Tagging of Atlantic cod (Gadus morhua) with intragastric transmitters: effects of forced insertion and voluntary ingestion on retention, food consumption and survival
Abstract
The effects of intragastric tagging on transmitter retention, food consumption and survival were investigated in Atlantic cod (Gadus morhua L.) under laboratory conditions. Cod (60–84 cm) were intragastrically tagged with two different sizes of dummy acoustic transmitters (16 × 45 and 16 × 82 mm) using the methods of forced insertion and voluntary ingestion. Cod that were tagged by forced insertion began to regurgitate transmitters the first day after tagging and continued to do so throughout the period of study. The percentage of fish retaining transmitters in this group dropped to 50% after 32 days. By comparison, cod that were tagged by voluntary ingestion exhibited a 44-day delay in the onset of initial regurgitation. This was followed by a rapid rate of regurgitation, with the percentage of fish retaining transmitters in this group dropping to 50% after 60 days. These retention times are prolonged in comparison to previous studies and have been attributed to the lower temperature regime used in this study. Transmitter size did not significantly affect the duration of retention. During the first 20 days following tagging, the average food consumption per fish was higher in the voluntary ingestion group compared with the forced insertion and sham groups. Mortality levels were 13, 44 and 56% for the voluntary ingestion, forced insertion and sham groups, respectively, over the period of study. We associate low food consumption and high mortality rates in the first 20 days of the experiment with high levels of stress due to handling. The findings indicate that voluntary ingestion is a more viable technique for the intragastric tagging of cod in field studies.
Introduction
A growing number of studies use radio and acoustic telemetry to monitor the free-ranging movements of fish. Consequently, various techniques for attaching transmitters to fish have evolved over the past few decades. These can be broadly classified as either external attachment or internal implantation into the peritoneal cavity, oviduct, or stomach (Winter 1983). Each technique has its own unique advantages and disadvantages, depending on the research objectives and species of interest. A fundamental criterion for any technique is the need to minimize adverse effects on the natural behaviour of the fish. If the presence of a transmitter affects a fish’s posture, swimming capability, or foraging activity, then the resulting tracking data may be biased. This has increased the concern regarding the ethical considerations of tagging (Smolowitz and Wiley 1998) and has encouraged the use of less invasive tagging techniques where possible.
Intragastric tagging entails placing a transmitter into the stomach cavity of a fish. This technique has been widely used in both marine (e.g. Priede and Smith 1986; Sarno et al. 1994; Godø et al. 1997; Løkkeborg et al. 2000; Wroblewski et al. 2000) and freshwater species (e.g. Kynard and Warner 1987; Gerlier and Roche 1998; Martinelli et al. 1998). Comparative studies conducted under laboratory conditions have indicated that this is one of the most viable techniques for short-term tagging studies (Mellas and Haynes 1985; Peake et al. 1997a). Transmitters placed in the stomachs of fish will lie near their centre of gravity, will not snag or cause increased drag while swimming, do not cause tissue abrasion, and are eventually regurgitated. Inserting the transmitters can be accomplished using either one of two methods: forced insertion or voluntary ingestion. The method of forced insertion has been widely used, particularly for its reduced handling time and quick recovery of the fish. Often taking less than 30 s to complete, the basic technique involves inserting a transmitter into the stomach cavity via the oesophagus (Hawkins and Urquhart 1983; Winter 1983). The method of voluntary ingestion, by comparison, relies on the ‘trickery’ of placing transmitters in baited packages and allowing fish to voluntarily ingest the contents in situ (e.g. Priede and Smith 1986; Bagley and Priede 1997; Godø et al. 1997; Løkkeborg 1998; Løkkeborg and Fernö 1999; Løkkeborg et al. 2000). The advantage of this method of intragastric tagging is that it is entirely non-invasive. This eliminates the stress associated with capture, including possible hydrostatic changes in the swimbladder, anaesthetization, and handling. By removing these stressors, it has been hypothesized that the natural behaviour of the fish would be less affected, survival would be improved, and the retention of transmitters would be prolonged (Armstrong et al. 1992a, 1992b).
The purpose of this study was to compare the two methods of intragastric tagging in Atlantic cod (Gadus morhua L.) under laboratory conditions. We test the null hypotheses that there are no differences in transmitter retention, food consumption or survival for cod tagged using either the method of forced insertion or voluntary ingestion. We also investigate the effect of transmitter size on the duration of retention. The temperature regime used in this study is typical for Newfoundland waters and is lower than those previously examined in intragastric studies using cod.
Materials and methods
Dummy transmitters were manufactured to meet the size and weight specifications of two sizes of acoustic transmitters commonly used in the field: 16 × 45 mm (diameter × length) with a mean weight of 25.14 g (SD=0.30) in air, and 16 × 82 mm with a mean weight of 39.48 g (SD=0.30) in air. The transmitters were cylindrical in shape and constructed of an epoxy-resin compound. Each transmitter was individually marked with an identification number for recognition.
Atlantic cod were captured by longline in the inshore waters of Trinity Bay, Newfoundland in March 1997. The fish were transported to St. John’s where they were held in flow-through tanks with a continuous supply of ambient temperature seawater and fed a diet of chopped Atlantic herring (Clupea harengus L.) once a day to satiation. The tank adaptation period was approximately 9 weeks. The experiment commenced in mid-May using a total of 40 cod (60–84 cm) in late-spawning or postspawning condition. The fish were starved for 8 days immediately prior to tagging to ensure the stomachs were sufficiently empty. They were then randomly distributed into five experimental treatments: (1) forced insertion with small transmitters, (2) forced insertion with large transmitters, (3) voluntary ingestion with small transmitters, (4) voluntary ingestion with large transmitters, and (5) sham forced insertion.
The forced insertion treatments each contained eight fish and were kept together in a round fibreglass tank with dimensions 1.7 × 1.5 m (diameter × depth). These fish were anaesthetized (2-phenoxyethanol, 1 ml : 8 L seawater) for the purpose of tagging and length measurement. Transmitters were carefully inserted into the stomach cavity via the oesophagus (Winter 1983). The cod implanted with small transmitters had a mean length of 68.4 cm (SD=5.6) while the cod with large transmitters had a mean length of 71.1 cm (SD=8.2).
The voluntary ingestion treatments each contained four fish and were kept together in a rectangular tank with dimensions 3.2 × 2.3 × 1.1 m (length × width × depth). For the purpose of tagging the fish, a small arena was established at one end of the tank using a temporary partition. Each fish was individually placed into the tagging arena and presented with a baited package for voluntary ingestion without the feeding competition of the other fish. The baited package consisted of a transmitter wrapped in a fillet of herring enclosed in a piece of cheesecloth. The most effective method for encouraging ingestion was to suspend the baited package in the water using a line of thread (similar to Løkkeborg 1998; Løkkeborg and Fernö 1999). The cod typically took either immediate interest in the baited package, tearing it off the suspension thread with little hesitation, or returned later to take the bait. Once the baited package was consumed, the partition was lifted and the fish was permitted to swim into the remaining area of the tank, i.e. unhandled, joining those fish already tagged. The cod which ingested small transmitters had a mean length of 73.3 cm (SD=5.0) while the cod with large transmitters had a mean length of 69.3 cm (SD=7.4).
The sham treatment was established to test for the artefacts of the anaesthetic and handling on food consumption and survival. These cod (n=16) were not tagged but underwent the anaesthetization and handling procedure similar to the forced insertion treatments. The cod in this treatment had a mean length of 69.7 cm (SD=7.5) and were kept together in a separate fibreglass tank with dimensions 1.7 × 1.5 m (diameter × depth).
Daily feedings of chopped Atlantic herring were resumed the day after tagging for all experimental treatments. The fish were fed to satiation by slowly throwing the feed into the tanks and allowing all fish to actively compete for the food. Feeding was terminated for each tank after three successive pieces of herring sank to the bottom and were not eaten after 20 s duration. The total weight of feed consumed by each tank was then recorded and each fish was assumed to have eaten an average share.
All tanks were inspected twice daily for regurgitated transmitters, mortality and water temperature. If transmitters were discovered, they were retrieved immediately and the date, time and transmitter identification number were recorded. If dead fish were found, they were immediately retrieved and dissected to determine their stomach contents and sex.
Results
Analyses of variance showed no significant effect of either transmitter size (F0.05 [1,19]=0.360, P=0.556) or fish length (F0.05 [1,19]=3.290, P=0.086) on the duration of transmitter retention. Hence, the data were pooled across the treatments for transmitter size within each tagging technique for further analysis.
The duration of transmitter retention varied between the two tagging techniques (Fig. 1). Cod in the forced insertion group began to regurgitate transmitters the first day after tagging and continued to do so throughout the period of study. The percentage of fish retaining transmitters dropped to 50% after 32 days. The longest period a transmitter was retained in this group was 87 days by both a 61 and 66-cm fish. By comparison, cod in the voluntary ingestion group exhibited a delayed onset of regurgitation, with the first occurrence recorded 44 days after tagging. This was followed by a rapid rate of regurgitation, with the percentage of fish retaining transmitters dropping to 50% after 60 days. The longest period a transmitter was retained in this group was 77 days by a 63-cm fish.
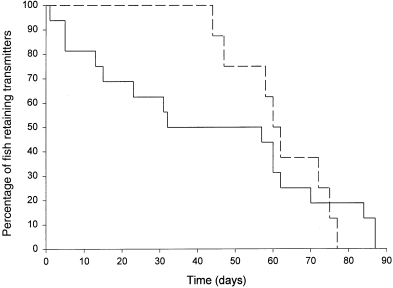
6)
Survival varied considerably among the experimental groups (Fig. 2). Mortality rates during the first 20 days were highest in both the forced insertion and sham groups. Overall, the mortality levels were 13, 44 and 56% for the voluntary ingestion, forced insertion, and sham groups, respectively. Cod which were tagged using voluntary ingestion exhibited the lowest mortality (one of eight), with only one fish dying on day 47. Dissection revealed that it had not regurgitated its transmitter prior to death and that it was a female in postspawning condition. In contrast, cod which were tagged by forced insertion suffered a 44% mortality (seven of 16), with three fish dying by day 7, six by day 25, and the seventh on day 84. Dissection revealed that all were female and were either in late- or postspawning condition. All but one fish had regurgitated their transmitters and they were all devoid of feed in the stomach at the time of death. In the sham group, nine out of 16 fish (56%) died over the period of study, two by day 7 and the remainder by day 21. Dissection revealed that all were female and were either in late- or postspawning condition. All were devoid of feed in the stomach at the time of death.
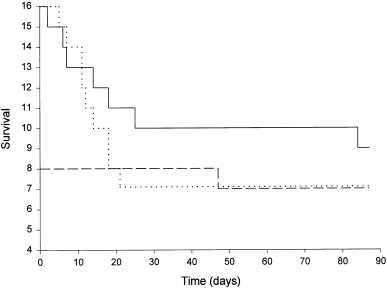
Number of fish surviving over the period of study in the forced insertion (solid line), voluntary ingestion (dashed line), and sham (dotted line) groups
Average daily consumption per fish was found to vary within and between experimental groups over the period of study. The data were pooled for each 5-day interval and are presented in Fig. 3(a) along with 95% confidence intervals. Most of the significant variation in feeding took place during the first 20 days following tagging. Average consumption levels in the sham and forced insertion groups initially decreased from day 6 to day 15 before beginning to increase. Average consumption levels in the voluntary ingestion group were noticeably higher during this period. By day 20, there was little difference in consumption levels between the three groups. From day 20 to day 55, the average consumption was slightly higher in the sham group and beyond day 55, all groups experienced a gradual increase in feeding.
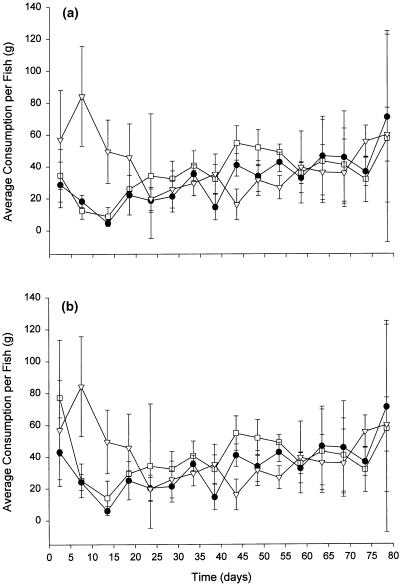
Average consumption of feed (g) per fish over the period of study for (a) the total number of fish in each tank, and (b) re-weighted for the number of fish potentially not feeding due to the onset of mortality. Values shown are the means for each 5-day interval plotted at the midpoint, with corresponding 95% confidence intervals: forced insertion (●), voluntary ingestion (▿), and sham (□)
Given the high mortality observed in the sham and forced insertion groups during the first 25 days, it is questionable whether all of the fish actually began feeding following the handling/tagging procedure. To determine whether our calculation of consumption levels may have been biased, we re-weighted the consumption data for the first 25 days, removing the data from those fish that eventually died during this period (Fig. 3b). Except for an increase in consumption by the forced insertion group during the first 5 days, the results are otherwise similar to Fig. 3(a), indicating that our estimates of average consumption per fish were not biased due to the onset of mortality in some fish. The effect of transmitter size on food consumption could not be assessed since these treatments were not isolated within separate tanks.
Ambient temperature of the incoming seawater supply to all tanks increased over the period of study (Fig. 4). Water temperatures ranged from 2.07 to 13.49 °C and were not significantly different between the experimental groups (ANCOVA, F0.05 [2,231]=0.043, P=0.958).
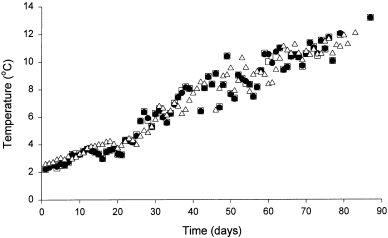
Ambient seawater temperatures observed over the period of study in the forced insertion (●), voluntary ingestion (▵), and sham (□) groups
Discussion
The duration of transmitter retention by cod which were tagged using forced insertion is prolonged in comparison with laboratory work by Lucas and Johnstone (1990), who reported a 50% retention time of only 5 days for North Sea cod (x=984.6 g) at 10 °C using transmitters of the size 16 × 56 mm. This is remarkably less than the 50% retention time of 32 days found in the current study. Lucas and Johnstone (1990) reported that the rate of regurgitation in their study decreased over time, with only one fish retaining its transmitter after 35 days. These authors also reported a higher regurgitation of transmitters within the first day following tagging (30%), compared to only 6% in the current study. Field studies using the forced insertion method have reported that cod are capable of retaining transmitters for reasonable periods of time with good success (e.g. Hawkins et al. 1974, 1980; Clark and Green 1990; Wroblewski et al. 1995; 2000). The longest retention time reported was 21 days by Clark and Green (1990) for cod (29–33 cm) in Newfoundland waters using transmitters of the size 17 × 57 mm. Among other gadoid species, retention times in the field have been reported as high as 19 days for pollack (Pollachius pollachius L., 43–44 cm) and 28 days for saithe (Pollachius virens L., 35–38 cm) using transmitters of the size 16 × 51 mm (Sarno et al. 1994).
The duration of transmitter retention by cod which were tagged using voluntary ingestion is also prolonged in comparison to previous laboratory work (Armstrong et al. 1992b). These authors showed that North Sea cod (67–92 cm), which voluntarily consumed transmitters (16 × 56 mm) wrapped in squid and held at 12 °C, had a 50% retention time of 8 days. Again, this is much less than the 50% retention time of 60 days observed in the current study. Both experiments, however, did note a delayed onset of initial regurgitation. In the Armstrong et al. (1992b) experiment, cod began regurgitating transmitters 6 days after tagging. Similar to the current study, they then exhibited a rapid rate of regurgitation with only one fish retaining its transmitter after 21 days. Field studies using the voluntary ingestion method have reported the occurrence of transmitter regurgitation by cod (40–55 cm) after 7–8 days using transmitters similar in size to the current study: 16 × 48 mm and 16 × 80 mm (Løkkeborg 1998; Løkkeborg and Fernö 1999). Maximum retention times in the field are unknown, but have been reported to be as high as 20 days for cod (~50 cm) using transmitters of the size 16 × 48 mm (Engås et al. 1998).
Water temperature has been hypothesized as a potential factor affecting the duration of transmitter retention (Armstrong et al. 1992a). These authors suggested that at low temperatures, transmitters might be retained longer due to a slowed digestive process; the results of the current study support this hypothesis. Low water temperatures observed during the beginning of this study may have been responsible for the prolonged periods of transmitter retention by cod in both tagging groups. In Newfoundland waters, cod are known to occupy temperatures ranging from – 1.5 °C (Wroblewski et al. 1994) up to 12.0 °C (Clark and Green 1990), depending on the region and season. In Trinity Bay, Newfoundland, intragastrically tagged cod have been tracked at temperatures well below 2 °C for periods up to 5 days (Wroblewski et al. 1995). If temperature has an effect on transmitter retention, then field studies conducted during low water temperatures should potentially benefit from an increased number of tracking days.
Hart and Summerfelt (1975) suggested that the presence of a transmitter in the stomach of a fish might affect its appetite. Evidence to support this hypothesis has been reported in juvenile Atlantic salmon (Salmo salar L., Armstrong and Rawlings 1993) and chinook salmon (Oncorhynchus tshawytscha Walbaum, Martinelli et al. 1998). However, laboratory and field studies have indicated that this is not the case for adult cod (Lucas and Johnstone 1990; Armstrong et al. 1992b; Løkkeborg 1998; Løkkeborg and Fernö 1999). Løkkeborg (1998) demonstrated that the natural foraging behaviour of cod in the ocean is not altered by the presence of a transmitter in the stomach. The results from the current study (Fig. 3a) support this finding. The similarity in consumption levels between our sham and forced insertion groups suggests that the presence of the transmitter in the latter group was not the reason for a decrease in feeding. We interpret the diminished appetites of cod in the sham and forced insertion groups during the first 20 days to be the result of the stress of the handling and the anaesthetic. A similar reduction in feeding may be expected to occur under field conditions, which could bias tracking data.
Experimental estimates of tagging-related mortality are variable. Mortalities as high as 13% (Cote et al. 2000) and 75% (Mellas and Haynes 1985) have been reported for surgical implantation, 50% (Mellas and Haynes 1985) and 93% (Ross and McCormick 1981) for external attachment, and 33% for oviduct insertion (Peake et al. 1997b). Studies using intragastric methods, by comparison, have generally reported lower mortalities. The method of voluntary ingestion yielded 13% mortality for cod in the current study and 0% in the Armstrong et al. (1992b) study. Studies using the method of forced insertion have also reported relatively low mortalities: 0% in Atlantic cod (Lucas and Johnstone 1990), chinook salmon (Adams et al. 1998) and rock fish (Sebastes sp., Starr et al. 2000), 13% in Atlantic salmon (S. salar, Armstrong and Rawlings 1993) and 17% in white perch (Morone americana Gmelin, Mellas and Haynes 1985). In the current study, mortality in the sham and the forced insertion groups occurred rapidly and simultaneously over the first 20 days before levelling off, as evident in Fig. 2. We explore arguments why this may have happened.
The tanks used for the sham and forced insertion groups were smaller than that used for the voluntary ingestion group. This is very similar to field situations where cod are generally held in small tanks on the deck of a vessel prior to intragastric tagging and are often returned to those tanks for an observation period prior to release (e.g. Wroblewski et al. 1995; 2000). Fish tagged using voluntary ingestion are not restricted spatially since they are tagged in situ. By giving our voluntary ingestion group a larger tank, we attempted to replicate natural field conditions. As a result, the estimated stocking densities for the sham and forced insertion groups (14.7 kg m−3) were higher than the voluntary ingestion group (3.3 kg m−3). Although we cannot completely rule out a tank effect on mortality, our stocking densities were well within the recommended range for rearing cod in aquaculture (e.g. Kvenseth 1993; Lambert and Dutil 2001). Aggressive behaviour among cod may also occur during the spawning season (Brawn 1961), but none was observed in our study. Rather, when compared with the voluntary ingestion group with only 13% mortality, we believe that the handling/tagging process itself contributed to the reduced survival in the sham and forced insertion groups. Both groups experienced capture, physical handling, and anaesthetization. Cod in the voluntary ingestion group did not experience this treatment. The process of death is unknown and is believed to be stress related and not immediate. The Study Group on Unaccounted Mortality in Fisheries reported that the onset of mortality in fish stressed from escaping otter trawl codends was delayed anywhere from 2 to 8 days (Anonymous 1997). Our first mortality occurred on day 2.
The findings from this study demonstrate the advantages of using the method of voluntary ingestion for the intragastric tagging of cod. We found that cod which voluntarily ingested transmitters exhibited a delayed onset of initial regurgitation, longer periods of retention, higher food consumption and lower mortality in comparison to fish that were tagged by the forced insertion method. Voluntary ingestion techniques should reduce the cost of tracking studies due to premature transmitter loss and minimize potential biases in natural behaviour such as feeding. Forced insertion, in contrast, should be used with caution. Given that regurgitation can occur within the first day and death within 2 days, we recommend holding cod for a minimum period of 48 h for observation prior to release.
Acknowledgements
We are grateful to Lotek Marine Technologies Inc. (St. John’s, Newfoundland) for providing the dummy transmitters needed to carry out the experiment. The manuscript benefited from reviews by Joseph Brown, Wade Hiscock and Kent Smedbol at Memorial University and by Barry McCallum and John Brattey at the Northwest Atlantic Fisheries Centre. Special thanks are given to C. Bonnell, P. Higdon, D. Orr, and M. Veitch for the collection and care of the fish. This project was funded through the Department of Fisheries and Oceans Strategic Science Project no. 9040. Financial support for PDW was provided by the Natural Sciences and Engineering Research Council of Canada.




