New results concerning the morphology of the most ancient dragonflies (Insecta: Odonatoptera) from the Namurian of Hagen-Vorhalle (Germany)
Abstract
enThe holotype specimen of the ‘protodonate’Erasipteroides valentini (Brauckmann in Brauckmann et al., 1985) and the paratype specimen K-13 of the giant ‘protodonate’Namurotypus sippeliBrauckmann and Zessin, 1989 from the Upper Carboniferous (Namurian B) of Hagen-Vorhalle (Germany) are redescribed, and a new specimen of Erasipteroides cf. valentini is described. The new evidence is used to refine the groundplan reconstruction of Odonatoptera and the reconstruction of odonatoid phylogeny. Prothoracic winglets for Erasipteroides and the absence of an archaedictyon are documented. Furthermore, a very long and sclerotized ovipositor with gonangulum is described from the female holotype specimen of Erasipteroides valentini, and it is proposed that it was not used for endophytic but for endosubstratic oviposition. The record of prothoracic winglets in early odonatoids, and their presence in fossil Palaeodictyoptera and ‘protorthopteres’, indicates that the groundplan of Pterygota indeed included three pairs of wings. A phylogenetic analysis suggests that the Palaeozoic giant Meganisoptera and “higher” odonatoids (incl. crowngroup Odonata) together form a monophyletic group which is here named Euodonatoptera. Erasipteroides and the other ‘Erasipteridae’ are shown to be more closely related to Euodonatoptera than to Eugeropteridae. The description of the male primary genital structures of Namurotypus sippeli is emended and a new interpretation is proposed, including new hypotheses concerning their function. The males of Namurotypus had a paired penis with a pair of lateral parameres, and a pair of leaf-like, but still segmented, gonopods. Segmented leg-like male gonopods are considered as a groundplan character of insects, while a paired penis is regarded as a putative synapomorphy of the palaeopterous insect orders Palaeodictyopteroida, Ephemeroptera, and Odonatoptera. It is proposed that Namurotypus did not mate by direct copulation but retained the archaic deposition of external spermatophores, just like the primarily wingless insects. The sigmoidal male cerci may have been placed behind the female head and used to drag the female over the spermatophore, which is remotely similar to the mating behaviour of some extant arachnids (e.g. Amblypygi). Three hypothetical scenarios regarding the evolution of secondary copulation in modern Odonata are proposed.
Abstract
deNeue Erkenntnisse zur Morphologie der ältesten Libellen (Insecta: Odonatoptera) aus dem Namurium von Hagen-Vorhalle (Deutschland)
Das Holotypusexemplar der ‘Protodonate’Erasipteroides valentini (Brauckmann in Brauckmann et al., Geol. Paläont. Westfalen 3, 1–131, 1985) und das Paratypusexemplar K-13 der riesenwüchsigen ‘Protodonate’Namurotypus sippeliBrauckmann and Zessin, 1989 aus dem Oberkarbon (Namurium B) von Hagen-Vorhalle (Deutschland) werden wiederbeschrieben. Die neuen Erkenntnisse werden zu einer Präzisierung der Grundplanrekonstruktion der Odonatoptera und für die Rekonstruktion der Libellenstammesgeschichte verwendet. Für Erasipteroides werden prothorakale Flügelchen beschrieben und das Fehlen eines Archaedictyons wird belegt. Des weiteren wird ein sehr langer und sklerotisierter Ovipositor mit Gonangulum für das weibliche Holotypusexemplar von Erasipteroides valentini beschrieben, und es wird vorgeschlagen, dass dieser nicht zur endophytischen Eiablage, sondern zur endosubstratischen Eiablage diente. Der Nachweis prothorakaler Flügelchen bei frühen Libellen sowie deren Vorkommen bei fossilen Palaeodictyoptera und ‘Protorthopteren’, deutet darauf hin, dass zum Grundplan der Pterygota drei Flügelpaare gehörten. Eine phylogenetische Analyse legt nahe, dass die riesenwüchsigen Meganisoptera des Paläozoikums und die ‘höheren’ Odonaten (inkl. Kronengruppe Odonata) gemeinsam eine monophyletische Gruppe bilden, die hier als Euodonatoptera benannt wird. Es wird gezeigt, dass Erasipteroides und die übrigen ‘Erasipteridae’ näher mit den Euodonatoptera verwandt sind als die Eugeropteridae. Die Beschreibung der primären männlichen Geschlechtsorgane von Namurotypus sippeli wird ergänzt, und eine neue Interpretation sowie neue Hypothesen zu deren Funktion werden vorgestellt. Die Männchen von Namurotypus besaßen einen paarigen Penis mit einem Paar lateraler Parameren und einem Paar blattartiger, aber noch segmentierter Gonopoden. Segmentierte, beinartige, männliche Gonopoden werden als Grundplanmerkmale der Insekten angesehen, während ein paariger Penis als potentielle Synapomorphie der paläopteren Insektenordnungen Palaeodictyopteroida, Ephemeroptera und Odonatoptera betrachtet wird. Es wird vorgeschlagen, dass die Paarung bei Namurotypus nicht durch eine direkte Kopulation ablief, sondern durch das Absetzen freier Spermatophoren, so wie bei den primär flügellosen Insekten. Die sigmoidalen männlichen Cerci könnten hinter dem weiblichen Kopf platziert worden sein, um das Weibchen über die Spermatophore zu dirigieren, ähnlich dem Paarungsverhalten mancher rezenter Spinnentiere (z.B. Amblypygi). Drei hypothetische Szenarien zur Evolution der sekundären Kopulation bei modernen Libellen werden vorgestellt.
Introduction
Without doubt, the brickworks quarry Hagen-Vorhalle (Ruhrgebiet, Germany) represents the most important locality yielding fossil insects from the lower Upper Carboniferous (Namurian B, Upper Marsdenian, about 310–315 Ma ago. Within the last 15 years more than 150 specimens with at least 16 species of five different orders have been discovered (Brauckmann 1988, 1991a; Brauckmann and Gröning 1998), many of them are completely preserved. Unfortunately, no further excavations at this locality will be possible. The three species of fossil ‘protodonates’ from this locality (viz Erasipteroides valentini (Brauckmann in Brauckmann et al. 1985); Namurotypus sippeliBrauckmann and Zessin, 1989 [first mentioned by Zessin (1989) and then figured in Zessin (1990); and Zessinella siopeBrauckmann 1988) belong to the oldest known representatives of the odonatoid clade (Brauckmann 1987; Zessin 1993; Brauckmann et al. (1996). As some of these ‘protodonate’ specimens show not only the wings, but also some other interesting details of the body morphology (head with antennae, spiny legs, thorax and abdomen), and two of them even have the male and female genitalia preserved, they are of outstanding scientific value for the reconstruction of the evolution of these body structures. This is even more important, as extant dragonflies exhibit a very complex mating behaviour (mating wheel) which involves a curious male secondary copulation apparatus on the basal abdomen as well as structures and mechanisms for removal of foreign sperm prior to sperm transfer. The evolution of these structures and behaviours was rather enigmatic, so that the fossils mentioned could play a key role in the solution of some evolutionary riddles (Zessin 1995; Bechly et al. in prep.).
The holotype of Erasipteroides valentini was described by Brauckmann et al. (1985) and afterwards again studied by Brauckmann and Zessin (1989) and Brauckmann (1991a). Meanwhile, a further preparation by Mr W. Sippel (Ennepetal) yielded numerous new details which require an amendment to the original description.
Brauckmann and Zessin (1989) and Brauckmann (1991a) described a unique specimen of the giant dragonfly Namurotypus sippeli which is the only known ‘protodonate’ specimen clearly showing the male primary genitalia. This specimen was also figured and discussed by Kukalová-Peck (1991). Our re-examination of the counterplate of this important fossil, which is meanwhile accessible for scientific study, yielded some new details and also revealed a few imprecise details in the original description. The latter were caused by the state of preservation of the only positive plate then available. Therefore, a revised description of the concerning structures was necessary and stipulated new considerations about their function and evolution.
Materials and methods
The three fossil dragonfly specimens from the Namurian of Hagen-Vorhalle, described in detail in this work, are deposited in the private collection of Dr M. Kemper (Bochum) (holotype of Erasipteroides valentini, and plate and counterplate of the paratype specimen K-13 of Namurotypus sippeli), and at the Westfälisches Landesmuseum – Planetarium Münster (Erasipteroides cf. valentini, specimen P 26 133).
The terminology of dragonfly wing venation is based on the interpretation of Riek and Kukalová-Peck (1984) and amendments by Kukalová-Peck (1991) and Bechly (1996). The classification is based on the new phylogenetic system of fossil and extant odonatoids by Bechly (1996, 1999a, b). Character analysis and interpretation as well as the reconstruction of phylogenetic relationships strictly follow the principles of consequent Phylogenetic Systematics (sensuHennig 1966, 1969; recently elaborated by Wägele 2000).
Results
Erasipteroides valentini (Brauckmann in Brauckmann et al. 1985) (‘Eomeganisoptera’: ‘Erasipteridae’), holotype
The following description is based on our examinations of the re-prepared holotype specimen which yielded several surprising new results (12Figs 145–6).
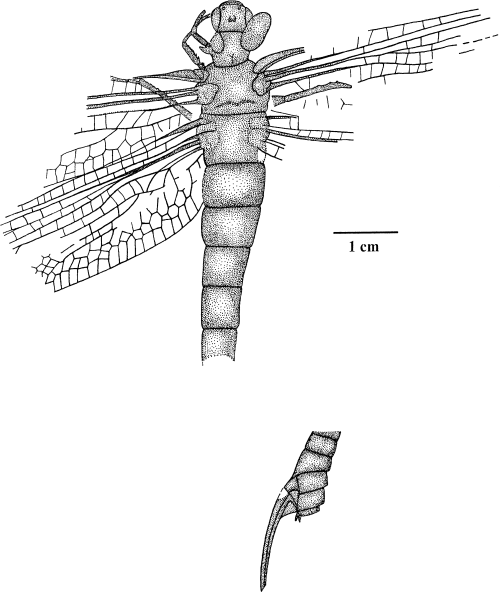
Erasipteroides valentini (Brauckmann in Brauckmann et al. 1985), holotype coll. Kemper, state after further preparation; Upper Namurian B, Hagen-Vorhalle (Ruhrgebiet, Germany)
Erasipteroides valentini (Brauckmann in Brauckmann et al. 1985), holotype coll. Kemper, state after further preparation. Photo by W. Sippel (under alcohol cover with polarized light)
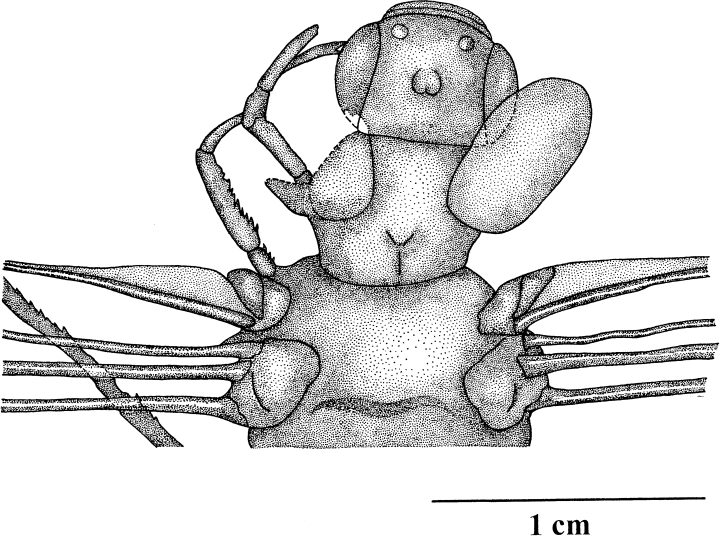
Erasipteroides valentini (Brauckmann in Brauckmann et al. 1985), holotype coll. Kemper, head and prothorax with prothoracic winglets; Upper Namurian B, Hagen-Vorhalle (Ruhrgebiet, Germany)
Erasipteroides valentini (Brauckmann in Brauckmann et al. 1985), holotype coll. Kemper, head and prothorax with prothoracic winglets. Photo by W. Sippel (under alcohol cover with polarized light)
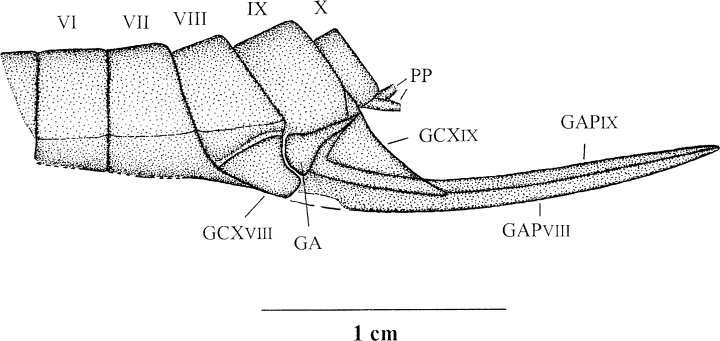
Erasipteroides valentini (Brauckmann in Brauckmann et al. 1985), holotype coll. Kemper, terminalia with ovipositor; Upper Namurian B, Hagen-Vorhalle (Ruhrgebiet, Germany). PP, bases of paraprocts?; GA, gonangulum; GAPVIII and GAPIX, gonapophyses of VIII and IX. abdominal segment, respectively; GCXVIII and GCXIX, gonocoxae of VIII and IX abdominal segments, respectively

Erasipteroides valentini (Brauckmann in Brauckmann et al. 1985), holotype coll. Kemper, terminalia with ovipositor. Photo by W. Sippel (under alcohol cover with polarized light)
Head
The head has a maximum width of 13.4 mm and a median length of 10.1 mm (Figs 3,4). The large compound eyes are clearly separated with a min. distance of 7.9 mm. In the middle or the dorsal surface of the head, there is a well-defined rounded bulge which is divided by a medio-longitudinal ridge in the posterior part. This structure most likely represents the ocelli which are located on such a bulge in the middle of the vertex in extant dragonflies as well. Lateral to this bulge there are two small round structures on the right side of the head, but these seem more likely to be diagenetic artefacts, as there are no traces of such structures visible on the left side. Additionally, two small circular structures are on the anterior part of the frons (lateral of the anterior end of the compound eyes) which presumably represent the bases of the antennae. Unfortunately, the antennae are not preserved. A projecting clypeus is clearly visible and is divided by an indistinct suture into ante- and postclypeus. The mouth parts are not visible.
Thorax
The prothorax is 10.7–12.6 mm wide and 10.7 mm long and bears laterally a prothoracic winglet on each side which is obliquely oriented into cranial direction, although the left winglet is only incompletely preserved (Figs 3,4). The completely preserved right winglet is of oval shape and is 13.1 mm long and a maximum of 7.0 mm wide. Venation and articulation may have been present, but are not clearly visible. Consequently, it is not possible to decide whether the prothoracic winglets were movable or if they were fused like paranotal lobes with the pronotum. The prothoracic winglets are also faintly visible in the specimen attributed to Erasipteroides cf. valentini housed in the collection of the Münster Museum (Figs 78–9). Meso- and metathorax each bear a pair of large wings (forewing length 80 mm, hindwing length 77 mm). The wing articulation is preserved and even shows the characteristic details of the ‘protodonate’ costal- and radio-anal plates on the mesothorax. The new preparation revealed several additional details of the wing venation which are illustrated in Fig. 1. The originally described archaedictyon in this species turned out to be a diagenetical artefact; neither the holotype nor the specimen at the Münster Museum possess an archaedictyon (reduction). The vestige of the media-stem in the left hindwing is somewhat longer than featured in the original description, since it extends up to the origin of CuA + 0. Only the three left legs and the base of the right foreleg are partly visible. Contrary to Namurotypus the legs are still rather short (a comparison with other insect groups suggests that this is most likely a plesiomorphy), which is also the case in the specimen from the Münster Museum. The legs are equipped with numerous strong and relatively short spines which are of different length and irregular distribution. As the latter character state is also present in the primitive Meganisoptera species Namurotypus sippeli, it seems to be a symplesiomorphic state, because Meganisoptera proves to be more closely related to the crowngroup Odonata than ‘Erasipteridae’ by several shared derived similarities (see below).
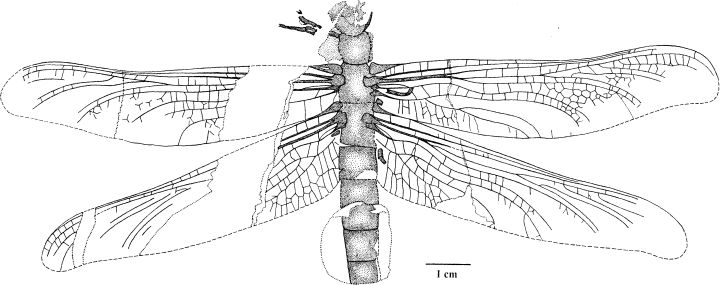
Erasipteroides cf. valentini (Brauckmann in Brauckmann et al. 1985), specimen P 26 133 Westfälisches Museum für Naturkunde – Planetarium Münster

Erasipteroides cf. valentini (Brauckmann in Brauckmann et al. 1985), specimen P 26 133 Westfälisches Museum für Naturkunde – Planetarium Münster, anterior part of body
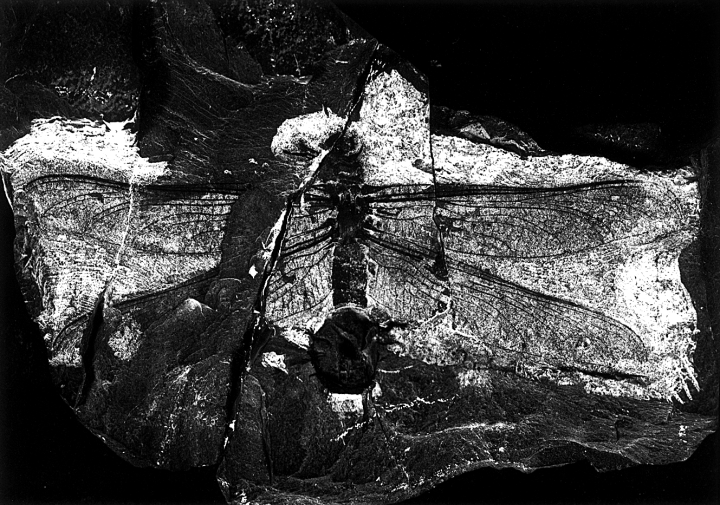
Erasipteroides cf. valentini (Brauckmann in Brauckmann et al. 1985), specimen P 26 133 Westfälisches Museum für Naturkunde – Planetarium Münster. Photo by G. Thomas, Münster
Abdomen
The basal abdominal segments I to V are preserved in connection with the body (Figs 5,6). Except for the shortened first segment (length 3.4 mm), all segments have about the same length (6.3–7.1 mm). The abdomen is a maximum of 10.3 mm wide. The distal half of the abdomen with segments VI (partial) to X is detached from the body and is situated about 15 mm obliquely right behind the body. This part of the abdomen is preserved in lateral aspect (the remaining body is embedded in dorsal aspect!) and has a strongly developed sable-like ovipositor. Considering the position, size, shape, and preservation of this detached abdomen it certainly belongs to the same dragonfly specimen as the rest of the body. Thus, it is a female specimen. The gonocoxae VIII and IX with their respective gonapophyses (valvulae of ovipositor) are distinctly visible. The gonapophyses are strongly sclerotized, only weakly curved, and extremely elongated (length 17.0 mm), so that the ovipositor is extending far beyond the end of the abdomen. A distinctly separated triangular sclerite is visible between the two gonocoxae, and obviously represents the gonangulum. A gonostylus is not preserved, and even a possible base of a stylus is not visible on gonocoxa IX. It cannot be decided whether the stylus was indeed reduced in this species, or is just not preserved in this particular specimen. The same applies to the lack of paired terminal appendages (cerci and paraprocts). However, on the ventral margin of the last abdominal segment (X) are two tiny structures visible which might represent remains of the cercal bases or rather (due to the ventral position) the paraprocts. The distal part of the ventral side of segment VII is posteriorly projecting up to the middle of gonocoxa VIII.
Erasipteroides cf. valentini at Münster Museum (catalogue no. P 26 133)
This specimen, which is herein described in detail for the first time, was discovered during excavations of the Westfälisches Museums für Naturkunde (Paläontologische Bodendenkmalpflege) (Figs 78–9). It was chosen as cover picture of the ‘Neujahrsgruss 1999’ of the Landschaftsverband Westfalen-Lippe, and was briefly discussed by Schöllmann (1999).
The fossil has a wing span of 165 mm (wing length 77.5 mm) and the length of the preserved part of the body is 62.5 mm. Contrary to the holotype, this specimen has a less distinctly preserved body, but rather well-preserved wings which only insignificantly differ in size and venation from those of the holotype. Minor differences in wing venation have to be expected in such a densely veined wing anyway (compare Zessin 1983: 67–68 for Meganeuridae; and Brauckmann 1991b for the Palaeodictyoptera species Homoioptera vorhallensis Brauckmann & Koch 1982). Consequently, we tentatively consider the two main differences in the wing venation (namely a shorter stem of media and a straighter course of AA) as intraspecific variation. Other differences, mainly in the proportions of head and prothorax, are explained by a taphonomic torsion of these body parts.
Preservation
The specimen was originally completely embedded and is visible in dorsal aspect. A few remains of the legs are still visible beneath the wings. The head and prothoracic region are somewhat angled to the left side and also turned, so that this area is obliquely visible in lateral aspect. This torsion also explains the different proportions compared to the holotype. A crack runs from the left side of the head obliquely backwards over the proximal parts of the wings. On the left side of this crack a band of 1.5 cm maximum width of the mesothoracic wing is broken off, whereas most of the metathoracic wing is completely gone, except for a small area near the apex. The mentioned crack also forms an ‘artificial’ lateral border of the left prothoracic winglet. Only a slim area of the base of the right prothoracic winglet is preserved, which could be explained by the torsion of this body area and the subsequent compression of the sediment. A further longitudinal crack is present at 1.7–2.0 cm distance, subparallel to the right side of the body; but no important parts of the right pair of wings are affected by this crack, so they are completely preserved (contrary to the holotype).
Namurotypus sippeliBrauckmann and Zessin 1989 (Meganisoptera: Namurotypidae)
Our re-examination of plate and counterplate of the specimen K-13 of Namurotypus sippeli yielded several new details of the male genitalia (Fig. 10) which allow a new interpretation of the structures concerned (1011Figs 10–13).
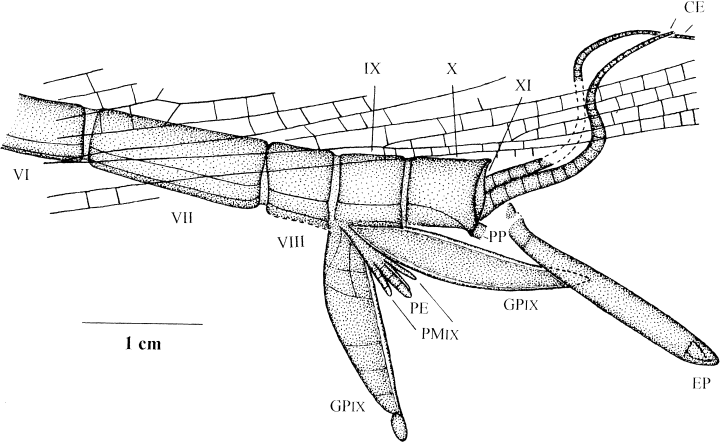
Namurotypus sippeliBrauckmann and Zessin 1989, paratype K-13 coll. Kemper, counterplate, terminalia with genital appendages (new interpretation); Upper Namurian B, Hagen-Vorhalle (Ruhrgebiet, Deutschland). CE, cerci; EP, epiproct (‘terminal filum’); GPIX, gonopods of IX. abdominal segment; PE, paired penis; PMIX, parameres of IX. abdominal segment; PP, paraproct?

Namurotypus sippeliBrauckmann and Zessin 1989, paratype K-13 coll. Kemper, positive plate. Photo by W. Sippel (under alcohol cover with polarized light)
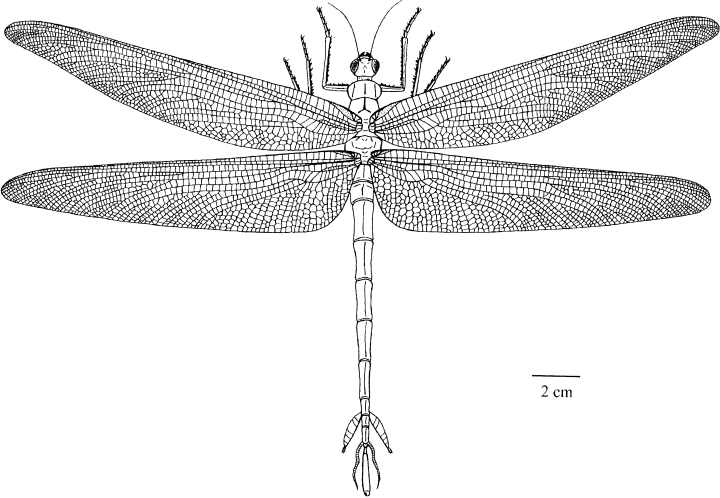
Reconstruction of a Namurotypus male (drawing by G. Bechly, based on a drawing of W. Sippel)
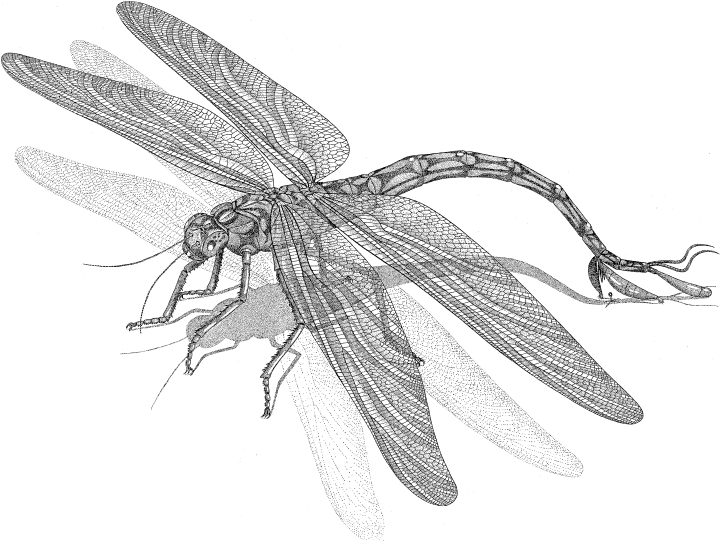
Reconstruction of a Namurotypus male depositing a spermatophore (drawing by E. Gröning)
Cerci
Both cerci (derivatives of leg appendages of segment XI) are segmented and filamentous, but rather thick. They both exhibit the same sigmoidal shape. The subsegments are relatively large and quadrangular (proximally 1.3 mm long and 1.3 mm wide). The shape of the cerci and the kind of segmentation suggest that the cerci were not totally rigid, but also not as flexible as the thread-like cerci of Ephemeroptera. Presumably, the sigmoidal shape was permanently established and not just due to diagenetical contingency (Whiting 1996).
Epiproct (‘terminal filum’)
Colour and position of the curious unpaired rod-like structure behind the end of the abdomen, between the gonopods with dorsally directed base, suggest that it corresponds to the epiproct (derivative of tergum XI), but not to a paraproct which should be paired, or to a diagenetical artefact, or even a coprolite or a plant remain (would be differently preserved). This epiproct is 20.8 mm long and 1.7–2.5 mm wide. Apparently, it was distally clubbed, since a diagenetic compression of such a clubbed tip would explain the conspicuous creased folds at the apex. A small but well-defined segment is visible close to the base of the epiproct. Obviously, the epiproct broke off at its very base and was embedded slightly dislocated.
Paraprocts
No unequivocal remains of the paraprocts (derivatives of sternum XI) are preserved. There is a small defined structure visible at the postero-ventral end of the abdominal segment X which might eventually represent a paraproct or its base. Because of the preservational limitations, it cannot be excluded that Namurotypus might have had large paraprocts.
Gonopods IX
The gonopods (gonocoxa + gonostylus) of abdominal segment IX are very large (length of left gonopod 18.9 mm, length of right gonopod 20.8 mm) and laterally flattened with asymmetrical lanceolate shape. The anterior and posterior margins of these gonopodal plates are distinctly thickened. There is a small ovoid segment at the distal end of the left gonopod (length 2.2 mm, maximum width 1.0 mm). The corpus of the gonopod plate is divided by weak sutures into at least six parts (= segments?). The most distinct suture is developed between the second and third ‘segment’. The right gonopod also shows the conspicuously thickened margins, but only a single indistinct suture in the basal corpus, which most likely corresponds to the suture between the second and third ‘segment’. The apex of the right gonopod is concealed by the epiproct. The flattened leaf-like shape of the gonopods and the weakly defined segmentation (reduction) suggest that the ‘segments’ of the corpus were fused, and thus not movable against each other, if the sutures should represent a vestigial segmentation at all and not just a secondary subdivision of a gonocoxa. Only the small distal segment (stylus?) presumably was still movable. The very different distinctiveness of the subdivision of right and left gonopods could be best explained by circumstances in which the right gonopod shows its inner side, whereas the left gonopod shows the outer side. Apparently, the vestigial segment borders were already mostly obliterated on the inner side, whereas they were still preserved as weak sutures on the outer side of the gonopods.
Whiting (1996) presumed that these gonopods must have had a grasping function. However, we regard such a function as hardly plausible because of the flattened shape of the gonopods. Therefore, we propose a new interpretation of their function (see below).
Parameres IX
The two parameres (homologues of male gonapophyses IX) are situated on the left and right side of the median paired penis [We restrict the term ‘parameres’ (sensu Nielsen 1957) to the homologues of gonapophyses; thus derivatives of coxal endites of the gonopods that seem to be serial homologous (homonomous) with eversible coxal vesicles on the pregenital abdominal segments of wingless insects. The so-called ‘parameres’ (sensu Snodgrass 1957) of the male genital apparatus of higher pterygotes (Eumetabola = Acercaria + Holometabola) should rather be called parandrites, since they seem to be neoformations and thus cannot be homologized with gonapophyses or coxal endites. In Eumetabola the primarily paired penis Anlage is secondarily subdivided; the lateral parts develop as parandrites, whereas the median parts fuse to an unpaired complex euphallus (aedoeagus). For this reason, the group Eumetabola was renamed as Phalloneopterata by some recent authors (Boudreaux 1979).]. They are segmented and somewhat shorter than the penis. Gonapophyses VIII (second pair of parameres) are absent, just as in all male Dicondylia including Palaeodictyopteroida. Among insects, only a few species of the Archaeognatha genus Machilis still possess male gonopods VIII, which are already much smaller than the gonapophyses IX, suggesting a convergent reduction within Archaeognatha and in the groundplan of Dicondylia. This mere fact alone, of course, precludes an eventual interpretation of the two pairs of median appendages in the male genital apparatus of Namurotypus as two pairs of true parameres (gonapophyses VIII and IX).
Penis
Obviously, the penis is paired and looks somewhat similar to the paired penis in the extinct Palaeodictyopteroida as well as in fossil and extant Ephemeroptera. The apices of the two penis lobes are darker coloured than the rest, indicating a stronger sclerotization of this part. Indistinct sutures seem to divide the penis into about four ‘segments’. A gonopore is not visible on the penis lobes. For functional reasons (spermatophore), the gonopore must have been unpaired and situated between the bases of the penis lobes, such as in the ontogeny of most insects and in adult Notoptera. The female genital opening almost certainly was also unpaired. The paired genital opening in both sexes of extant Ephemeroptera and the position of the male gonopores on the paired penis lobes seem to be an autapomorphy related to the evolution of a true copulation.
Terminalia
The terminal abdominal segments VIII to X together are only slightly longer than the pregenital segment VII.
Abdominal segments II and III
These segments are preserved in lateral aspect and clearly show that Namurotypus did not yet possess any secondary genital apparatus. Contrary to the opinion of Whiting (1996) we regard it as highly unlikely that this absence is only apparent due to preservational circumstances, since the body region concerned is sufficiently well-preserved. Whiting (1996) also tried to show with a three-dimensional model constructed by him, that the giant ‘protodonates’ were unable to mechanically establish a mating wheel, since the female abdominal end could not bend up to the anterior abdominal segments of the male. However, this argument is not very well-founded, as in the figure concerned (Whiting 1996: Fig. 4) only a minor change in the position of male and female are necessary to theoretically allow the formation of a mating wheel. Furthermore, the influence of the intersegmental membranes must be considered when estimating the flexibility of the abdomen. Nevertheless, we are also convinced that Namurotypus did not copulate in wheel position, although because of the very different reasons explained below.
In discussion with colleagues, we have often been questioned as to whether this specimen could not rather be a female with four annulated gonapophyses (ovipositor) and a pair of flattened gonostyli. Therefore, we here present in detail the reasons why we believe that this specimen can only be a male. A sclerotized and curved ovipositor with tightly joined valves (mortise and tenon like) is present among extant Odonata in all Zygoptera, in Epiophlebiidae, and in basal Anisoptera, nmely in Petaluridae, Austropetaliidae, and Aeshnidae. This character state is also known from the Mesozoic Tarsophlebiidae that most likely represent the sistergroup of all extant Odonata. Such an ovipositor is also present in most Neoptera (if the ovipositor is not reduced) and obviously belongs to the groundplan of this monophylum. This type of ovipositor is also known from the extinct Palaeodictyopteroida and Palaeozoic stemgroup representatives of Ephemeroptera. A phylogenetic interpretation of this character distribution leads to the most parsimonious conclusion that a distinctly sclerotized curved ovipositor belongs to the groundplan characters of Pterygota. Furthermore, two groups of fossil dragonflies which have a more basal position in the phylogenetic tree of Odonatoptera than Namurotypus (see below), also have a similar ovipositor to that documented by the new specimen of Eugeropteridae (see Fig. 14) and our revised description of Erasipteroides (Erasipteridae). Thus, if the specimen of Namurotypus were interpreted as female, a distinctly sclerotized and curved ovipositor with tightly joined valves would have to be interpreted as convergently evolved for at least six times and even three times within Odonata. Since this seems to be as unlikely as the assumption that such an ovipositor could be reversed into a thysanuran-like ovipositor with flexible and less tightly joined valves (filamentous and annulated), the specimen of Namurotypus obviously can only be a male. Although, the example of Neuroptera shows that a pterygote ovipositor secondarily can become flexible, their valves are by no means similar to the filamentous and annulated ovipositor valves of ‘thysanurans’.
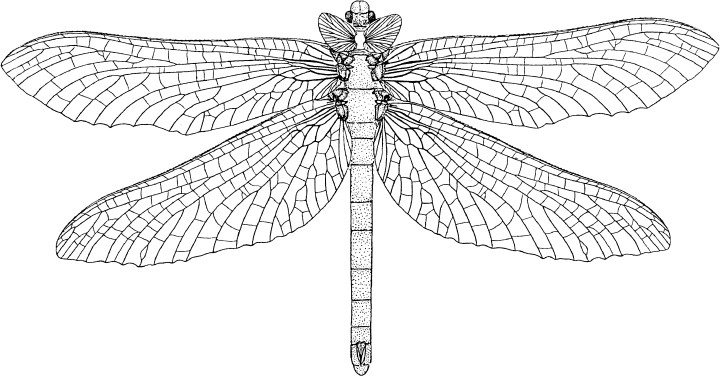
Undescribed Eugeropteridae gen. et spec. nov., Upper Carboniferous of Argentina (reconstruction drawing by J. Kukalová-Peck)
Finally, we would like to mention an important amendment to the description of Brauckmann and Zessin (1989): The holotype (specimen N 1000) of Namurotypus sippeli seems to possess narrow but distinctly delimited paranotal lobes on the prothorax (compare Brauckmann 1991a: Plate 14) which could be interpreted as vestiges of prothoracic winglets.
Discussion
Erasipteroides valentini
The following three here newly described characters of Erasipteroides valentini are of particular interest:
1. Prothoracic winglets
Prothoracic winglets (Figs 3,4) have recently been recorded for the first time in an early stemgroup representative of Odonata (Fig. 14; Wootton et al. 1998; Wootton and Kukalová-Peck 2000). The prothoracic winglets in Erasipteroides are distinctly smaller than in this new specimen of Eugeropteridae [This statement is based on the new discovery of a complete female specimen (in coll. Muzón) of Eugeropteridae from the Upper Carboniferous of Argentina (Wootton et al. 1998) which is currently being studied and described by Wootton and Kukalová-Peck (2000). Fortunately, we were given the opportunity to thoroughly study a very good cast of this specimen, and now can independently confirm that this animal indeed has prothoracic wings with venation and articulation. The mobility of these structures is also documented by the fact that the left prothoracic wing is embedded parallel to the surface of the plate, whereas the right one is obliquely directed into the sediment. Incidentally, this most interesting specimen also confirms the wing venation interpretation of Riek and Kukalová-Peck (1984) beyond any doubt, and it shows an ovipositor which looks very similar to that of extant dragonflies (sclerotized and curved, but not prolonged as in Erasipteroides).]. However, they have a very similar shape and the same oblique position in the cranial direction. Considering the large prothoracic winglets in Palaeodictyoptera and basal fossil Neoptera (‘protorthopterans’), the larger state of these structures in Eugeropteridae has to be regarded as a plesiomorphy, which could support the position of Eugeropteridae as the most basal branch in the phylogenetic tree of dragonflies (Bechly 1996, 1999a,b) and thus the closer relationship of Erasipteroides with ‘higher’ odonatoids. The reduction of the size of the prothoracic winglets in Erasipteroides could well represent an intermediate stage towards the total reduction of these organs, which would then be a putative synapomorphy of the Palaeozoic giant dragonflies (Meganisoptera s.str.) and ‘higher’ odonatoids (incl. Odonata s.str.). The oblique position of the prothoracic winglets could be a derived groundplan character of Odonatoptera, since it is present in Eugeropteridae and ‘Erasipteridae’, whereas the winglets are perpendicularly extending from the prothorax in Palaeodictyoptera as well as in ‘protorthopteres’. The oblique position in ‘basal protodonates’ might have increased the freedom for movement of the (mesothoracic) forewings and thus supporting a better manoeuvrability, which, for example, could have been an adaptation to the predatorial habits (please note: since ‘protodonates’ presumably were not yet capable of an independent movement of fore- and hindwings, because of the still preserved mechanical coupling of the thoracic segments, the mentioned oblique orientation of the prothoracic winglets could indicate they had already become rather immobile due to the reduction of the original articulation, as only in this case would they have been a significant hindrance for the radial movement of the mesothoracic forewings).
2 Ovipositor
The extremely prolonged ovipositor of Erasipteroides (Figs 5,6) is quite similar to the secondarily prolonged ovipositor of extant Cordulegastridae and the Mesozoic taxa Tarsophlebiidae (sistergroup of crowngroup Odonata), Steleopteridae (a basal representative of Zygoptera or the ‘anisozygopteroid’ grade), and Aeschnidiidae (sistergroup of crowngroup Anisoptera). The mentioned distribution of character states of course could suggest an interpretation as symplesiomorphy, thus the assumption of a prolonged ovipositor in the groundplan of dragonflies. However, such a hypothesis is strongly contradicted by the shared presence of a similar short, curved ovipositor in all extant Zygoptera, in Epiophlebiidae (the sole living ‘anisozygopteres’), and the basal groups of extant Anisoptera, namely Petaluridae, Austropetaliidae, and Aeshnidae. The most primitive known fossil dragonflies also had a short ovipositor, as is documented by the new complete female specimen of Eugeropteridae. The same type of ovipositor is also found in Palaeodictyoptera and many extant Neoptera. Consequently, we have to conclude that a short and curved ovipositor and endophytic oviposition belong to the groundplan characters of winged insects and dragonflies (see above), whereas a distinctly prolonged and rather straight ovipositor evolved as multiple convergence within dragonflies. Such a long and straight ovipositor is hardly suited for endophytic oviposition in plant tissues. Extant Cordulegastridae use their long ovipositor to deposit single eggs into the soft ground of shallow brooks. Such an endosubstratic oviposition has to be assumed for the mentioned fossil taxa (including Erasipteroides) as well. An endosubstratic oviposition might have an adaptational advantage by shortening the duration of oviposition, since these large insects are very vulnerable to predators such as large fishes or amphibians during this process.
Namurotypus sippeli
The present revised description and new interpretation of the male genitalia of Namurotypus sippeli (Fig. 10) allows important conclusions and hypotheses about the evolution of the male genitala within Odonatoptera.
1 Segmented leg-like male gonopods IX belong to the plesiomorphic groundplan characters of Pterygota (Kukalová-Peck 1997; contra Willmann 1997)
This interpretation is supported by the presence of leg-like gonopods in Palaeodictyopteroida (Kukalová-Peck 1991), segmented gonopods (‘genital claspers’) in fossil and extant Ephemeroptera, and the vestigial segmentation of the gonopods in Namurotypus (the single known ‘protodonate’ with preserved male genitalia).
The hypothesis of Willmann (1997) that the segmentation of the gonopods in extant Ephemeroptera should just be a secondary subdivision is contradicted by the facts that the most ‘primitive’ fossil stemgroup representatives of Ephemeroptera (namely Syntonopterodea and Protereismatidae) possess a even more distinct leg-like segmentation, and that only some ‘advanced’ and very subordinated groups of extant Ephemeroptera do (secondarily) possess unsegmented gonopods (Staniczek, personal communication). In our view, a secondary subdivision of unsegmented gonostyli into leg-like segmented gonopods is not plausible in this case, even though it is not entirely impossible. Therefore, we conclude that these structures are not neoformations in palaeopterous insects, but are groundplan features of insects which have been retained by these basal pterygote taxa, and which were convergently reduced in Entognatha, Archaeognatha, Zygentoma, and Neoptera. Obviously, the male gonopods are derived from an undifferentiated pair of leg-appendages of the ninth abdominal segment in the ‘myriapod’-like ancestor of insects.
The leaf-like morphology of the male gonopods in Namurotypus is most likely not an autapomorphy of this particular genus, but rather a derived groundplan character of dragonflies. The latter interpretation is supported by the fact that in all extant dragonflies the male gonopods are reduced to small flat genital flaps surrounding the genital porus. The flattened gonopods in ‘thysanurans’ do not seem to be homologous though, since Palaeodictyopteroida and Ephemeroptera still have preserved the plesiomorphic leg-like gonopods within Pterygota.
We also considered the possibility that the terminal segmented legs with paired claws in some fossil wingless insects from the Upper Carboniferous of Mazon Creek could be homologous of gonopods. However, these legs have been homologized with cerci by Kukalová-Peck (1997) who therefore classified these strange insects in a new taxon Cercopoda. Our re-examination of the original fossils and of very good casts of some of these fossils has strongly confirmed this interpretation as cercal legs. The articulation of this terminal pair of legs is clearly situated in the middle of the hindmargin of the tenth abdominal segment. These animals also only possess a single filamentous anal appendage (epiproct or terminal filum), and female specimens possess a distinct ovipositor with leg-like gonopods VIII (additional to the cercal legs!) which indeed also still bear paired claws. Contrary to Kukalová-Peck we presume that the taxon Cercopoda does not belong to Dicondylia, but rather to the stemgroup of insects, since Diplura (Campodeidae), Archaeognatha, Zygentoma, Palaeodictyopteroida, Ephemeroptera, basal Odonatoptera (Namurotypus) and Neoptera (e.g. Plecoptera) all share very similar filamentous cerci. Such cerci would have to be assumed to be evolved at least three to four times by convergence, if Cercopoda would be attributed to the stemgroup of Dicondylia or even to the stemgroup of Zygentoma. The original attribution to Dicondylia was based on the alleged presence of a gonangulum and a dicondylic mandible in some of the fossils, but we could not unequivocally confirm these structures in the material that was available for our re-examination. Our new interpretation of course implies that an ovipositor is secondarily absent (completely reduced) in Entognatha, or at least in Diplura if Entognatha should not be monophyletic. In the latter case an ovipositor could be primarily absent in Ellipura (Protura + Collembola).
2 The paired penis represents a putative autapomorphy of Palaeoptera
A paired penis, previously only known from the fossil Palaeodictyopteroida (Kukalová-Peck 1991) and fossil and extant Ephemeroptera (including the very ‘primitive’ Permian stemgroup mayflies Protereismatidae), is now also documented for a basal stemgroup representative (Namurotypus) of Odonata. In extant dragonfly males the primary penis is strongly reduced to an eversible membranous structure, correlated with the development of a secondary copulatory apparatus on the second and third abdominal segment. Therefore, the vestigial penis is only visible during the filling of the sperm vesicle. According to the principle of parsimony, a paired penis has to be interpreted as a putative synapomorphy of palaeopterous insects, since all primarily wingless insects and Neoptera (except Notoptera and many Dermaptera) possess an unpaired penis. The pseudo-paired penis in Dermaptera must have evolved by convergence, since this taxon has a very subordinated position within the phylogenetic tree of Neoptera. A convergence is also suggested by the different ontogeny and morphology of the dermapteran penis which is rather distally forked than truly paired. The convergent evolution of a paired penis with unpaired gonopore between the phallomeres in Notoptera (Grylloblattodea) could be easily explained by a paedomorphotic suppression of the ontogenetic fusion of the paired penis Anlage, since such a paired Anlage is present in the early ontogeny of this organ in all ectognathous insects.
The common presence of paired penis and parameres on the ninth abdominal segment excludes an evolutionary derivation of the penis from parameres (= gonapophyses = endites), unless one postulates more than one pair of endites per segment and pair of legs in the groundplan (Kukalová-Peck 1991, 1997), or another complex nature such as an inclusion of leg homologues of the tenth segment (Nielsen 1957). Since there is no unequivocal evidence for the latter assumptions, we prefer the more simple interpretation that the penis of ectognathous insects is a secondary differentiation of the cuticle in the area of the opening of the ductus ejaculatorius. This hypothesis of course implies that the paired anlage and the apparent segmentation of the penis are considered as secondary phenomena, even though one may not forget that the two mentioned facts indeed represent conflicting evidence which could instead support the interpretation of Kukalová-Peck (1991, 1997).
3 The direct copulation of extant Ephemeroptera and Neoptera most likely is a convergence, since we have good reasons to believe that in the groundplans of Pterygota and Odonatoptera the transfer of sperm was still accomplished by means of external spermatophores or droplet-spermatophores
This hypothesis, which was already endorsed by Brinck (1962) and Carle (1982), is supported by the transfer of spermatophores in all primarily wingless insects (‘apterygotes’), and the reasonable assumption that such a transfer of spermatophores must have been still present in the early ancestors of dragonflies. Although the latter assumption is neither strictly verifiable, nor falsifiable, it can be justified with the following three arguments:
(a) The male genitalia of Namurotypus have greater similarities with those of some primarily wingless insects (Archaeognatha and Zygentoma) than with any of Ephemeroptera or Neoptera. Although this similarity seems to be mostly based on symplesiomorphies, it is of significant importance, as it is more plausible that two similar homologous structures also have similar functions, rather than dissimilar functions in spite of their structural similarity. Since Archaeognatha and Zygentoma both deposit free spermatophores, the same mode has to be assumed for Namurotypus (Fig. 13) rather than a direct copulation as in Ephemeroptera and Neoptera. Contrary to the opinion of Whiting (1996) it is evident that the presence of a penis does not necessarily imply a direct copulation at all.
(b) The complex secondary copulation apparatus on the second and third abdominal segment of extant dragonfly males cannot be derived from ancestors with a direct copulation without assuming unnecessary complex scenarios (see below), but can easily be derived from ancestors with free spermatophores (Bechly et al. in prep.). Rather absurd scenarios have indeed been suggested by Fraser (1939), who postulated a ‘jumping’ of the tandem grip from the base of the abdomen (completely hypothetical) over the wing articulation area to the thorax, and by Moore (1960), who suggested an overturn of the female into a backward position with a paralysis during mating and a subsequent somersault into an upright position. Carle (1982) avoided the assumption of such weird behaviours, but had to imply (just like any scenario that is based on primary copulation) that droplets of sperm were accidentally displaced on the basal abdominal sternites of the male after a successful copulation, and were subsequently actively absorbed by the female even though it was already inseminated. This very process is hardly plausible or even impossible from the viewpoint of evolutionary biology, since it involves no selective advantages that could drive the evolution of a secondary copulation apparatus.
Contrary to the mentioned problematic scenarios, the hypothesis of an indirect mating with spermatophores would only require the assumption of one hypothetical intermediate step, in which the spermatophore is attached on the third abdominal sternite of the male instead of being deposited on the ground. This would immediately cause an adaptational pressure for the development of fixation structures for the spermatophore (with vesicula spermalis) and the female ovipositor (with hamuli anteriores) (Zessin 1995; Bechly et al. in prep.).
The groundplan of extant Odonata includes a secondary male genital apparatus with an unpaired vesicula spermalis on the venter of the third abdominal segment, and an unpaired ligula, paired hamuli anteriores and paired hamuli posteriores on the venter of the second segment. All these substructures are present in all three major monophyla of extant Odonata (namely Zygoptera, Epiophlebiidae and Anisoptera), and certainly have to be regarded as homologues. However, an active transfer of liquid sperm (instead of transmission of a spermatophore) with a secondary copulatory organ (=functional analogue of a penis) apparently evolved independently as a triple parallelism within Odonata. This surprising conclusion is strongly suggested by the strange fact that in each of the three mentioned groups different non-homologous substructures of the secondary genital apparatus are transformed into sperm transfer devices (an enlarged and complex ligula in Zygoptera; strongly enlarged hamuli posteriores in Epiophlebiidae; and an enlarged and subsegmented outgrowth of the vesicula spermalis in Anisoptera). None of these highly complex types of copulatory devices can be derived from the other without assuming nearly impossible scenarios, thus none of them can actually represent the plesiomorphic state for Odonata. This of course implies that the sophisticated behaviour of removal of foreign sperm prior to sperm transfer must have evolved three times by convergence, since the devices for sperm removal are situated on non-homologous organs. The most parsimonious interpretation of the character pattern leads to a reconstruction of the secondary genital apparatus in the groundplan of extant Odonata, which curiously does not include any structures that are modified as copulation organs, so that an active transfer of liquid sperm by the male would be impossible, but only an active absorption of a spermatophore or droplet-spermatophore by the female would remain as a possible process. This theoretical reconstruction was recently perfectly confirmed by the discovery of a male specimen of Mesozoic Tarsophlebiidae (the sistergroup of crowngroup Odonata) with preserved secondary genitalia which exactly match the mentioned groundplan reconstruction: It has simple plate-like hamuli anteriores and posteriores, a small and simple ligula, and a short unsegmented vesicula spermalis with a very wide opening (Bechly 1999b; Bechly et al. in prep.).
(c) The copulation behaviour of extant Ephemeroptera strongly differs (male in ventral position beneath the female) from that in the groundplan of extant Neoptera (male in dorsal or terminal position in relation to the female). Only a few highly derived groups of Neoptera also have a copulation behaviour with the male in ventral position (e.g. Pulicidae) or in facies-ad-faciem position (e.g. Bittacidae). According to Willmann (personal communication) even the homology of the copulation organs in Ephemeroptera and Neoptera has to be regarded as questionable.
The hypothesis preferred by Whiting (1996), that ‘protodonates’ had a direct copulation in flight, is one we regard as highly unlikely, also because the capabilities for active flight of these giant insects could have hardly been sufficient for such sophisticated manoeuvres. Whiting (1996) did not believe in a transfer of spermatophores mainly because there are no other pterygote insects known to still share this mode of mating. Nevertheless, his argument is not well founded from the viewpoint of phylogenetic systematics, as it does not consider the mating behaviour of primarily wingless insects, such as Zygentoma, which after all are the closest relatives (sistergroup) of Pterygota. Furthermore, even numerous extant Pterygota (e.g. Orthoptera) do not transfer liquid sperm during copulation, but still spermatophores! The fact that extant dragonflies do not transfer free spermatozoae with their seminal liquid, but packages of so-called spermatozeugmata, could well be regarded as reminiscent of the original transfer of spermatophores.
4 In dragonfly mating behaviour, the formation of a tandem position is a phylogenetically older behaviour than the formation of a mating wheel
This hypothesis is based on the following arguments. There is good reason to believe that Namurotypus still deposited free spermatophores (see item 3 and 5). Males of Namurotypus did not yet possess a secondary copulation apparatus (Brauckmann and Zessin 1989; Zessin 1995), and the male abdominal appendages (i.e. cerci) obviously were not suited for an active grasping and holding of the female (Whiting 1996). Consequently, Namurotypus certainly was unable to mate in the wheel position. On the other hand, the curious sigmoidal shape of the cerci, which can hardly be a diagenetic artefact, requires a plausible explanation. The thickness of the cerci, as well as the shape and size of its segments, suggest that these cerci were rather stiff, with the sigmoidal shape being permanently established. Male cerci of this type would have been perfectly suited to be firmly placed behind the head and compound eyes of the female (maybe also touching the prothorax), so that the female could be directed by the male over the spermatophore deposited on the ground, similar to the behaviour in some extant whipspiders (Amblypygi) (Weygoldt 1969). Such a ‘drag off’ behaviour could have represented a suitable intermediate stage towards the evolution of a strong tandem grip, since all extant dragonfly males use their forcep-like cerci (and paraprocts or epiproct) to grasp the female head and/or prothorax.
Whiting (1996) postulated a grasping of the female by the male ‘protodonates’ with the male gonopods being positioned between the female pro- and mesothorax, and the male epiproct lying on the prothoracic tergum of the female, while the male cerci are held beneath the female wings. However, this hypothetical mechanism would hardly allow the continuous evolution towards a tight grip of the female head and/or prothorax with the male anal appendages (cerci and epiproct or paraprocts). Considering the much more plausible alternatives (see above) we regard this hypothesis as too speculative and overly complicated.
5 The possible function of the clubbed epiproct
Brauckmann and Zessin (1989) discussed a putative aerodynamic function with respect to the adaptations for gliding flight in these Carboniferous dragonflies. Taking into account our new hypotheses about the mating behaviour of Namurotypus (see above), we here suggest another possible explanation for the peculiar shape of the epiproct, without excluding an additional aerodynamic function or even an entirely different function (e.g. as signal structures in mate recognition; Lindeboom, personal communication). Our new hypothesis is based on the following two conspicuous facts:
(a) The epiproct is strongly sclerotized and thickened which indicates the need for enforcement against mechanical stress in longitudinal direction.
(b) The epiproct has about the same length as the two gonopods IX.
Both points could be well-explained with the following hypothesis. The two gonopods IX and the epiproct served as a kind of tripod for the deposition of the (maybe stalked) spermatophore, while sensory hairs on the apex of the gonopods may have checked the condition and suitability of the substrate. Furthermore, the gonopods might even have assisted in the manipulation and application of the spermatophore. This interpretation implies that Namurotypus did not yet attach the spermatophore to the venter of abdominal segment III, but deposited the spermatophore on the ground or the surface of fallen trees or large leaves. This particular interpretation of course has to be considered as very speculative and hardly testable, but we think it is more plausible than an exclusively aerodynamic function, which could not be convincingly explained with our current knowledge about insect flight aerodynamics.
The here proposed re-interpretation might also explain the following striking fact: In the imaginal stage of extant Odonata an epiproct is only present in Epiophlebiidae and Anisoptera, and it is reduced in Zygoptera (unfortunately it is still unknown whether the fossil Tarsophlebiidae did have an epiproct or not). The ontogeny shows that the apparent imaginal epiproct of [Epiophlebiidae + Anisoptera] is not homologous with the genuine epiproct (terminal filum) at all, since this original epiproct is successively reduced in the subsequent larval stages, while the so-called epiproct of the adults is successively developed from a ventral outgrowth (epiproct process) of the larval epiproct. Indeed, the original epiproct seems to be reduced in the groundplan of (imaginal) Odonata, and was replaced by an autapomorphic neoformation in [Epiophlebiidae + Anisoptera]. This reduction of the imaginal epiproct in the groundplan of Odonata could hardly be explained if this epiproct played an important role in the original tandem grip, but it could be easily explained if the ‘protodonate’ epiproct had a function in connection with the deposition of the spermatophore. In the latter case, the evolution of a secondary copulatory apparatus and the mating wheel would have made the epiproct superfluous, which could have resulted in its reduction. The neoformation of an epiproct process in [Epiophlebiidae + Anisoptera] must be correlated with an improvement of the tandem grip on the female head. The character pattern indeed could suggest that a tandem grip on the female head represents the apomorphic state, whereas a tandem grip on the female prothorax represents the plesiomorphic state, since the latter state is present in all Zygoptera (and maybe in Tarsophlebiidae), although the most basal groups of Anisoptera (Petaluridae and Aeshnidae) have a dual grip on the female head and prothorax. The exclusive grip on the female head in Epiophlebiidae is of course conflicting evidence that would have to be interpreted as a convergence to ‘higher’ Anisoptera.
Scenarios for the evolution of the secondary genital apparatus
The evolution of a complex behavioural pattern is a difficult topic for which fossils, even if they are very well-preserved, usually cannot contribute much. One of the exceptions is Namurotypus whose genital structures are so well-preserved that they allow detailed interpretations and conclusions. Below we compare and discuss three alternative models or scenarios concerning the evolution of dragonfly copulation.
Scenario 1 by Zessin (1995)
The following scenario was developed and first presented by Zessin (1995). However, this scenario was still based in the latter publication on the assumption of a primary copulation in the groundplan of dragonflies, whereas it is here combined with the hypothesis of spermatophore deposition in the groundplan of Odonatoptera (see above). This modified scenario of Zessin involves the following considerations.
That male dragonflies grab a female and drag it off to an undisturbed location for mating seems to be a phylogenetically very old strategy of dragonflies (see above) which may be explained with the strong intraspecific competition of the males. Extant dragonfly males use various methods to minimize such competition (e.g. many Anisoptera mate in flight). If the relatively stiff and characteristically bent cerci of Namurotypus are correctly interpreted as devices for tandem formation (see above), either for directing the female over the spermatophore, or to move it out of the competition area of another male, any improvement of this behaviour would have implied a potential improvement of the mating success.
In extant dragonflies, the tandem formation generally starts with the male landing on the back of the female that is initially only grasped with the legs, which could be a primitive behavioural trait. Afterwards, the males establish the tandem by a tight coupling to the female head and/or prothorax with their forcep-like terminal abdominal appendages. To achieve this, the male must of course bend the abdomen anteriorly so that it can reach the head of the female with the end of the abdomen. Since the genital opening comes close to the basal venter of the abdomen in this position, it is quite possible that such a bending of the male abdomen was a suitable pre-adaptation of ‘protodonates’ with respect to the later evolution of active transfer of a spermatophore (or sperm droplets) on the third abdominal sternite.
Extant dragonfly females often show distinct defence movements with the forelegs and the tip of the abdomen against the ‘rape-like’ grabbing by the male. The females strongly bend their abdomen anteriorly to push away the body of the male. Such a defence behaviour could primarily represent a test for the fitness of the males, but could also have been a suitable pre-adaptation for the evolution of the mating wheel, since the female gonopore comes very close to the basal abdominal sternites of the male during this behaviour. Consequently, the defence movements of the female could even promote a more rapid achievement of the sperm transfer. Any shortening of the duration of the copulation would have had a selective value by reducing the risk for disturbances, especially by conspecific males as well as predators.
As dragonflies flying in the wheel position are less exposed to the competition of conspecific males than those that fly in tandem position (Rüppell, personal communication), the evolution of the secondary male genital apparatus on abdominal segments II and III could be related to the improvement of a tight coupling in mating flight. However, this assumption is contradicted by the fact that neither Zygoptera, nor Epiophlebiidae generally fly in the wheel position, so that this behaviour can hardly be regarded as a putative groundplan character of Odonata.
Scenario 2 by Bechly et al. (in prep.)
Against the mentioned scenario of Zessin one could generally object that the grasping of the female did not primarily need to be done with the male legs (male landing on the female’s back), but was achieved from the very beginning with the male’s cerci (male with straight extended abdomen in front of the female). Grasping with the legs does not make much biological sense anyway if there is no sophisticated coupling mechanism present. Therefore, this mode hardly could have been the primary state. Maybe in the beginning, the male’s cerci were only loosely positioned behind the female’s head to direct the female over the spermatophore, since such a behaviour would easily allow a continuous evolution towards a tight forcep grip. Presumably, the position of the cerci behind the female’s head was not precisely fixed but rather variable in those early stemgroup representatives of dragonflies.
The main problem with the described scenario of Zessin could be the involved assumption that a sperm transfer could just be promoted by a defence behaviour of the female against the male. This appears to be an evolutionary biological contradiction that can hardly be resolved. An alternative scenario that avoids such problems could be as follows: the male deposited the spermatophore more and more anterior beneath his own abdomen on the ground, since it was better protected there. Later in the evolution, the spermatophore was attached to the venter of the third abdominal segment, maybe in the beginning only accidentally or facultatively so. This implied a selective advantage due to even better protection of the spermatophore which was not lost if the male had to flee due to disturbance by predators or competing males; an event which would otherwise require the deposition of a further spermatophore. The successive anterior shifting of the spermatophore position would allow a continuous evolution of a corresponding bending of the female abdomen during the ‘precopula’ and would thus also explain the evolution of the mating wheel, since the female has to bend the abdomen anteriorly beneath the male abdomen to take up the spermatophore.
Scenario 3, according to Willmann (personal communication)
In this scenario it would be possible that the transfer of the spermatophore was achieved while the animals were hanging on a twig, since in case of mating on the ground there would hardly be sufficient space for the female’s abdomen between the ground and the abdomen of the male. However, this hypothesis of course greatly complicates the explanation of the evolution of the tandem position. Therefore, we consider a mating on the ground as the more plausible alternative, and think that the flexibility of the abdomen of both sexes would certainly allow such a mating position.
Footnotes
Acknowledgements
We are much obliged to Dr M. Kemper (Bochum) for the loan of the holotype of Erasipteroides valentini and the counterplate of specimen K-13 of Namurotypus sippeli for our re-examinations. We are most grateful to Mr W. Sippel (Ennepetal) for the excellent further preparation of the holotype of Erasipteroides valentini and for prints of his photos of two of the fossil dragonflies. Most of the unique fossil discoveries from the Upper Carboniferous of Hagen-Vorhalle were only possible due to many years of volunteer efforts by Dr M. Kemper and Mr W. Sippel. We also thank Dr L. Schöllmann and Dr A. Hendricks and the complete excavation and preparation team of the Westfälisches Landesmuseum – Planetarium Münster who have supported our work by a constant flow of information and especially by the loan of the new specimen of Erasipteroides valentini. They also provided photos of this specimen. We are very grateful to Professor Dr J. Kukalová-Peck (Ottawa) and Dr J. Muzón (La Plata) for a cast of the new complete specimen of Eugeropteridae, and to Professor Dr J. Kukalová-Peck (Ottawa) also for casts of fossil ‘apterygotes’ from Mazon Creek. We are obliged to Professor Dr J. Kukalová-Peck (Ottawa), Professor R. Willmann (Göttingen), Professor G. Rüppell (Braunschweig), Dr M. Lindeboom (Tübingen), Dipl.-Biol. A. Staniczek (Tübingen) and J. G. Whiting (Ottawa) for numerous interesting discussions and important hints. Last but not least we thank Mr A. Ilg (Düsseldorf) for carefully proofreading this manuscript, and two anonymous reviewers for comments. We would like to emphasize that the authors are alone responsible for the presented hypotheses and that the mentioned colleagues partly hold other opinions.
Appendix
Appendix: Phylogenetic Systematics
Phylogenetic system of dragonflies (see Fig. 15)
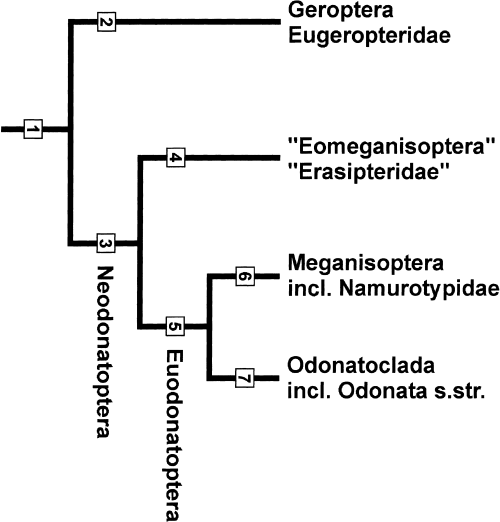
Phylogenetic tree (for the concerning synapomorphies see Appendix)
1 Odonatoptera Martynov 1932
2 Geroptera Brodsky 1994 (incl. Eugeropteridae)
3 Neodonatoptera Bechly 1996
4 ‘Eomeganisoptera’ Rohdendorf 1962 (incl. ‘Erasipteridae’)
5 Euodonatoptera taxon nov.
6 Meganisoptera s.str. Martynov 1932 (incl. Namurotypidae)
7 Odonatoclada Bechly 1999 (incl. Odonata s.str.)
List of synapomorphies concerning this phylogenetic system
1 Odonatoptera: wings with reduced anal area, especially in the forewing; MP-secondarily unbranched; subbasal cubito-anal-anastomosis (‘anal brace’) with a Z-kink in CuP-; ScP- fusing with anterior margin basal of wing apex; wing articulation with two large sclerite plates (costal plate and radio-anal plate); double pleural wing articulation (fulcrum), apparently correlated with a double pleural suture and crest in the groundplan; abdomen long and slender; male gonopods not leg-like anymore, but developed as flattened plates with reduced segmentation (however, the character state of Eugeropteridae and Erasipteridae is still unknown, thus it could eventually only be an autapomorphy of Euodonatoptera).
2 Geroptera (Eugeropteridae): archaedictyon suppressed (convergent to Erasipteroides and Euodonatoptera); ScP- more distinctly shortened (except in the new species of Eugeropteridae; convergent? to ‘Erasipteridae’, Paralogidae and Odonatoclada except Lapeyriidae). The monophyly of Geroptera is currently only weakly founded and far from certain.
3 Neodonatoptera: wings very long and slender; RA + and RP-basally running very closely parallel or even fused; base of MA + fused with RP-, and lost its connection to the medial stem; M + and Cu- at least shortly fused; MP- and CuA + undulating or even kinked; AA2 secondarily with several parallel posterior branches in the hindwing; prothoracic winglets smaller or completely reduced.
4 ‘Eomeganisoptera’ (‘Erasipteridae’): no synapomorphies known yet, with possible exception of one weak and homoplastic character: Shortening of ScP- that fuses with the anterior margin in midwing position (convergent? to Geroptera, Paralogidae and Odonatoclada except Lapeyriidae). Consequently, this taxon might well turn out to be paraphyletic.
5 Euodonatoptera taxon nov. Complete suppression of prothoracic winglets; archaedictyon secondarily absent (synapomorphy with Erasipteroides?; convergent to Geroptera); legs prolonged (still short in Erasipteroides).
6 Meganisoptera: Wing span larger than 200 mm, correlated with a strongly increased number of cells in wing venation (al least about 500 cells) and an increased body size (reversed in Paralogidae); longitudinal veins crowded along anterior wing margin.
7 Odonatoclada: RA + and RP basally fused to a ‘double-barrelled’ radial stem; ScP-shortened (except in Lapeyriidae) since it fuses with the anterior wing margin at midwing position (convergent? to Geroptera, ‘Erasipteridae’ and Paralogidae); branching of MA + strongly reduced; compound eyes enlarged. The monophyly of crowngroup Odonata is of course supported by numerous further autapomorphies (see Bechly 1996, 1999a, 1999b).




