Effects of Indomethacin on Salmonella Typhimurium- and Cholera Toxin-induced Fluid Accumulation in the Porcine Small Intestine
Abstract
The effect of the cyclooxygenase and prostaglandin E2 (PGE2) synthesis inhibitor, indomethacin, on the secretory responses induced by Salmonella serotype Typhimurium (ST) and cholera toxin (CT), in the porcine small intestine was investigated. ST (1010 colony-forming units) and CT (56 μg) were instilled in tied-off intestinal loops in young anaesthetized pigs receiving intravenous indomethacin in a total dose of 7.5 mg/kg, or saline. The accumulated fluid in the loops and the luminal content of endogenous secretagogues PGE2 and 5-hydroxytryptamine (5-HT) were measured. ST induced fluid accumulation in the jejunum, whereas CT induced fluid accumulation in the jejunum and ileum. Indomethacin had no effect on the secretory responses. Indomethacin had a significant effect on the luminal content of PGE2 in jejunal ST and CT loops, whereas no effect of indomethacin was observed on the luminal content of 5-HT in ST and CT loops. In ST and CT loops, an increased content of PGE2 and 5-HT compared with test loops infused with Ringer’s solution was observed. These results indicate that the porcine jejunal secretory response to ST and CT does not involve prostaglandins although indomethacin has an influence on the luminal release of PGE2 but not of 5-HT.
Introduction
Salmonella serotype Typhimurium (ST) induces diarrhoea in domestic animals and man. The pathophysiological mechanisms behind the diarrhoea are incompletely understood and seem complex, where several mechanisms and virulence factors may play a part (Jones, 1997). ST invades the intestinal epithelium but this does not necessarily elicit a fluid secretion per se (Giannella et al., 1973; Wallis et al., 1986), as the presence of polymorphonuclear leucocytes is required to induce intestinal fluid secretion (Wallis et al., 1989). However, an invasion of ST is a key element in the induction of secretion, as mutations in the ST invasion invH genes reduce ST-induced enteritis in cattle (Watson et al., 1998). Numerous studies have demonstrated the presence of an enterotoxin in ST isolates (Molina and Peterson, 1980; Wallis et al., 1986; Duebbert and Peterson, 1985; Chopra et al., 1991), which is structurally, functionally, and immunologically related to Escherichia coli heat-labile enterotoxin and cholera toxin (CT) (Finkelstein and Mauro, 1963; Molina and Peterson, 1980; Chopra et al., 1991; Khurana et al., 1991, 1992; Prasad et al., 1992).
It is assumed that ST liberates the enterotoxin after entering the epithelium (Stephen et al., 1993). In bovine ligated ileal loop assays, mutation in the gene encoding the enterotoxin reduces neither secretion nor inflammatory responses (Watson et al., 1998). The involvement and precise role of the enterotoxin in ST-induced diarrhoea in other animal models still remain to be defined. In contrast to ST, the pathophysiology of CT-induced diarrhoea is better established (Lundgren and Jodal, 1997). CT binds to those cells that express GM1 ganglioside receptors. This leads to an increase in intracellular adenosine 3′,5′-cyclic monophosphate (cAMP), and release of 5-hydroxytryptamine (5-HT), accompanied by prostaglandin E2 (PGE2) release (Peterson et al., 1983; Beubler et al., 1989; Grøndahl et al., 1998), and activation of the enteric nervous system (Brunsson, 1987). An increase in luminal cAMP and PGE2 has also been observed during ST stimulation (Peterson et al., 1983; Grøndahl et al., 1998). Apparently, CT can stimulate the enterocytes directly and indirectly. The direct pathway increases intracellular cAMP of the enterocyte. The indirect pathway stimulates the enterochromaffin cells to release 5-HT and PGE2, mainly from the fibroblasts, which will activate effector neurones, via cholinergic and non-cholinergic inter-neurones, to liberate vasoactive intestinal peptide and probably acetylcholine at the bases of the enterocytes [see review by Hansen and Skadhauge (1995)]. In rodents, the ST- and CT-induced fluid secretion can be attenuated by treatment with the non-steroid anti-inflammatory agent and prostaglandin synthesis inhibitor, indomethacin, suggesting an involvement of prostaglandins with the main focus on PGE2 (Giannella et al., 1977; Beubler et al., 1989).
The pig was used in this study because of its significance in veterinary and agricultural science. Because of the gastrointestinal anatomical, physiological, and cardiovascular physiological similarities to man (Miller and Ullrey, 1987), the porcine small intestine seems to be an appropriate model for the human small intestine, and is often used for the study of the pathophysiological mechanisms of diarrhoea. It is also important to note that our knowledge of most of the mechanisms that cause diarrhoea largely comes from observations in rodents. The aim of this study was therefore to investigate the effect of a clinically recommended dose of indomethacin on ST- and CT-induced fluid accumulation, and luminal content of PGE2 and 5-HT in ligated loops in the porcine small intestine. Also investigated was whether there was a correlation between the instilled concentration of ST and CT and the induced fluid accumulation, and the luminal content of PGE2.
Materials and Methods
Animals
Danish Landrace/Yorkshire crossbred 18–20 kg (9–10 weeks) female pigs on commercial standard diet were used. Before the experiments the animals were tested for Salmonella infections according to serum antibodies and faecal cultures, and observed for any clinical signs of diarrhoea. Before surgery, the animals were fasted overnight with free access to sterile drinking water containing D-glucose (55 g/l).
Bacterial strain and preparation of inocula
The inoculum of log-phase bacteria was prepared from a single colony of Salmonella enterica subspecies enterica serotype Typhimurium (Salmonella Typhimurium; abbreviated ST) 3389–1 (DT12) as previously reported (Grøndahl et al., 1998). Cells were concentrated by centrifugation (1935 g for 10 min at 30°C) in a Heraeus Megafuge 1.0R and resuspended in 2 ml of ‘Argenzio-4’ Ringer’s solution, without D-glucose (Argenzio, 1980) (mmol/l: Na+, 115; K+, 10; Cl−, 80; HCO3−, 45; pH 7.4). Viable counts of colony-forming units (cfu/ml) of inoculum were determined by a standard plate count technique.
Anaesthesia
Immediately before general anaesthesia and surgery the pig was sedated by an intramuscular injection of azaperonum (Stresnil, 5 mg/kg, Janssen Pharmaceuticals, Belgium). General anaesthesia was induced by an initial intravenous injection of mebumal (50 mg/ml, Pharmacy of The Royal Veterinary and Agricultural University). After intubation, anaesthesia was maintained by α-chloralose (15 mg in 100 ml of saline; Pharmacy of The Royal Veterinary and Agricultural University) by an intravenous bolus every 2 h.
The pig was connected to a semiclosed circle anaesthetic machine (Dameca Ventilator MCM 801, Finland) with artificial controlled ventilation using a tidal volume of 200 ml, and a frequency of 12 cycles/min and 100 % oxygen in a flow of 500 ml/min. The rectal temperature was monitored, and kept at 39°C using a thermostat-controlled water mattress (Gaymar T-pump, TP 400 series, Gaymar Industries, New York, USA). Systolic and diastolic blood pressure and heart rate were continuously monitored (Dameca CH-2, Datex-Engstrom, Finland) via a catheter placed in the right arteria saphena. All cardiovascular values were within normal range during the experiment. A catheter placed in the left arteria saphena supplied blood samples for partial pressure of blood gases and pH measurements. Blood samples obtained 30 min after anaesthesia, at the time of instillation of CT and ST, and 2, 4, 6, and 8 h after instillation, were immediately centrifuged at 480 g for 10 min and plasma was stored at −20°C.
To prevent dehydration, the animal was supplied with a continuous intravenous infusion of isotonic NaCl at a rate of 15 ml/kg in the first hour and thereafter 7.5 ml/kg each hour through an ear vein.
The long-acting local analgesic, Marcain (bupivacaine, 0.5 ml/kg, 0.5 %, Astra, Sweden), was infiltrated into the skin and underlying tissues at the incision line to avoid reactions from the nociceptive system.
After the experiment the animal was killed by an intravenous overdose of pentobarbital sodium.
Surgical procedure
A midline incision was made in the abdomen and ligated (tied-off) loops in the jejunum (the proximal loop placed 30 cm distal to the ligament of Treitz) and in the ileum (the distal loop placed 60 cm proximal to the ileocaecal valve) were prepared by ligating between the mesenteric vascular arcades to ensure full blood supply. A series of 11 or six loops, in the jejunum and ileum, respectively, with a length of 20 cm, departed with 3 cm, were left unfilled, or instilled with 56 μg of CT per loop (Sigma, St. Louis, MO, USA) or ST (approximately 1010 cfu) in 10 ml of ‘Argenzio-4’ Ringer’s solution (as described above, now including 80 mM D-glucose, and hereafter designated ‘test solution’). The loops were meticulously returned to the peritoneal cavity, the abdomen was closed with towel clamps, and covered with a sterile sheet.
To ensure an effective dose of indomethacin, 2.5 mg/kg (Confortid, Dumex, Denmark), freshly prepared, was injected intravenously immediately before anaesthesia and again after 4 h, as the half-life of Confortid in human plasma is 4–11 h (communication by Dumex A/S, Denmark). Each pig was given a total dose of indomethacin of 7.5 mg/kg throughout the experimental period. Pigs serving as controls for the treatment of indomethacin were given saline.
After 8 h of incubation the loops were carefully removed from the peritoneal cavity, and weighed separately immediately with (T), and without (V) the fluid content, and then dried for at least 24 h at 80°C to constant dry weight (t). Thus, fluid accumulation (remaining fluid) in the loop was calculated from the ratio (T − V)/t, and expressed as mg/mg loop dry weight/8 h. Before emptying the loops, 5 ml samples were collected from the accumulated fluid by syringe. Empty loops were washed with 5 ml of test solution before sampling. All samples were centrifuged at 45 000 g for 20 min at 4°C (Sorvall Instruments RC5C, DuPont, USA) and supernatants of the accumulated luminal fluid were frozen immediately at −20°C until analysis for PGE2 and 5-HT.
Analytical procedures for samples
The PGE2 content was measured by radioimmunoassay (Christensen and Leyssac, 1976) and the 5-HT content was measured by enzyme immunoassay (Immunotech, Marseille, France). Blood gases (i.e. P O2, P CO2), and pH (ABL-300, Radiometer, Copenhagen, Denmark and IRMA Series 2000, Diametrics Medical, Inc, St. Paul, USA) were determined from blood samples every second hour.
Statistics
The results are presented as means ± standard error of the mean (SEM). N and n are the numbers of animals and loops, respectively. The data were analysed with Student’s t-test. P < 0.05 was considered significant.
Results
Fluid accumulation
Figure 1a shows that jejunal test loops instilled with test solution were practically empty, indicating that the intestine had normal absorptive capability and capacity. The jejunal unfilled loops in saline-treated and indomethacin-treated pigs were empty, as the weights were 0.20 ± 0.11 mg fluid/mg loop dry weight × 8 h (n=11; N=8) and 0.37 ± 0.09 mg fluid/mg loop dry weight × 8 h (n=12; N=8), respectively. In jejunal tied-off loops instilled with ST (mean concentration 1.8 × 1010 ± 1.1 × 1010 cfu in n=34; N=8) or CT (56 μg/loop in n=12; N=8) for 8 h, fluid accumulation was observed (Fig. 1a). These doses of ST and CT have previously been shown to induce a maximal fluid accumulation in the porcine small intestine (Grøndahl et al., 1998; Maltbæk et al., 1998). Treatment with indomethacin had no effect on the ST- (mean concentration 1.1 × 1011 ± 9.6 × 1011 cfu in n=32; N=8) and the CT-induced (56 μg/loop in n=12; N=8) jejunal fluid accumulation, although a minor, non-significant effect was observed in the CT-induced fluid accumulation.
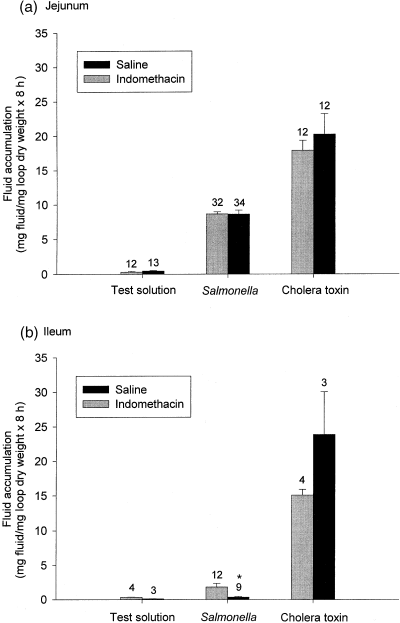
Effect of indomethacin on Salmonella Typhimurium- and cholera toxin-induced fluid accumulation in the porcine jejunum (a) and ileum (b). Test: instillation of Ringer’s solution in tied-off loops. The numbers indicate the number of loops in eight animals treated with saline or indomethacin, respectively, in the jejunum, and three or four animals treated with saline or indomethacin, respectively, in the ileum. Indomethacin was given in a total dose of 7.5 mg/kg in three doses.
The ileal unfilled loops in saline-treated and indomethacin-treated pigs were also empty, as the weights were 0.20 ± 0.06 mg fluid/mg loop dry weight × 8 h (n=3; N=3) and 0.58 ± 0.31 mg fluid/mg loop dry weight × 8 h (n=4; N=4), respectively (Fig. 1b). The test loops instilled with test solution were practically empty, indicating that the ileum had normal absorptive capability and capacity. CT induced a significant fluid accumulation, which was not significantly (P=0.156) altered by indomethacin. However, almost no ileal fluid accumulation was observed in ST-instilled loops (mean concentration 3.5 × 1010 ± 2.8 × 1010 cfu in n=9; N=3) by saline-treated pigs, and a lesser significant fluid accumulation was observed compared with indomethacin-treated pigs (mean concentration 21.5 × 1010 ± 19.0 × 1010 cfu in n=12, N=4).
There was no significant difference (P=0.296) between the CT-induced fluid accumulation in the ileum and the jejunum in indomethacin-treated pigs.
Because the aim of the present study was to investigate the effect of indomethacin on ST-induced fluid accumulation, with CT as a control for the secretory capacity, the lack of ST-induced fluid accumulation in the ileum was not considered further.
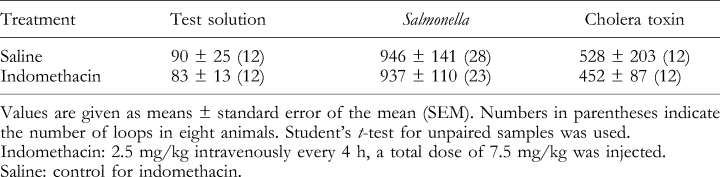
Because the instilled concentration of ST varied slightly from pig to pig due to the procedure for the preparation of bacterial inoculum, it was investigated whether the fluid accumulation was dependent on the instilled concentration of ST. The results in Fig. 2a illustrate no correlation between the instilled concentration of ST and the observed fluid accumulation in the jejunum. The estimated fluid accumulated per 1010 cfu ST in the jejunal loops of saline-treated pigs (N=8) was 11.6 ± 2.2 mg fluid/mg loop dry weight × 8 h and 15.4 ± 3.6 mg fluid/mg loop dry weight × 8 h in the loops of indomethacin-treated pigs (N=8). Although the instilled concentration of CT was constant (56 μg/loop), the observed fluid accumulation varied (Fig. 2b). In the ileum, the estimated fluid accumulated per 1010 cfu ST of saline-treated pigs (N=3) was 0.5 ± 0.4 mg fluid/mg loop dry weight × 8 h and 2.2 ± 1.7 mg fluid/mg loop dry weight × 8 h in the loops of indomethacin-treated pigs (N=4).

Fluid accumulation in porcine jejunal tied-off loops as a function of (a) the instilled concentration of Salmonella Typhimurium and (b) cholera toxin. The circles represent the fluid accumulation in a single loop. Eight animals were treated with saline or indomethacin.
Content of PGE2 and 5-HT in the accumulated fluid
Because only the jejunum responded to ST by secreting fluid, the jejunal content of PGE2 and 5-HT in loops instilled with test solution, and in the accumulated fluid of ST or CT loops were determined. The results showed that indomethacin induced a significant effect on the luminal content of PGE2 in test, CT, and ST loops (Table 1), and not on 5-HT (Table 2). The results in Fig. 3a illustrate no correlation between the instilled concentration of ST and the observed content of PGE2. CT also induced a varied luminal content of PGE2 (Fig. 3b). Furthermore, no correlation between the instilled concentration of ST, and CT (56 μg/loop), and the observed content of 5-HT was observed (data not shown).

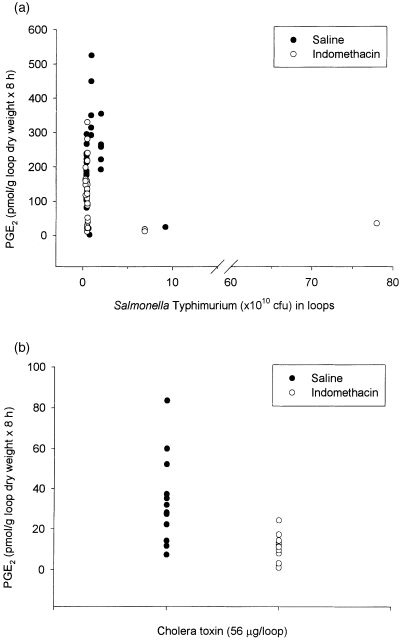
Content of prostaglandin E2 (PGE2) in porcine jejunal tied-off loops as a function of (a) the instilled concentration of Salmonella Typhimurium and (b) cholera toxin. The circles represent the fluid accumulation in a single loop. Eight animals were treated with saline or indomethacin.
Discussion
In the present study, we sought to answer the questions: can the PGE2 synthesis inhibitor, indomethacin, influence: (1) ST- and CT-induced fluid accumulation and (2) the release of the endogenous secretagogues PGE2 and 5-HT in the porcine small intestine?
The secretory responses to CT and ST in the small intestine are often compared because they both induce fluid and electrolyte secretion (Klimpel et al., 1995; Grøndahl et al., 1998). The major difference in the pathophysiology between CT and ST is that CT induces no inflammatory or morphological changes (Klimpel et al., 1995; Hansen et al., 1996). This complicates a direct comparison of the potency of CT and ST.
Epithelial exposure to ST or CT has been shown to induce a production of PGE2 in the small intestine, which is believed to play a significant role in mediating the secretory response (Duebbert and Peterson, 1985; Beubler et al., 1989; Grøndahl et al., 1998). It has furthermore been suggested that PGE2 induces fluid accumulation in rat jejunum via secretory reflexes in the enteric nervous system (Brunsson et al., 1987). This could account for an involvement of 5-HT.
Fluid accumulation
Indomethacin had no effect on the ST- and CT-induced fluid accumulation in the present study with pigs, indicating that prostaglandins are not involved, or are at least not a major component, in the secretory response of ST and CT in the porcine jejunum. This is in contrast to observations in rats (Beubler et al., 1989). Besides a species difference, the use of a lower dose of indomethacin in this study may explain the contradictory results. The intravenously administered dose of indomethacin in the present study was the maximal dose recommended for use in man (communication by Dumex, Denmark). Gastrointestinal side-effects (ulcer, crypt losses, lesions) have been reported in higher, pharmacological doses (Masferrer et al., 1994; Ettarh and Carr, 1996).
In contrast to previous observations (Grøndahl et al., 1998), ST failed to induce ileal secretion. The reason for this inconsistency might be the use of different anaesthetics, α-chloralose in the present study, and isoflurane in the study by Grøndahl et al. (1998). The fluid accumulation to CT in the porcine small intestine depends on the anaesthetic (Maltbæk et al., 1998). Apart from different effects on the nervous system and the synaptic mechanisms (Griffiths and Norman, 1993), anaesthetics additionally influence the endogenous production of PGE2 (Denson et al., 1996). The CT-induced fluid accumulation in the ileum indicates that this tissue has a secretory mechanism. The apparently minor enhanced ST-induced secretion in indomethacin-treated pigs could suggest a diminished absorption or stimulated secretion in the ileum. In addition, the mean concentration of instilled ST in the ileum was higher in indomethacin-treated pigs compared with saline-treated pigs.
The fluid accumulation varied significantly within a relatively small difference in the instilled concentration of ST, and with a constant concentration of CT. This could be due to segmental and inter-species differences. For ST, it could in addition be due to differences in sensitivity to the invasion of ST.
Luminal content of PGE2 and 5-HT
The release of the endogenous secretagogues PGE2 and 5-HT into the intestinal lumen represents an indirect measure, and reflects an ‘overflow’ from the mucosa into the gut lumen (Speelman et al., 1985). It is therefore difficult to make a direct correlation between the luminal content and the accumulated fluid (Beubler et al., 1989). Nevertheless, it is convenient and advantageous, at least for PGE2, to measure the release luminally as it avoids a contribution of prostaglandins by aggregating platelets in the intestinal blood stream (Bukhave and Rask-Madsen, 1979).
The present study demonstrated that ST and CT induced a significant luminal release of PGE2 and 5-HT. Previous studies showed that CT had no influence on PGE2 released to the lumen in rat (Smith et al., 1985); and indomethacin failed to reduce fluid secretion in actively purging patients (Rabbani and Butler, 1985). The indomethacin in the present study was given immediately after anaesthesia to inhibit the cyclooxygenase early. The content of PGE2 in ST loops was significantly increased compared with the content of PGE2 in CT loops, which may reflect the inflammatory response induced by the invasion of ST. An alternative explanation could be that most of the prostaglandin receptors are saturated with locally produced prostaglandins, even in the presence of an adequate concentration of indomethacin, or the formation of all mediators in the intestine may be at maximum in the secretion process (Rabbani, 1995). Treatment with indomethacin had a significant influence on the luminal release of PGE2 in test, ST and CT loops but not on 5-HT. Regarding 5-HT, this is in accordance with studies in the rat (Beubler et al., 1989). However, indomethacin had no effect on fluid accumulation. The failure to reduce fluid accumulation significantly in ST and CT loops by indomethacin despite an inhibition of PGE2 release and the presumable ‘overflow’ of PGE2 content, might reflect the activation of the other cyclooxygenase isomere, cyclooxygenase-2, which is induced by mechanisms such as cytokines and endotoxins in inflammatory cells (Herschman, 1996). It has been suggested that the inducible form of the cyclooxygenase, i.e. cyclooxygenase-2, is responsible for the elevated production of prostaglandins during inflammation (Masferrer et al., 1994; Seibert et al., 1994). Thus, a simultaneous expression of the constitutive form of the cyclooxygenase, cyclooxygenase-1, responsible for the physiological production (housekeeping) of prostaglandins, and the inducible cyclooxygenase-2 of the two cyclooxygenase isoforms might inevitably cause a marked rise in the local PGE2 synthesis accounting for the measured residual content of PGE2 in indomethacin-treated pigs. It has previously been observed that the CT-induced secretion is not mediated by locally produced prostaglandins in rat ileal loops (Smith et al., 1985). A higher content of PGE2 in the jejunum than in the ileum has previously been demonstrated, which further corroborates the segmental differences in the secretory response induced by ST (Grøndahl et al., 1998). This further emphasizes the importance of studying enteropathogenic infections in several segment1s, and under different experimental conditions, because diarrhoea is a complex phenomenon (Stephen et al., 1985; Jones, 1997; Lundgren and Jodal, 1997). Other products may in addition be involved in the secretory cascade, and it has previously been observed that 5-HT is involved in the secretory pathway of CT and ST in the porcine small intestine (Grøndahl et al., 1996, 1998). This might explain the failure of indomethacin to reduce the fluid accumulated in the intestine. Endogenous substances in the intestine, such as gut hormones from the enterochromaffin cells and inflammatory mediators, i.e. cytokines, may also induce secretion by direct stimulation and/or neuronal stimulation of the enterocyte (Hecht, 1999). Interestingly, it has been observed that biologically active prostaglandin-like compounds, the isoprostanes, are formed by free radical-catalysed peroxidation independent of cyclooxygenase activity under pathophysiological conditions (Morrow et al., 1990), and they can induce a secretory response in the porcine small intestine (Unmack et al., 1998).
Conclusion
ST and CT induced a significant secretory response in the porcine jejunum in vivo, which was not reduced by indomethacin, an inhibitor of the PGE2 synthesis pathway, despite a significant reduction in the intraluminal release of PGE2. Other inflammatory mediators may therefore contribute to the fluid accumulation in the porcine small intestine. However, the ST- and CT-induced fluid accumulation could be caused either by the residual content of PGE2 and/or by 5-HT, or other inflammatory mediators.
Acknowledgements
We thank B. Holle, C. T. Larsen, T. Otto, I. Thomsen, D. S. Jensen, L. Djurhuus, B. Sørensen, T. Bønnelycke, and G. Frederiksen for skilled technical assistance, and Dr S. E. Jorsal (Danish Veterinary Laboratory) for testing the animals for Salmonalla infections before the experiments. M. A. Unmack was supported by grants from The Hannibal Sander’s Foundation (Margrethe Brinch’s Grant), Simon Fougner Hartmann Family’s Foundation, Else and Mogens Wedell-Wedellsborg’s Foundation, King Christian IX and Queen Louise’s Anniversary Foundation, Dagmar Marshall’s Foundation, Ingeborg Roikjer’s Foundation, Landed Proprietor Viktor A. Goldschmidt’s Foundation (Department B), Merchant Mr Sven Hansen and Mrs Ina Hansen’s Foundation, Director Mr Jacob Madsen’s and Mrs Olga Madsen’s Foundation, King Christian X’s Foundation, Novo Nordisk Foundation, Carlsberg Foundation (The Royal Veterinary and Agricultural University, Denmark). M. B. Hansen was supported by a grant from The Lundbeck Foundation. A preliminary report of this work was presented at the Second International Symposium on Epidemiology and Control of Salmonella in Pork, Copenhagen, Denmark, August 1997. The experiments were conducted according to the ethical guidelines approved by the Danish animal experiment inspection.




