophectoderm of the trilaminar omphalopleure have fused to form a syncytium (Figs 4b, 5a). Cell borders are rare. The thin basal lamina underlying the single layered trophectoderm measures about 150 nm. The syncytium is covered with microvilli and shows pinocytosis, intracellular vesicles and filaments (Fig. 3d), indicating active transepithelial transport of materials. It is rich in rER, polyribosomes and lipid droplets (Fig. 3d) and multivesicular bodies. Nuclei are located in syncytial knots (2–5 nuclei per knot) (Fig. 4b). Between the syncytial knots are thin lamellae of syncytial epithelium (Fig. 4b). Occasionally, cell borders were found within the syncytial lamellae. Lipid droplets and accumulations of glycogen are mainly located in the knots, cell organelles are rare. The trophectoderm is in close association with the uterine epithelium with an intimate interdigitation of trophoblastic and endometrial microvilli (Fig. 3d). The height of the microvilli does not exceed 1.6 μm. The invasive trophoblast penetrates the maternal epithelium at regular intervals, and reaches the maternal endothelium (Figs 3e, 5a). To the side of the invasive areas, the trophoblast is intimately interconnected with the uterine epithelium by newly formed cellular adhesions (Fig. 5a). The invasive trophoblast displaces the uterine epithelium at either small (Fig. 3e) or expanded areas (Fig. 5a). Thus, a mixed epithelio-endothelio-chorial yolk sac placenta is formed. At the invasive areas, the maternal endothelium, the trophoblast (with thin basal lamina underneath), interconnected cells of the connective tissue of the yolk sac, the extracellular matrix and the foetal endothelium form the layers, through which the foeto-maternal exchange of material and gases takes place. Owing to the thinning of foetal layers, the barrier between foetal and maternal blood does in some areas not exceed 2.5 μm. In these areas, the uterine epithelium is close to the cells of the foetal connective tissue near the vitelline vessels (Fig. 4b). Bilaminar parts of the yolk sac are rare, probably due to fusion of yolk sacs of adjacent embryos and subsequent loss of these areas (Freyer in prep.). In the bilaminar yolk sac wall, fusion of trophectodermal cells could not be found (Fig. 5b–d). Thinning of the trophoblast and accumulations of lipid droplets are similar to the trophoblastic syncytium (Fig. 5b–d). The trophoblast of the bilaminar yolk sac walls were closely attached to the surface of the maternal epithelium. However, invasive areas could not be found.
The endometrium is strongly folded. The largest folds form chambers for single embryos and their foetal appendices. As pointed out above, the height of the microvilli is reduced. The thickness of the endometrial glandular pad is reduced to 0.2–0.4 mm. The thickness of the myometrium remains unchanged at 0.1 mm.
At the 15th day after copulation, the propria is highly invaded by polymorphonuclear granulocytes, macrophages and lymphocytes (Fig. 3f). Granulocytes are also found in the glandular tubes. This is followed by the parturition of the placenta.
Discussion
In the present study of the grey short-tailed opossum, M. domestica, the early ontogeny and placentation from ovulation to parturition are described. To confirm and complement previously published detailed studies and timetables of early embryonic development (Harder et al. 1993; Mate et al. 1994; Selwood et al. 1997), the development of the oocyte and zygote, the embryonic development, as well as changes of the embryonic membranes and the endometrium during ontogeny are considered. Special emphasis is placed on the ultrastructural changes of the placenta, and the development of structures is interpreted functionally, where possible, by correlating data obtained from LM, EM, histochemistry and immunohistochemistry. The following discussion is aimed at reconstructing the marsupial morphotype based on the character distribution of own findings and literature data.
Tubal passage
The entrance of the zygote into the uterus at 29 hpc in M. domestica relates well to the times given by Mate et al. (1994) and Selwood et al. (1997) (24 hpc and 30 hpc, respectively). This short time for passing the oviduct (about 7 h) is equivalent to that found in Macropus eugenii (Macropodidae) with 7–13 h (Renfree and Lewis 1996) and Antechinus stuartii (Dasyuridae), in which the zygote enters the uterus 12 h after fertilization (Selwood 1980). In Didelphis virginiana (Didelphidae), the zygote enters the uterus between 15 and 24 h after ovulation (Hartman 1924; Rodger and Bedford 1982). Thus, a short tubal passage lasting 1 day at maximum can be regarded as a character of the marsupial morphotype.
Egg coats
Zona pellucida and corona radiata
The oocyte of M. domestica is surrounded by the zona pellucida (Phillips and Fadem 1987; Baggott and Moore 1990; Selwood et al. 1997; personal observations). Phillips and Fadem (1987) describe the zona pellucida as 2–3 μm thick and filamentous. The zona pellucida, which is also found in monotremes (Hughes 1984) and eutherians, is present in all marsupial taxa so far examined (cf. Tyndale-Biscoe and Renfree 1987: Didelphidae, Dasyuridae, Peramelidae, Phalangeridae, Macropodidae; Ward and Renfree 1988: Acrobatidae) and is, consequently, a plesiomorphic character of the marsupial morphotype. As already summarized by Tyndale-Biscoe and Renfree (1987), the thickness of the zona pellucida ranges from 1 μm in Didelphis marsupialis (McCrady 1938) to 6.3 ± 1.4 μm in M. eugenii (Hughes and Hall 1984). The width of the zona pellucida, like that of the mucoid coat and shell coat, may change by pressures set up by the extrusion of the yolk mass and the first two cleavages as observed in A. stuartii (Selwood and Young 1983). The disappearance of the zona pellucida by expansion of the blastocyst as we observed in M. domestica (5 dpc) is also shown for D. virginiana (Hartman 1928) and Acrobates pygmaeus (Ward and Renfree 1988). Bancroft (1973) also reports the absence of the zona pellucida at the blastocyst stage of Petauroides volans. Ovulated eggs of M. domestica (Phillips and Fadem 1987; Baggott and Moore 1990; this article), D. virginiana (Krause 1998), Dasyurus viverrinus (Hill 1910) and Sminthopsis crassicaudata (Breed and Leigh 1990) lack a corona radiata. Thus, the absence of the corona radiata can be assumed for the marsupial morphotype.
Mucoid coat
The mucoid coat of M. domestica consists of concentric layers (Phillips and Fadem 1987; this article). Spermatozoa are entrapped in the mucoid coat (Phillips and Fadem 1987; Baggott and Moore 1990; Selwood and Vandeberg 1992; this article). This is also described for other marsupial taxa (D. viverrinus: Hill 1910; M. eugenii: Renfree and Shaw 1996; Schoinobates [Petauroides] volans: Bancroft 1973; A. pygmaeus: Ward and Renfree 1988), which suggests that it may prevent polyspermy in addition to the zona reaction (Selwood 1982; Baggott and Moore 1990). In addition, Hartman (1919) assumes a nutritive function of the mucoid coat in D. virginiana. Furthermore, Selwood (2000) suggests that the mucoid coat as well as the molecules that are trapped in it are taken up by the trophoblast and may thus be a nutritional source for the embryo. The mucoid coat is also suggested to act as an osmotic stabiliser (Selwood 2000). The secretion of the mucoid coat by oviductal cells as described for several other marsupial taxa as well as for monotremes (monotremes: Hughes and Carrick 1978; Trichosurus vulpecula: Hughes 1974; S. crassicaudata: Roberts et al. 1994), is also observed in M. domestica (Phillips and Fadem 1987; Baggott and Moore 1990; this article). Thus, mucoid coat secretion by oviductal cells is a plesiomorphic character of the marsupial morphotype. Accordingly, the oviduct consists of ciliated and non-ciliated secretory cells (Phillips and Fadem 1987; Baggott and Moore 1990; this article). The secretion of mucoid coat precursors in S. crassicaudata is shown to be restricted to the oviduct (Roberts et al. 1994). Similar to D. virginiana (Hartman 1928), T. vulpecula (Hughes 1974), D. viverrinus (Hill 1910), Bettongia cuniculus [B. gaimardi] (Kerr 1935) and A. pygmeus (Ward and Renfree 1988), the mucoid coat in M. domestica disappears, with the zona pellucida, when the blastocyst expands.
Shell coat
Although the marsupial shell coat is suggested to consist of keratin, namely ovokeratin (Hughes 1974; Krause 1998), it was impossible to verify the presence of keratin by immunohistochemistry in M. domestica. This could be due to the absence of keratin, a change of epitopes during condensation or a masking effect.
The shell coat is secreted within the uterus in all marsupial taxa so far examined (M. domestica: Baggott and Moore 1990, this article; T. vulpecula: Hughes 1974, 1977, 1984; Hughes and Hall 1984; S. crassicaudata: Roberts and Breed 1996; A. stuartii: Selwood 1982; personal comm.; D. virginiana: Hartman 1916; McCrady 1938; Dasyurus, Perameles, Trichosurus, Macropus, Petrogale, Phascogale, Acrobates, Phascolarctos, Bettongia: Hill 1910; M. eugenii: Tyndale-Biscoe and Renfree 1987). Thus, at least uterine shell coat secretion can be assumed to be a character of the marsupial morphotype.
Inconsistent data exist concerning a possible oviductal shell coat secretion in addition to the uterine shell coat secretion. Whereas Hill (1910) reports that the shell coat is laid down in the oviduct in Dasyurus, Perameles, Trichosurus, Macropus, Petrogale, Phascologale [Phascogale], Acrobates, Phascolarctos and Bettongia, the shell coat of M. domestica (this article, Baggott and Moore 1996) and T. vulpecula (Hughes 1974, 1977, 1984; Hughes and Hall 1984) was found to be secreted by uterine cells only. As Hughes (1974) points out, although oocytes recovered from the oviduct of T. vulpecula may possess a shell coat, shell glands are restricted to the uterus, suggesting a retrograde flow of uterine shell secretions. However, Tyndale-Biscoe and Renfree (1987) argue that such a retrograde flow is impossible in M. eugenii and other macropodids. In S. crassicaudata, as in M. domestica, only uterine embryos have a shell coat, whereas those recovered from the oviduct have no shell coat (Roberts and Breed 1996). Immunohistochemically, shell coat precursors of S. crassicaudata are found to be restricted to the anterior region of the uterus, but also to the utero-tubal junction and adjacent glands (Roberts et al. 1994). In D. virginiana (Hartman 1916; McCrady 1938) and A. stuartii (Selwood 1982; personal comm.), zygotes recovered from the ‘lower part of the oviduct’ were covered with shell coat material. In the ‘lower oviduct’ of D. virginiana, there are numerous mucosal glands (Andersen 1928); the posibility that they are shell glands cannot be excluded as the function of these is unknown (Tyndale-Biscoe and Renfree 1987). It can be assumed, that the part described as ‘lower oviduct’ in D. virginiana and A. stuartii corresponds to the utero-tubal junction that was found to secrete shell coat precursors in S. crassicaudata. Although Hill (1910) does not mention which part of the oviduct secretes the shell coat, tubal shell secretion can be excluded distal to the isthmus of the oviduct in didelphids (this study; Baggott and Moore 1996), phalangerids (Hughes 1974, 1977, 1984; Hughes and Hall 1984) and dasyurids (Roberts and Breed 1996). Based on this distribution, shell secretion distal to the isthmus of the oviduct can be excluded for the marsupial morphotype. Since the shell coat is secreted by cells of the utero-tubal junction in dasyurids (Roberts et al. 1994) and didelphids (McCrady 1938; Hartman 1916), this can be assumed for the marsupial morphotype as well. In conclusion, the shell is secreted by the utero-tubal junction and the uterus in the marsupial morphotype.
The monotreme and marsupial shell coats have been suggested to be partly homologous (Hughes 1977). The monotreme shell coat was found in eggs recovered from the ‘lower oviduct’, but it is also found to be secreted in the uterus (Caldwell 1887). The presence of shell coat glands in the lower third of the Fallopian tube and within the uterus (Hughes 1977) shows that this is due to both tubal and uterine shell coat secretion. Thus, shell secretion by cells of both uterus and the proximal oviduct (but not distal to the isthmus) may be a plesiomorphic character of the marsupial morphotype.
Yolk arrangement and cleavage
Yolk arrangement
Our observations agree with that of Baggott and Moore (1990) in that the peripheral yolk-poor cytoplasm, which is the formative cytoplasm, surrounds the central yolky cytoplasm (deutoplasm) in the unfertilized oocyte of M. domestica. As this arrangement is also found in unfertilized oocytes of D. virginiana (Krause 1998), S. crassicaudata (Breed and Leigh 1990) and A. stuartii (Selwood and Young 1983), this can be concluded to be a character of the marsupial morphotype. In M. domestica, we additionally observed a central portion of yolk-poor cytoplasm enclosed in the deutoplasm. The yolk bodies of compound yolk and lipid yolk in D. virginiana, as Krause (1998) points out, move toward the peripheral cytoplasm. This probably leads to the three-layer arrangement of central and peripheral yolk-free cytoplasm and subperipheral yolky cytoplasm (Hartman 1916; McCrady 1938).
In the fertilized oocyte (zygote), the yolky cytoplasm is polar-oriented in M. domestica (Baggott and Moore 1990; Selwood and Vandeberg 1992; Breed et al. 1994; Selwood et al. 1997; Zeller 1999a), D. viverrinus (Hill 1910), S. crassicaudata (Breed and Leigh 1990; Selwood and VandeBerg 1992), A. stuartii (Selwood 1982; Selwood and Sathananthan 1988), Phascolarctos cinereus (Caldwell 1887) and T. vulpecula (Frankenberg and Selwood 1998). Thus, polarity of the zygotic yolk is concluded to be a character of the marsupial morphotype. The polarized features in the zygote are suggested to identify the future embryonic and abembryonic poles (Selwood 1994); the side of the pronuclei is suggested to be the embryonic hemisphere, whereas the yolky cytoplasm is located in the abembryonic pole (Frankenberg and Selwood 1998). Although the yolk mass becomes eccentric prior to ovulation in dasyurids (D. viverrinus: Hill 1910; S. crassicaudata: Breed and Leigh 1990) an P. cinereus (Caldwell 1887), fertilization enhances the prior polarity (cf. Merry et al. 1995). The zygote of D. virginiana shows no yolk polarity (McCrady 1938; Hartman 1916), but lipid droplets can be located more on one pole (Hartman 1916; McCrady 1938). However, Hartman (1916) and Hill (1918) agree that ‘an inherent, if nonvisible, polarity probably does exist in the unsegmented ovum’ (Hill 1918; p. 105). Although the pronuclei are first eccentrically located (Hartman 1916), they later migrate to the centre (Hartman 1916; McCrady 1938). The non-polarized yolk arrangement of the zygote is a derived character of D. virginiana with regard to the proposed marsupial morphotype.
Cleavage
In all marsupials so far examined cleavage starts in the uterus (Selwood 2000), probably due to the shortness of the tubal passage. This can also be assumed for the marsupial morphotype.
In M. domestica, the first cleavage is meridional and accompanied by segmentation of most of the yolk and subsequent extrusion of smaller yolk vesicles (Baggott and Moore 1990; Fromm and Zeller 1997; Selwood et al. 1997). The subsequent two-cell stage (46–53 hpc: Selwood et al. 1997) is characterized by apocrine extrusion of further yolk vesicles (Fromm and Zeller 1997). The second cleavage is asynchronous, meridional (Selwood et al. 1997; Fromm and Zeller unpublished) and sometimes latitudinal (Baggott and Moore 1990; Selwood and Vandeberg 1992; Selwood et al. 1997) depending on the amount of yolk. This cleavage is accompanied by further yolk extrusion (Baggott and Moore 1990; Selwood and Vandeberg 1992; Selwood et al. 1997; Fromm and Zeller unpublished). Separated yolk and deutoplasmolysis are also found in D. virginiana (Hartman 1916, 1928), dasyurids (D. viverrinus: Hill 1910; A. stuartii: Selwood and Young 1983; S. crassicaudata: Selwood 1987), phalangerids (T. vulpecula: Frankenberg and Selwood 1998) and acrobatids (A. pygmaeus: Ward and Renfree 1988). Consequently, a cleavage including deutoplasmolysis is a character of the marsupial morphotype. The absence of any separated yolk in the zygote of macropodids (grey kangaroos, M. eugenii: Renfree and Lewis 1996) and the correlated cleavage without deutoplasmolysis is a derived character of this group and may be a synapomorphy of macropodids. The mode of deutoplasmolysis is the same in M. domestica (Didelphidae) and dasyurids (Sminthopsis macroura, S. crassicaudata, A. stuartii). In these taxa, most of the deutoplasm (yolk) is separated off at one pole during the first cleavage, lesser quantities are subsequently extruded as numerous vesicles during the first two cleavages (Selwood 1982; Selwood 1987; Baggott and Moore 1990; Selwood and VandeBerg 1992; Fromm and Zeller unpublished). En bloc segmentation of deutoplasm (yolk) during the first cleavage is also described for D. viverrinus (Hill 1910). Based on character distribution, this mode is a character of the marsupial morphotype. Didelphis virginiana (Hartman 1916, 1919; McCrady 1938) and D. marsupialis (Hill 1918) show no localized en bloc segmentation of deutoplasm (yolk). Instead, deutoplasm is separated off by generalized extrusion of vesicles. This is a synapomorphy of D. virginiana and D. marsupialis with regard to the proposed marsupial morphotype.
As pointed out by Selwood (1982), yolk quantity, yolk polarization and the mode of deutoplasmolysis may be correlated. This correlation is based on the assumption that oocyte dimension is determined by the amount of yolk. Then, large oocytes such as those of D. viverrinus (Hill 1910), S. crassicaudata and S. macroura (Selwood 1987) with 240, 254 and 343 μm diameter, respectively, contain great yolk quantities. Medium-sized oocytes of A. stuartii (Selwood and Young 1983) and M. domestica (this investigation) with 158 and 170 μm contain medium amounts of yolk. Small eggs, as found in D. virginiana (Hartman 1916) and D. marsupialis (Hill 1918) with 135–165 and 144 μm, respectively, are poor in yolk. Early yolk polarization, i.e. before fertilization, was found in species with yolk-rich oocytes, namely Dasyurus and Sminthopsis. In those species, the morphotype mode of deutoplasmolysis was found. The same applies to species with oocytes with a medium amount of yolk (A. stuartii, M. domestica). Both types differ in the time of yolk polarization. In yolk-rich oocytes, polarization first occurs in the ovary oocyte (Hill 1910; Breed and Leigh 1990), whereas in oocytes with a medium amount of yolk, polarization is only found at the pronuclear stage (this paper; Selwood 1982, 1992). The yolk-poor oocytes of D. virginiana and D. marsupialis do not exhibit yolk polarity and the deutoplasmolysis is reduced to a generalized extrusion of vesicles. The smallest oocytes, 126 μm in diameter (M. eugenii: Tyndale-Biscoe and Renfree 1987), are found among macropodids which lack a separated deutoplasm (yolk).
In M. domestica, the asynchronous, third (Baggott and Moore 1990; Selwood and Vandeberg 1992; Selwood et al. 1997; Fromm and Zeller unpublished) and fourth cleavage (Baggott and Moore 1990; Selwood and Vandeberg 1992; this paper) are latitudinal or meridional, the fifth cleavage was found to be latitudinal (Selwood and Vandeberg 1992). The sequence of meridional and latitudinal cleavages leads to a blastocyst at 6 dpc (this paper). At this stage, the blastocoel contains little yolk (this paper; Fromm and Zeller unpublished). We assume that the yolk has been taken up by blastomeres.
In M. domestica, the cells are closely apposed to the zona pellucida at the two-cell stage (Fromm and Zeller unpublished) and eight-cell stage (Baggott and Moore 1990; Selwood et al. 1997; Fromm and Zeller unpublished), whereas no cell contact is established. Baggott and Moore (1990) report first intercellular junctions between blastomeres at the 16-cell stage in M. domestica. Apposition of blastomeres to the zona pellucida prior to intercellular association and connection by tight junctions was also found in A. stuartii (third + fourth cleavage, Selwood and Young 1983), S. crassicaudata (second cleavage, Selwood et al. 1997) and T. vulpecula (second cleavage, Frankenberg and Selwood 1998). In M. eugenii, the blastomeres are in close association with one another during the first cleavages, but no tight junctions are present at least until the eight-cell stage is reached (Renfree and Lewis 1996). This is probably a derived character of macropodids. This could be correlated with the absence of separated yolk in the zygote (see above). The apposition of blastomeres to the zona pellucida prior to intercellular association, and the consequent absence of a morula stage (Selwood 1996) is a character of the marsupial morphotype, since this is present in ameridelphian didelphids, as well as in australidelphian dasyurids and phalangerids.
Placentation
Our observations agree with that of Harder et al. (1993) concerning both the close apposition of the trophectoderm to the uterus at 13 and 14 dpc and the microvillous surface of the endo- and trophectoderm in M. domestica. Our observed increase of foldings at 12 dpc is confirmed by the increase of the index of epithelial surface area from 1.66 at 9 dpc to 6.9 at 13 dpc measured by Harder et al. (1993). We can further confirm, that M. domestica possesses a choriovitelline placenta (Harder et al. 1993; Roberts and Breed 1994a) during the entire development from implantation to birth. The observed drop in uterine gland density in M. domestica between 9 and 14 dpc (Harder et al. 1993) can be correlated with the decreasing thickness of the uterine gland pad from 3 mm at 10 dpc, 1.5 mm at 12 dpc to 0.2–0.4 mm at 14 dpc. This indicates the shift from histiotrophic to haemotrophic nutrition of the embryo.
Invasiveness of the placenta
Occurrence and degree of invasion of uterine epithelium by the trophectoderm of the bilaminar and trilaminar yolk sac varies among marsupials as summarized in Table 4.
Contrary to earlier reports given by Harder et al. (1993) and Roberts and Breed (1994a), who report the trophectoderm of M. domestica to be non-invasive, our electron-microscopic observations clearly demonstrate that the trophoblastic syncytium of the trilaminar yolk sac penetrates the uterine epithelium and is thus invasive (Fig. 3e, Fig. 4b). An invasive trophectoderm in a didelphid was only known for Philander opossum (Enders and Enders 1969).
The variation between invasive and non-invasive trophectoderm among closely related species may be explained by either interspecific variation or a lack of information. The latter suggestion is supported by the findings of an invasive trophectoderm in M. domestica, which was formerly described as possessing a non-invasive trophectoderm. Thus, this character needs to be re-examined during the entire ontogeny from implantation to birth, and by EM.
Formation of an allantochorion and yolk sac placenta
The different conditions of the allantois relative to the chorion found in different marsupial taxa were often mentioned and grouped into four placental types (Sharman 1959; Hughes 1984; Tyndale-Biscoe and Renfree 1987), which are summarized in Table 5.
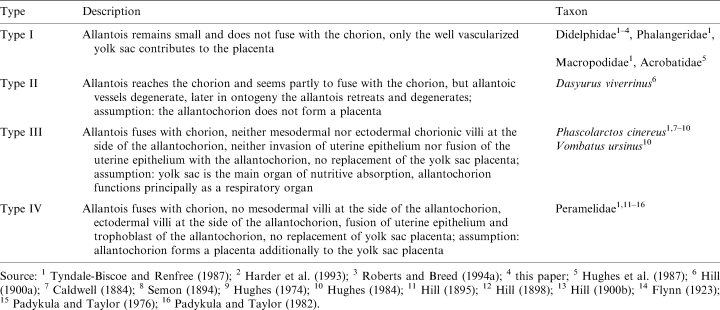
However, it must be kept in mind that this classification is an artificial grouping of different characters, namely the contact between allantois and chorion, the size of allantois and yolk sac, their vascularization and the function of both allantois and yolk sac. This is the reason why a character polarization based on these types has failed. For a phylogenetic interpretation of these findings, a polarization of homologous characters is needed. To date, the insufficient and inconsistent data concerning the allantochorion in marsupials can only be polarized by comparison with the sistergroup (eutherians) of marsupials and the outgroup of the Theria (monotremes). Since a fusion between chorion and allantois was found in Dasyuridae, Vombatiformes and Perameloidea, and this is a character also found in eutherians (Wooding and Flint 1994) as well as in monotremes (Caldwell 1887), this may be a plesiomorphic character of the marsupial morphotype. Thus, the absence of a chorioallantois in didelphids on one side and australidelphian taxa (acrobatids, macropodids, phalangerids) on the other side is a convergent reduction. The parsimony of this assumption, however, is weakened by the absence of an allantochorion in the dasyurid S. crassicaudata (Roberts and Breed 1994a). In a morphotype reconstruction using parsimony (see Cunningham et al. 1998), the downpass reconstructed marsupial morphotype remains ambiguous, but the uppass reconstruction suggests an allantochorion to be present in the marsupial morphotype (Fig. 6). The absence of an allantochorion in phalangeroids (phalangerids, acrobatids) and macropodids may thus be synapomorphic. Since the allantochorion is vascularized in monotremes (Caldwell 1887), placentals (Wooding and Flint 1994), peramelids and vombatiforms, it is to date parsimonious to assume a reduction of this vascularization in D. viverrinus derived from a vascularized allantochorion in the marsupial morphotype. This preliminary polarization, however, demands a re-examination of recent findings, the examination of the ontogeny from implantation until birth in further species and the re-evaluation of marsupial systematics, which are in progress (Freyer, Zeller and Renfree in prep.). We are aware that our hypothesis appears to contradict with the interpretation of Luckett (1977; p. 490), who assumes a ‘retardation in expansion of the exocoelom and allantois’ in the marsupial morphotype.
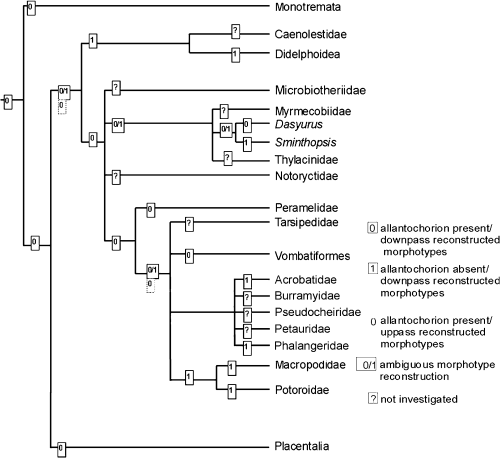
Reconstruction of the allantochorion in the marsupial morphotype using parsimony as described by Cunningham et al. (1998)
Although the chorioallantoic placenta in marsupials never forms mesodermal villi and never replaces the yolk sac placenta as that of eutherians (Luckett 1977), it can be assumed that the allantochorion in vombatiforms and peramelids serves at least respiratory function (Luckett 1977; Semon 1894; Hughes 1984; Tyndale-Biscoe and Renfree 1987). A respiratory function of the vascularized allantochorion in the marsupial stem species seems likely. However, it is unknown whether this allantochorion formed a placenta which also allowed the exchange of material.
All marsupials so far examined possess a yolk sac placenta for most of the time after implantation (cf. Tyndale-Biscoe and Renfree 1987). Thus, a yolk sac placenta can be assumed for part of the ontogeny in the marsupial morphotype (Luckett 1977), irrespective of the formation of an allantois placenta at near-term stages.
Formation of a trophoblastic syncytium
Zeller (1999a) provides the first evidence of a trophoblastic syncytium ever observed in a marsupial by EM. Here, we give a detailed description of the ultrastructure of this syncytium by presenting original electron micrographs. We assume that the aggregations of nuclei in plasma accumulations are syncytial knots rather than plasmodia, i.e. nuclear division. This assumption is based on the constant nuclear sizes from 11 to 14 dpc (Fig. 4a versus 4b), the absence of evidence on nuclear divisions at any stage, and the great extension of the syncytial parts relative to former cell sizes and regular cell arrangements with tight junctions between single cells (Fig. 4a versus 4b). Hill (1900) reported a trophoblastic syncytium in D. viverrinus by LM. As the cell boundaries can hardly be seen by LM, this description needed to be verified by EM. However, the electron microscopic findings in M. domestica presented herein make a syncytium in D. viverrinus highly probable. There is no report of fusions between cells of the trophoblast or extended parts of it in any other marsupial species. If further examinations confirmed a trophoblastic syncytium to be present in D. viverrinus, a trophoblastic syncytium could be assumed for the marsupial morphotype.
The description of trophoblastic giant cells in M. domestica by Roberts and Breed (1994a) may represent syncytial knots of the trophoblastic syncytium not identified by the authors. The question whether the trophoblastic giant cells as observed in other marsupial species, e.g. S. crassicaudata (Roberts and Breed 1994a), are syncytical knots can only be answered through further examination.
Formation of a feto-maternal syncytium
Until now, there has been no evidence for a foeto-maternal syncytium in M. domestica. A foeto-maternal syncytium is known for the bilaminar yolk sac of Dasyurus sp. and P. cinereus (Flynn 1923). In S. crassicaudata, cells of the trilaminar omphalopleure partly fuse with uterine epithelial cells (Roberts and Breed 1994b). In peramelids (Isoodon macrourus), the trophoblast of the chorioallantois ‘may have fused with maternal homokaryons to create placental heterokaryons’ (Padykula and Taylor 1982; p.95). This data base is insufficient for a morphotype reconstruction.
Parturition
The timing of parturition in M. domestica determined to be 15 dpc in this study equals that of 15.2 ± 0.3 (n=16) found by Harder et al. (1993) and almost matches that of 13.5–14 days after fertilization found by Moore (1992).
At the time of parturition, the propria of M. domestica was found to be highly invaded by polymorphonuclear granulocytes, macrophages and lymphocytes (Fig. 3f). Granulocytes are also found in the glandular tubes. In a single specimen of S. crassicaudata, which had given birth 3–7 h before the time of death, ‘the nuclei of the trophoblast giant cells had become pyknotic and there was a massive infiltration of granular leucocytes and lymphocytes into the endometrial stroma’ (Roberts and Breed 1994a, p.109). In late pregnancy (CRL 2.9–3.5 mm), scattered neutrophils, lymphocytes and macrophages were found in the endometrial stroma of S. crassicaudata, ‘particularly below the invasive trophoblast giant cells’ (Roberts and Breed 1994a; p. 108). Although Cruz and Selwood (1993) report that lymphocytes are clustered in a dense mat in the uterine stroma adjacent to the basal lamina of the endometrial epithelium in both pregnant and nonpregnant specimens of A. stuartii, it is striking that the number of lymphocytes was found to be significantly greater in pregnant than non-pregnant animals on the first and 15th day after ovulation. In addition, the lymphocyte density decreases in non-pregnant animals, but increases in pregnant animals from the fourth to the eighth day after ovulation. An infiltration of the placenta by polymorphonuclear leucocytes is also reported for two specimens of Perameles obesula [Isoodon obesulus] (Flynn 1923). Whether this inflammatory reactions is in any way related to birth is still unknown.
Conclusions
This study is a contribution to the reconstruction of the morphotype of recent marsupials by providing current findings, a character polarization and a phylogenetic interpretation. This is based on marsupial systematics as proposed by Luckett (1994).
Our data support most of the previous examinations on the early ontogeny and placentation of M. domestica. We describe both the ultrastructure of a trophoblastic syncytium and an invasive trophoblast for the first time in M. domestica by presenting original electron micrographs. Table 6 summarizes characters that are concluded to be present in the marsupial morphotype and those that are derived, based on the character distributions within marsupials (Fig. 7).
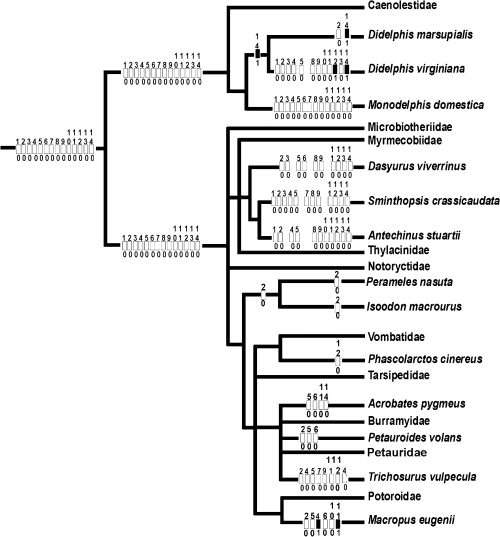
Reconstruction of the early ontogeny in the marsupial morphotype using parsimony as described by Cunningham et al. (1998). For character coding see Table 6. Numbers above rectangle are character numbers; the numbers below rectangle are the character states
To date, polarization of most characters of the marsupial placenta is impossible due to insufficient and incongruent data. Obviously, a yolk sac placenta is established at early stages of placentation in the marsupial morphotype. In a preliminary parsimony analysis, a vascularized allantochorion can be assumed for the marsupial morphotype. The contribution of this allantochorion to placentation in the marsupial morphotype has not yet been established.
The early ontogeny and placentation in the stem species of recent marsupials may have occurred as follows: When released from the oviduct, the oocyte was covered by the zona pellucida and a corona radiata was lacking. The yolky cytoplasm was centrally located and surrounded by the formative, yolk-poor cytoplasm. Fertilization occurred in the oviduct. Prior to or shortly after fertilization, the yolky cytoplasm migrated to one pole (abembryonic pole) opposite the formative cytoplasm (embryonic pole). During the passage down the oviduct, the oocyte became surrounded by the mucoid coat, which was secreted by non-ciliated oviductal cells. The remaining spermatozoa were entrapped into the mucoid coat, preventing polyspermy. The mucoid coat also prevented direct contact between the oocyte and the tubal epithelium and may also have functioned as an osmotic stabilizer. With the assistance of ciliated oviductal cells, the conceptus reached the uterus within 1 day after ovulation. There was no cleavage during the tubal passage. Cells of both the utero-tubal junction and uterus secreted shell coat precursors, which polymerized into the shell coat on the mucoid coat. The shell coat may have consisted of keratin which differed in its chemical characteristics to known human keratins. The meshes of the shell coat could be passed by transferrin, nutrients and gases, but it also prevented direct contact of the conceptus with the uterine epithelium. The cleavage of the zygotes that contained moderate to large amounts of yolk started in the uterus. Most yolk was separated off at one pole during the first cleavage. Lesser quantities were subsequently extruded during the first two cleavages. A sequence of equatorial and latitudinal cleavages lead to the unilaminar blastocyst. Blastomere–zona adhesions preceded blastomere–blastomere connections. Thus, a morula stage was omitted.
Subsequently, the yolk was reabsorbed by the blastomeres and the blastocyst expanded. The zona pellucida and the mucoid coat disappeared during this process. The uptake of mucoid coat and entrapped molecules may have served as a nutritional source for the embryo. Before implantation the embryo was nourished by secretions (histiotrophe) of the uterine glands. Within the last third of pregnancy, the shell coat broke down and the trophectoderm apposed to the uterine epithelium. In certain areas the trophoblast may have penetrated the uterine epithelium. Early at implantation, the placenta was solely formed by the yolk sac. Histiotrophic nutrition may have been shifted toward an exchange of material and gases between foetal and maternal vessels (haemotrophic nutrition). Later in ontogeny, a vascularized allantochorion was formed, but did not replace the yolk sac placenta. It is unknown, whether this allantochorion served material transfer beside its respiratory function.
We regard the reconstruction of the marsupial morphotype as a first step toward an evolutionary (holistic) scenario that explains mammalian evolution arround the Cretaceous– Tertiary boundary.
Acknowledgements
We thank Dr H.-J. Kuhn for his support in maintaining the breeding colony of Monodelphis domestica at the Institute of Anatomy, University of Göttingen, until 1994 and for providing research facilities. We are indebted to the University of Mainz, Institute of Zoology, for providing research facilities during Dr Zeller’s stay there from 1994 to 1996. We are also grateful to the Humboldt-University Berlin for the substantial support in building new research facilities including animal housing at the Museum für Naturkunde since 1996. We thank Mrs. J. Zeller for technical assistance. We are indebted to Dr M. Ade, Dr S. Frahnert and Dr A. Mess for critical comments and fruitful discussions. We especially thank Dipl. Biol. P. Giere for his assistance with linguistic corrections. We thank Dr P. Luckett, Dr L. Selwood, Dr M. B. Renfree and Dr G. Shaw for critical comments and fruitful discussions. We especially thank Dr M. B. Renfree for her kind permission to use her research facilities during Dr Zeller’ s stay there from 3–16 October 2000.