Immunocytochemical studies of the gonadotropic cells in the pituitary gland of male mullet, Mugil cephalus, during the annual reproductive cycle in both natural habitat and captivity
Summary
Using antiserum specific for the β subunit of coho salmon gonadotropic hormone II (GTH II), an immunocytochemical study of Mugil cephalus (L.) pituitaries was conducted during the annual reproductive cycle of the male in both natural habitat and captivity. The gonadotropic potency of the pituitary gland in general underwent an obvious increase during testicular development, reaching a peak at the time of reproductive maturity. During the testicular cycle of M. cephalus, the GTH cells showed an increase in immunoreactive staining intensity, granulation, hypertrophy and hyperplasia during sexual maturation. However, degranulation, vacuolization, and weakened immunoreactivity of these cells occurred during spawning. The GTH cells in the pituitary gland of M. cephalus males reared in captivity appeared with high synthetic and secretory activity but the reproductive activity declined, as reflected in the form of low values of the gonadosomatic index (GSI) and earlier resorption of the testes.
Introduction
In recent years, immunocytochemical techniques have greatly enhanced the potential for investigating the reproductive physiology of fishes. Immunohistochemical studies of cell type distribution of the pituitary gland of mullet (Mugil cephalus L.) revealed that there is only one gonadotropin-producing cell type (Mousa and Mousa 1997) which secretes the gonadotropic hormone, GTH IIβ. Coincidental with gametogenesis, marked changes in the serum gonadotropin level in mullet (M. cephalus) occur with sexual maturity (Zaki et al. 1995).
Despite the research carried out on the artificial propagation of mullet in captivity, farming still relies on the capture of juveniles from the sea (Lee and Tamaru 1988). Most investigations have concentrated on the induction of mullet spawning in natural conditions at the peak of their normal breeding season (Kulikova and Gnatchenko 1987; Lee et al. 1987; Suzuki et al. 1991). Because males reared in captivity fail to undergo final spermatozoa production (i.e. spermiogenesis) and the information on the reproductive hormones during gonadal maturation for wild broodstocks is limited, more information regarding the reproductive endocrinology of M. cephalus in both natural habitat and captivity is needed.
The present study examined the GTH IIβ of the pituitary gland of the male M. cephalus at different stages of the testicular cycle in both natural habitat and captivity to gain insight into the endocrinology of the mullet and to add to our understanding of the inhibitory effects of captivity on reproduction. Knowledge of mullet reproductive physiology may help to improve the management of this species which has an interesting economic potential.
Materials and methods
Fish collection
From El-Bardawill Lagoon (natural marine habitat) and El-Manzalah freshwater fish farm, live mature males of M. cephalus (standard length + 28 cm; body weight 700–1500 g) were collected at intervals of about 1 month throughout the year. However, during the prespawning and spawning season (September and October), fish were collected at intervals of about 15 days to ensure that all stages of gonad maturation were included.
The gonadosomatic index (GSI = (gonad weight/gutted weight) × 100) and histological appearance were used as indices of maturity. Five stages of testicular activity were identified.
Histological appearance
The pituitary gland with the brain attached was immediately removed after decapitation and fixed in Bouin's fluid for 24 h. The fixed pituitary was thereafter dehydrated through an ascending series of increasing concentrations of ethanol, cleared and embedded in paraplast (m.p. 56–58 °C). Consecutive median sagittal sections of the pituitary gland were made at 3–5 µm thickness. Testes were also removed, weighed and prepared for histological examination using the same method as for the pituitaries. Sections of gonads were stained with Harris's alum haematoxylin and Heidenhain's iron, according to Mallory (1938), and an aqueous solution of eosin was used as a counterstain.
Immunocytochemistry
Immunocytochemical staining for the sections of the pituitary gland was generally performed with a Vectastain ABC (avidin-biotin-peroxidase complex) kit (Vector Laboratories, Burlingame, CA, USA). In brief, sections were deparaffinized in xylene, rehydrated through graded ethanol and washed in phosphate-buffered saline (PBS; pH 7.4) twice for 10 min. Unless otherwise stated, all incubation was at room temperature and PBS was used for washing after each step. Sections were incubated with the antiserum to chum salmon GTH Iβ subunit (lot no. 8707) or chum salmon GTH IIβ subunit (lot no. 8506), diluted 1:5000 for 18 h at 4 °C. The antisera to chum salmon GTH Iβ and GTH IIβ subunit were obtained from Dr H. Kawauchi (School of Fisheries Science, Kitasato University, Iwate, Japan). Thereafter, the sections were incubated with the biotinylated secondary antibody (Vector Laboratories) for 1 h and with avidin-biotin-conjugated peroxidase for 45 min. Finally, the sections were washed and stained with 3′,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO, USA) including 0.01% H2O2 in 0.05 M Tris-buffered saline (pH 7.6) for 3–5 min. After the enzyme reaction, the sections were washed in tap water, dehydrated in alcohol, cleared in xylene and mounted in DPX.
Because a heterologous GTH antiserum was used in this study, it was essential to gain a perspective on the specificity of this antiserum. To prove the specificity of staining, various controls were used. The GTH antiserum was preabsorbed with an excess of antigen. Primary or secondary antibodies and the avidin-biotin-peroxidase complex were also omitted.
Analysis of data
All values are expressed as the mean ± the standard error of the mean. Comparisons were made using analysis of variance ANOVA followed by the Dunett test. Differences were considered significant when P < 0.05.
Results
Testicular cycle
In natural habitat (saline water)
On the basis of seasonal changes encountered in the histomorphology and GSI (Table 1), the seasonal changes in testicular activity of male M. cephalus in saline water can be classified into five stages. Stage I consists of fish having immature testes with small-sized lobules; the main lobule components are sperm mother cells and spermatogonia (Fig. 1a); the GSI was 0.045 ± 0.015. Fish in stage II are in a stimulated spermatogenic stage characterized by the predominance of spermatocytes and the appearance of spermatids as well as small clusters of spermatozoa (Fig. 1b); the GSI was 0.072 ± 0.04. Stage III consists of fish with rapid spermatogenic testes, the spermatogenic activity is at its peak, and the lobules become filled with cysts of germ cells in different stages of maturation (Fig. 1c). Further, this stage is characterized by the predominance of spermatids and spermatozoa; in such cases, the GSI was 0.50 ± 0.30. Males in stage IV have ripe (mature) testes with seminiferous lobules fully packed with mature spermatozoa (Fig. 1d); the GSI was 3.6 ± 1.5. Fish in stage V have spent testes which are marked by an almost total cessation of spermatogenic activity; this is indicated by an increase in the thickness of the interlobular septa and by the presence of spermatozoa in the lumen of some seminiferous lobules after spermiation (Fig. 1e); the GSI was 0.58 ± 0.33.
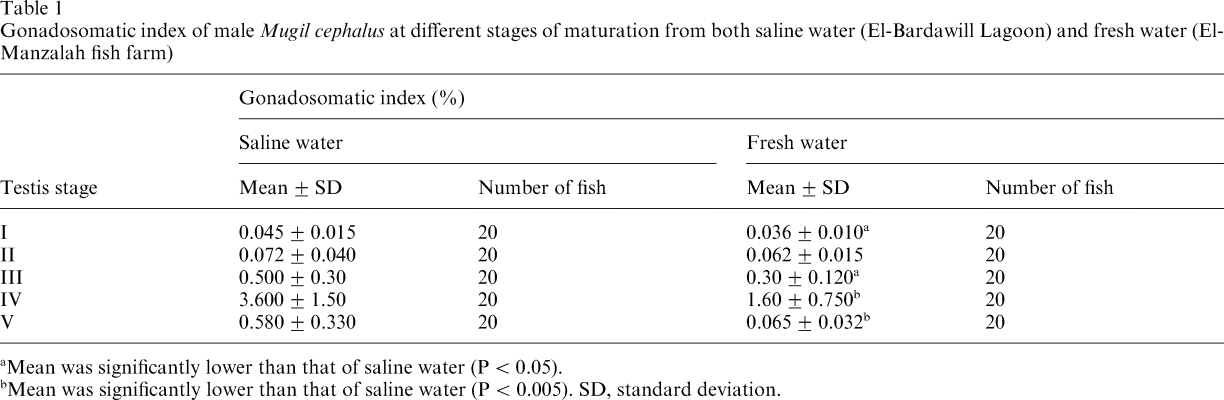
Sections of Mugil cephalus testis in different stages of development stained with haematoxylin and eosin (×400). (a–e) Fish obtained from natural habitat. (a) Immature testis, showing seminiferous lobules containing spermatogonia (SG). (b) Testes of fish obtained during the period of stimulated spermatogenesis, showing the germ cells at various stages of maturation: spermatogonia (SG), spermatocytes (SC), spermatids (ST) and spermatozoa (SZ). (c) Testes of fish obtained during the period of rapid spermatogenesis, showing the predominance of spermatids (ST) and spermatozoa (SZ) in the seminiferous lobules. (d) Ripe testis, showing seminiferous lobules filled with spermatozoa (SZ), and the thin interlobular septa (ILS). (e) Spent testis, showing the thick interlobular septa (ILS), the presence of spermatozoa (SZ) in the lumen of some seminiferous lobules (LU), and Sertoli cells (Se C). (f) Testes of fish obtained from captivity at the resorbed stage, showing the seminiferous lobules with low spermatogenic activity or in a degenerative stage
In captivity (fresh water)
In freshwater fish, the testes development followed more or less the same course as that of fish in saline water. Nonetheless, the monthly appearance of different stages was considerably delayed in fresh water, with relatively lower GSI (Table 1). Spawning did not occur in the fresh water and the resorption of ripe testes (Fig. 1f) was observed.
Cyclic changes in the GTH cells
The GTH cells occupied the major part of the proximal pars distalis (PPD) and were contained in numerous granules which were bound strongly and specifically by the antichum salmon GTH IIβ subunit (Figs 2 and 3). The GTH cells exhibited numerical variation both in number and size. They also displayed a marked degree of granulation, degranulation, vacuolization and devacuolization accompanying the different stages of spermatogenic cycles, as represented in Figs 2 and 3.
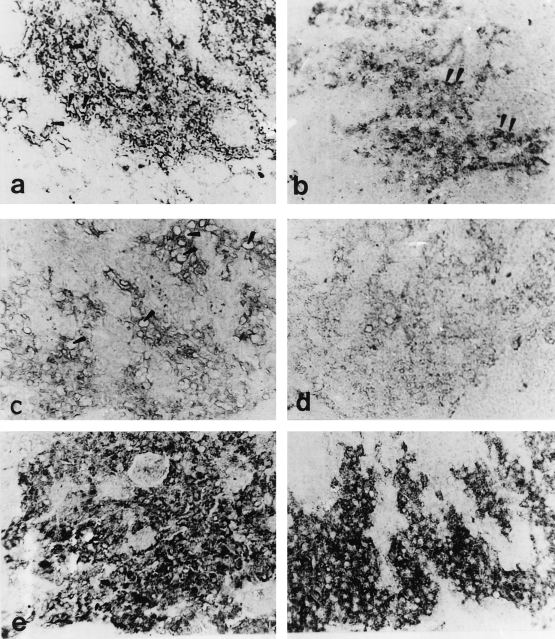
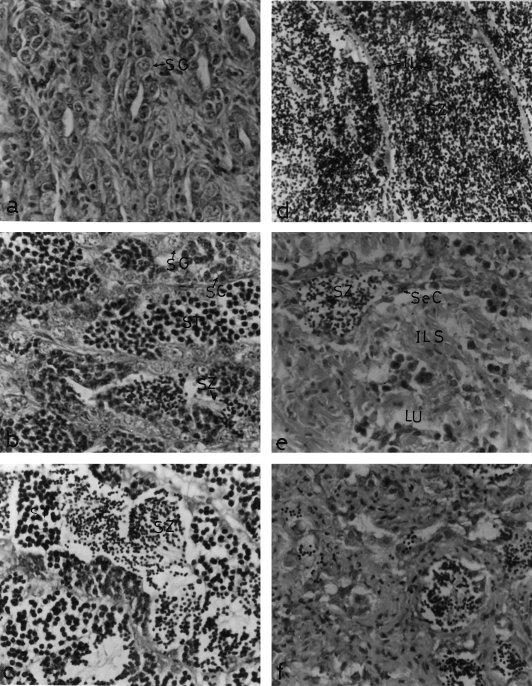
Midsagittal sections through the proximal pars distalis (PPD) of male Mugil cephalus, stained with antichum salmon gonadotropic hormone IIβ (GTH IIβ subunit) (×400) (a) Immature male obtained from a natural habitat (saline water). Note the small size and moderate immunoreactivity of the GTH cells and the presence of some vacuoles (arrowheads). (b) Immature male obtained from captivity (fresh water). The GTH cells are small in size and number. Note the accumulation of immunoreactive granules in some GTH cells (arrowheads). (c) Male obtained during the period of stimulating spermatogenesis. The GTH cells are few in number with many vacuoles (arrowheads). Note the degranulation and weak immunoreactivity of the GTH cells. (d) Male obtained during the period of stimulating spermatogenesis from captivity. The GTH cells are degranulated with very few immunoreactive granules. (e) Male obtained during the period of rapid spermatogenesis from a natural habitat. The number of GTH cells has increased and have variable immunoreactivity. Note the revival of immunoreactive granules. (f) Male from captivity obtained during the period of rapid stimulating spermatogenesis. The GTH cells are granulated with intensely immunoreactive granules
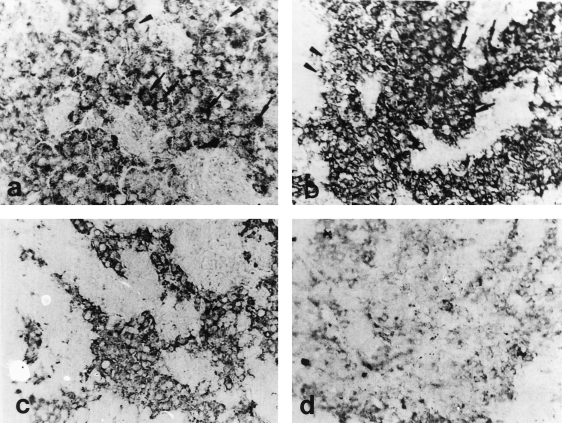
Midsagittal sections through the proximal pars distalis (PPD) of male Mugil cephalus stained with antichum salmon gonadotropic hormone IIβ (GTH IIβ subunit) (×400). (a) Ripe male obtained from a natural habitat. The GTH cells are hypertrophied and vacuolated (arrowheads). Note the accumulation of immunoreactive granules in most of the GTH cells (arrows). (b) Ripe male obtained from captivity. The GTH cells are small in size with variable immunoreactivity. Note the presence of both degranulated (arrowheads) and granulated cells (arrow). (c) Spent male obtained from a natural habitat. The GTH cells are small in size and number, degranulated and vacuolated. Note the moderate immunoreactivity of the GTH cells. (d) Male obtained during the period of testis resorption from captivity. The GTH cells are small in size and degranulated. Note the scarcity of immunoreactive granules
In natural habitat (saline water)
In the prespermatogenic fish (immature), the staining of the GTH cells was faint or moderate (Fig. 2a). With the onset of spermatogenesis (stimulating spermatogenesis), the GTH cells appeared degranulated with many vacuoles and showed weak immunoreaction (Fig. 2c). Thereafter, as spermatogenesis progressed (rapid spermatogenesis), the GTH cells stained more intensely and appeared to be granulated, degranulated and vacuolated (Fig. 2e). During the ripening stage of the testes, the synthetic and secretory activity of GTH cells showed an increase, reflected in the form of hypertrophy and hyperplasia (Fig. 3a). At this stage, some GTH cells appeared to empty their secretory contents as indicated by their vacuolized appearance, while others exhibited accumulation of immunoreactive granules (Fig. 3a). In the fish with spent testes, the activity of GTH cells decreased as represented by the decrease in area, small sizes and lower immunoreactivity compared with that of the ripe fish. In this stage the GTH cells became degranulated and vacuolated.
In captivity (fresh water)
During both the prespermatogenic and stimulating stages, apparent intensities of staining and the number of GTH cells were comparable with that observed in natural habitat males (Fig. 2b,d). Thereafter, as spermatogenesis progressed (rapid spermatogenesis and ripe stages), the GTH cells stained more intensely and appeared to be heavily granulated with few vacuoles (Figs 2f, 3b). During the resorption stage of the testes, the activity of GTH cells appeared greatly reduced, reflected in complete degranulation with rarified immunoreactive granules (Fig. 3d).
Discussion
Mousa and Mousa (1997) we described the presence of only one GTH-producing cell type in the pituitary gland of M.cephalus which secretes GTH II. The present study confirms this observation in all stages of reproductive development examined. Also, only one cell type of gonadotrops, which secretes maturational GTH, was observed in Micropogonias undulatus, Cynoscion nebulosus and Sciaenops ocellatus (Yan and Thomas 1991). During the reproductive cycle of male M. cephalus, the GTH cells displayed quantitative and qualitative changes. During the resting (immature) period, when the spermatogonia form the main bulk of the testes, the GTH cells showed low synthetic activity. The poor concentration of the immunoreactive granules was shown by their faint or moderate immunoreactivity. The immature testis therefore does not appear to depend upon the activity of the pituitary gland. In describing the pituitary-gonad (testes) relationship in Glyptothorax pectinopterus (Pant and Bisht 1973), Schizothorax richardsonii (Bisht 1975) and in M. cephalus (Mousa 1994), similar views were expressed.
By the beginning of spermatogenesis (stimulating spermatogenic stage) with the increased activity of the spermatogonia and their conversion into spermatocytes, the GTH cells revealed a low secretory activity in the reflected form of degranulation, vacuolization and weak immunoreactivity. During the period of active spermatogenesis, when the spermatocytes are converted into spermatids and sperm, the GTH cells exhibited a strong immunoreactivity, and granulated, degranulated or vacuolated appearance, which suggests the implication of high secretory activity of these cells in the ripening of testes or the formation of sperm. These results are consistent with the classic histological studies of Sundararay (1960) inHeteropneustes fossilis,Robertson and Wexler (1962) in Salmo gairdneri,Pant and Bisht (1973) inG. pectinopterusand Mousa (1994) inM. cephalus.
During the spawning period, the ripe male pituitary appeared to be gonadotrophically potent because of the accumulation of immunoreactive granules in the cytoplasm of the GTH cells. Later on, with the approach of postspawning and regression phases of the testes, the GTH cells showed moderate or weak immunoreactivity with the discharge of their secretory contents. This feature points out that the granulated GTH cells degranulated and vacuolated, releasing the hormone necessary for spermiation during spawning. This is in conformity with the immunocytochemical observation of Nozaki et al. (1990) inSalmo gairdneri irideusand the histological observations of Van Overbeeke and McBride (1967) inOncorhynchus nerka, Pant and Bisht (1973) inG. pectinopterus, Tan (1985) inChanos chanos, Krishnan and Diwan (1990) in Etroplus suratensis, and Mousa (1994) inM. cephalus.Our results also support the physiological studies of Mousa (1994) and Zaki et al. (1995) who reported that the beginning of spermatogenesis (stimulating spermatogenesis) of male M. cephalus was accompanied by low levels of gonadotropin when testosterone was increased, but as spermatogenesis progressed, the levels of both gonadotropin and testosterone increased. The high level of testosterone recorded by Mousa (1994) and Zaki et al. (1995) during the ripening stage possibly caused an accumulation of the pituitary gonadotropin by a positive feedback mechanism. The accumulation of immunoreactive granules and strong immunoreactivity of the GTH cells in ripe male were observed in the present study. Low levels of serum gonadotropin in ripe males were observed by Mousa (1994) and Zaki et al. (1995). In the postspawning stage, the low level of serum testosterone observed by Mousa (1994) and Zaki et al. (1995) in spent males of M. cephalus may have caused the release of gonadotropin from the pituitary, shown by the moderate or weak immunoreactivity of the GTH cells in the present study and the high level of serum GTH in spent males (Mousa 1994). Thus, the changes in the number and immunoreactivity of the GTH cells in M. cephalus males in a natural habitat reflect the fluctuations in the pituitary content and circulating blood levels of this hormone.
In spite of the high synthetic and secretory activity of the GTH cells in the pituitary of M. cephalus males reared in captivity (freshwater ponds), reproductive activities declined, reflected in the form of low values of GSI and earlier resorption of the testes soon after maturation. There are at least two possible explanations for the decline in reproductive activity of M. cephalusmales: (1) the enhanced prolactin (PRL) production under freshwater conditions which may interfere with gonadal functions (Blanc-Livni and Abraham 1970); (2) defects in steroidogenesis (Mousa 1994). The first explanation is not acceptable as there is increasing evidence that the growth hormone (GH) and PRL possess steroidogenic and gonadotropic activity in some teleosts such as Fundulus heteroclitus(Singh et al. 1988) and Pleuronectes platessa(Power 1992). However, the significance of these effects of teleost GH and PRL is not known. The accepted possible explanation for the low activity of testes in captivity is that the rate of testosterone production by the testes is low. This explanation received good support from the results of Mousa (1994) who observed high levels of serum gonadotropin and low levels of testosterone, which were much lower in ripe males than were the corresponding values in saline water fish. The recorded low level of testosterone in ripe males caused the release of GTH from the pituitary because of the weak immunoreactivity of the GTH cells which became completely degranulated with rarefied immunoreactive granules. The low level of serum testosterone and the high level of GTH in ripe males reared in captivity caused earlier resorption of the testes soon after maturity (Mousa 1994; Zaki et al. 1995. This is also in conformity with the observation of Nayyar et al. (1976) who indicated that high doses of testosterone can prevent regression of testes in hypophysectomized catfish, H. fossilis. Thus, it appears that in M. cephalus, water salinity may play an important role in the reproductive cycle through the effects on steroidogenesis. Similarly, Lee and Weber (1986) concluded that salinity is an important factor controlling the maturation of male grey mullet, M. cephalus.
Acknowledgements
The authors are extremely grateful to Dr H. Kawauchi (School of Fisheries Science, Kitasato University, Iwate, Japan) for kindly donating the GTH Iβ and GTH IIβ antisera.
References
Author's address: Dr Shaaban Mousa, Freie Universität Berlin, Universitätsklinikum Benjamin Franklin, Klinik für Anaesthesiologie und Operative Intensivmedizin, Hindenburgdamm 30, D-12200 Berlin, Germany E-mail: [email protected]




