The reaction of young coho Oncorhynchus kisutch to declining oxygen levels during long-term exposure
Abstract
The respiration of coho salmon, Oncorhynchus kisutch, weighing between 20 and 45 g was measured at gradually declining oxygen levels and at temperatures ranging between 14 and 17 °C. The maximum and minimum oxygen concentrations tested were 235 and 41 μmol/L, respectively. Respiration rates were measured for 24 h at 235 μmol/L before the oxygen concentration was lowered stepwise to 157 and 81 μmol/L. In one single trial, the oxygen level was lowered to 66, 53, and 41 μmol/L. Respiration was highly variable in time. Peak activities always occurred during the night. The standard metabolic rate at normoxic conditions was estimated to be around 4 μmol oxygen/g/h. The highest rates reached values close to 15 μmol oxygen/g/h. At reduced oxygen levels the standard oxygen demand slightly increased to 4.5 μmol oxygen/g/h, indicating a higher demand for vital metabolic functions. Due to the decrease of swimming activity, the maximum oxygen uptake rates dropped to < 8 μmol oxygen/g/h below 81 μmol/L oxygen concentration. Under long-term conditions, physiological and behavioural adaptations play an important role for survival and need to be considered for the design and operation of fish farm facilities.
Introduction
The rapid growth of aquaculture has raised concerns about the potential environmental impact on production sites. However, the progressive deterioration of many inshore waters appears to be of more severe impact, threatening not only natural fish stocks but also aquaculture operations where fish encounter toxic substances and hazardous pathogens. A very high risk for aquaculture operations is also the lack of dissolved oxygen along the sea coast.
Regarding physiology and behaviour, Pacific salmon is probably one of the most investigated families ( Groot 1995) . However, there is still a lack of information on the long-term effects of reduced environmental oxygen and possible measures to mitigate this impact with respect to aquaculture. In addition to physiological adaptations, behavioural changes in fish may also serve to enhance their survival. Several studies have shown that coho salmon, Oncorhynchus kisutch, were very tolerant to low dissolved oxygen levels of around 62 μmol/L and survived for several hours ( Katz 1959 ; Davis 1963 ; Dahlberg 1968 ; Doudoroff 1970) . However, these studies focused more on basic physiology and experimentation and did not compare typical conditions in aquaculture, i.e. high stocking density in a confined environment such as net cage structures. One study focused on the avoidance reaction of salmonids ( Whitmore 1960) , another studied the energetics of coho salmon at decreasing ambient oxygen levels ( Waller 1997) . However, these investigations focused on short-term reactions; long-term effects were not investigated nor were endurance limits established.
Therefore, the aim of this study was to achieve a better understanding of energetic demand and behaviour in a fish farm environment and to assess the impact of decreasing dissolved oxygen levels. The results of a series of laboratory experiments are presented together with data from field studies in net pen farms. The experiments were conducted with coho salmon, O. kisutch, a species heavily exploited in aquaculture along the coast of British Columbia.
Materials and methods
The fish used for the experiments were young coho salmon (O. kisutch, live weight 20–45 g, total length 13–16 cm) obtained from the Big Qualicum River Hatchery, British Columbia. The fish were acclimated and maintained in an indoor 200 L circular tank illuminated by natural daylight and with a continuous water flow-through (temperature: 14–17 °C, salinity: 29.5 ± 0.2 standard deviation (SD)). Food pellets were provided every second day at a rate of 2% wet weight.
The technique used to investigate the respiration rate was as described by Waller et al. (1997) , and only some brief comments are given here. Measurements were carried out in a computer-controlled intermittent flow respirometer made up of a circular measuring chamber ( Fig. 1) attached to a small recirculation system. Oxygen saturation was continuously monitored. When oxygen dropped below a selected saturation, aerated seawater was supplied by peristaltic pump from a reservoir. When the oxygen level returned to the preset saturation the pump was turned off. Determined by the operation time of the pump, the oxygen concentration of the seawater and the total weight of the fish, respiration rates were estimated.
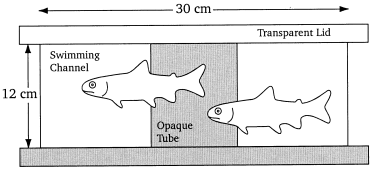
Diagram of the 200 L measuring chamber. Fish (total length 14–17 cm) swam in a channel allowing spontaneous forward and backward movements. A centrifugal pump recirculated the water inducing a current of 9–10 cm/s at the periphery of the channel. At the inner diameter, the current velocity was typically less than 2 cm/s
The measuring chamber was continuously exposed to natural daylight. A video camera was mounted on top of the chamber to permit behavioural observations without disturbing the fish. Behaviour was observed between 09.00 and 13.00h (Pacific Standard Time). Locomotory activity was estimated as the number of swimming bouts per minute a fish (swimming bouts/individual/min = B/ind/min) swam and changed position by at least half a body length or one body diameter, regardless of the swimming direction. Each swimming activity lasting 15 s or less was counted as one bout of spontaneous activity; when swimming activity lasted longer than 15 s, additional bouts were counted. Activities were usually monitored over 10 5 min periods.
The swimming speed of the fish was estimated by timing the duration of 10–20 tailbeats when the fish were moving forward against the current. Tailbeat frequency is expressed as tailbeats/s in this study.
The experiments lasted from 24 to 120 h and were carried out at six different oxygen levels: 235, 157, 81, 66, 53, and 41 μmol/L. The water temperature was between 15 and 17 °C and salinity levelled around 29.5 ± 0.2. The experiments started at around 235 μmol/L oxygen concentration; at 24 h intervals the oxygen concentration was lowered stepwise to 157 and 81 μmol/L. In one single trial the oxygen level was lowered to 66, 53, and 41 μmol/L. Because the intervals between successive oxygen levels were lengthy, 1–2 h were needed before the new oxygen level was reached and the measurement of oxygen uptake rates could be resumed. Therefore, each new oxygen concentration step was preceded by a short adaptation period.
Additional observations were made on the Eagles Landing Fish Farm located in the Sansum Narrows area, Vancouver Island, British Columbia. To establish an inventory of behavioural patterns for farmed Pacific salmon (Oncorhynchus sp.), behaviour was observed during six field trips of 2–3 days each. Surface events like jumping and splashing were counted during different times of the day. All observations obtained during a single field trip were standardized (Ri = (Xi − Xmean)/Xmean, Ri = relative count, Xi= observed value, Xmean = average count for observation period (2–3 days)).
Results
In the long-term experiments the oxygen uptake of coho juveniles was measured for 24 h at 41, 53, 66, 81, 157, and 235 μmol/L oxygen concentrations. Combinations of two to five animals with an average weight of 24–42 g were used. Temperatures were maintained between 15 and 17 °C and experimental salinity was 29.5 ppt.
At the highest oxygen level tested (235 μmol/L) the average respiration for the five experiments was 5.5 ± 1.1 μmol oxygen/g/h, with a minimum of 4.3 and a maximum of 14.5 μmol/g/h. A steep increase in the respiration rate was always preceded by an outburst of spontaneous activity and often agonistic behaviour. Respiration rates in trials L3 and L6 ( Fig. 2) showed no obvious changes during the 24 h experiment. Oxygen uptake rates were 5.4 ± 0.46 and 4.8 ± 0.56 μmol oxygen/g/h, respectively. In experiments L4 and L5, respiration rates increased markedly between 18.00 and 02.00h; for short periods respiration exceeded 14.5 μmol/g/h ( Fig. 2). The average rates for the entire L4 and L5 experimental periods (24 h) were 5.8 ± 2.25 and 5.6 ± 1.1 μmol oxygen/g/h, respectively. In experiment L7, coho slightly increased activity in the morning around 08.00h and the afternoon around 14.00h, whereby respiration increased to around 6.5 μmol oxygen/g/h. However, the average rate was 5.7 ± 0.74 μmol oxygen/g/h.
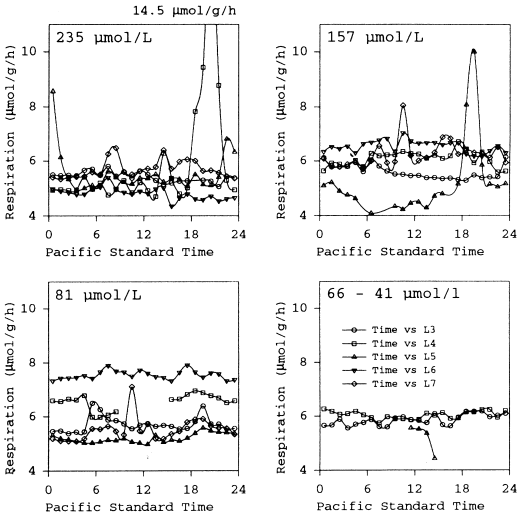
Respiration of young Oncorhynchus kisutch (n = 2–5, live weight 15–50 g, total length 12–17 cm) at stepwise reduced levels of oxygen concentration. Temperature 14–17 °C, salinity 29.5 ± 0.2 standard deviation (SD). Data points represent average hourly rates
At 157 μmol/L oxygen concentration ( Fig. 2) average hourly respiration rates of coho were in the range of 4–10 μmol oxygen/g/h ( = 6.1 μmol/g/h), slightly higher when compared with rates recorded under normoxic conditions (235 μmol/L oxygen concentration). In experiments L3 and L5, the activity of the test animals increased during the evening and at night. Whereas in experiment L3 only a minor increase was recorded (≤ 6.2 μmol oxygen/g/h) at 24.00h, the oxygen uptake markedly increased (≤ 10.0 μmol oxygen/g/h) between 18.00 and 21.00h in experiment L5; average rates were 5.6 ± 0.55 and 6.6 ± 0.71 μmol oxygen/g/h, respectively. In test trial L7, a short outburst of activity (8.0 μmol oxygen/g/h) was recorded around 11.00h. This was assumed to have been an external disturbance as it occurred at a time of the day when outbursts of activity were not observed in any other experiment. In experiments L4 and L6, respiration remained constant during the entire experiment. The average rates for trials L7, L4 and L6 were 6.3 ± 0.96, 6.1 ± 0.83, and 6.6 ± 0.71 μmol oxygen/g/h, respectively.
At 81 μmol/L oxygen concentration ( Fig. 2) the average respiration was slightly higher (6.2 ± 0.91 μmol/g/h) compared with normoxic conditions. However, the maximum uptake remained on a lower level (7.9 μmol oxygen/g/h) compared with the peak values obtained at the higher oxygen concentrations. Only once (experiment L3)( = 5.7 ± 0.51 μmol oxygen/g/h) was an increase of oxygen uptake recorded at night. This peak was not very pronounced (6.4 μmol oxygen/g/h) nor did it last long. In experiment L7 ( = 5.5 ± 0.69 μmol oxygen/g/h) an increase in oxygen uptake rates was recorded around 11.00h (7.1 μmol oxygen/g/h). As noted above, it was probably caused by an external stimulus and not related to an endogenous rhythm. In general, the maximum oxygen uptake of coho juveniles began to decrease at this concentration level. Outbursts of spontaneous activity increasing the respiration rate were seldom observed. The average rates of the three remaining experiments L4, L5, and L6 were 6.6 ± 0.49, 5.2 ± 0.34, and 7.5 ± 0.56 μmol oxygen/g/h, respectively.
In experiment L7 ( Fig. 2), coho were exposed to oxygen concentrations as low as 66, 53, and 41 μmol/L. The mean respiration rates were 5.9 ± 0.20, 6.0 ± 0.14, and 5.2 ± 0.14 μmol oxygen/g/h and remained generally at a constant level. Whereas the fish survived the 24 h experiment at 66 and 53 μmol/L, they lost their equilibrium after 1.5 h and died at the lowest concentration. The respiration rates therefore are only an incomplete estimate emphasizing the failure of vital metabolic processes.
The nonparametric test (Kruskal–Wallis, α = 0.05) revealed no significant differences among experiments with the exception of those made at the highest oxygen concentration (235 μmol/L). The average normoxic respiration rate differed from those obtained at 157, 81, 66, and 53 μmol/L (χ2-test of differences between average ranks). Because few data were available at the 41 μmol/L oxygen concentration, these results were not included in the statistics tests. Figure 3 summarizes the average respiration for all measurements. Because respiration was measured in spontaneously active fish, the average rate gives an estimate for the routine metabolic demand. To show the differences among treatments, average maximum and minimum respiration is indicated by the arithmetic mean for all rates either exceeding the 90th percentile (very restless behaviour, active rate) or below the 10th percentile (resting, inactive fish), respectively.
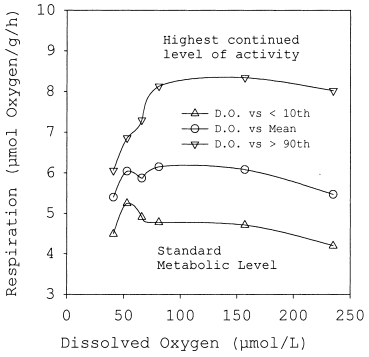
Summarized average respiration rates for the various oxygen treatments. To emphasize the differences among treatments the maximum and minimum rates are indicated by the average for all respiration rates that exceeded the 90th percentile or fell short of the 10th percentile, respectively. Rates for the 41 μmol/L treatment represent only 4 h of measurement and thus may be highly biased in fish that had already lost their equilibrium
With the exception of the three lowest oxygen levels (41, 53 and 66 μmol/L) all other results showed a gradual increase in the minimum respiration rates (10th percentile)( Fig. 3) over the concentration interval. Because these rates represent the respiration rate of unfed and resting animals, it is assumed that they compare well with the standard respiration rate. Standard respiration steeply increased at 66 and 53 μmol/L oxygen concentrations, indicating an increased demand for basic metabolic processes. At 41 μmol/L the standard respiration dropped to very low values, indicating that this oxygen concentration was not tolerated.
The highest values (> 90th percentile)( Fig. 3) were similar in the range from 81 to 235 μmol/L oxygen concentration. It can be assumed that these rates represent the energy demand for intense spontaneous activity. At lower oxygen levels (66, 53, 41 μmol oxygen/L) the rates decreased sharply, indicating a reduced spontaneous behaviour. However, these values comprised the increasing standard metabolic demand at decreasing oxygen concentrations. Figure 4 shows the range of activity estimated as R>90th percentile − R<10th percentile. The range of activity, i.e. the oxygen available for spontaneous behaviour, decreases slightly from 235 to 81 μmol oxygen/L followed by a steep decline at the lowest oxygen levels tested (66, 53, and 41 μmol/L).
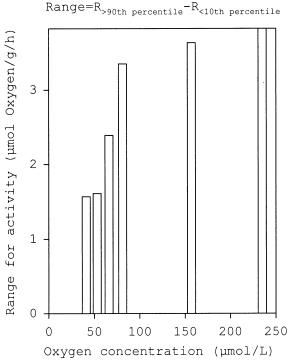
Respiration range of freely swimming coho (Oncorhynchus kisutch) at different oxygen concentrations. The range is given as the difference between the maximum respiration of active fish and the standard rate of resting, inactive animals: range = R>90th percentile– R<10th percentile
The behaviour of coho was monitored between 09.00 and 13.00h. At 235, 157, and 81 μmol/L oxygen concentrations the fish were frequently swimming in the chamber. The average activity in all three experiments was 0.7 ± 0.5 movements/individual/s. At the lowest oxygen levels (41, 53, and 66 μmol/L oxygen concentration) no activity pattern fulfilling the criteria used in this study was observed; these experiments were therefore excluded from further observations.
Coho were never observed as being permanently active. Figure 5 shows the typical behavioural pattern of coho in the measuring chamber. The fish usually rested at the inner diameter of the measuring chamber, where the water flow was slow ( Fig. 5; position 1). When a fish resumed swimming it moved towards the outer part of the swimming channel (positions 2 and 3) where current velocities were 0.6–0.7 body lengths/s. The fish thereafter returned to rest again in the inner area of the measuring chamber (position 4).
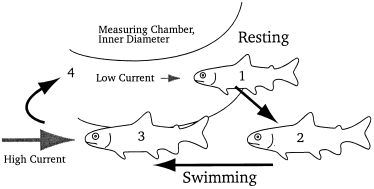
Typical behavioural pattern of coho salmon (Oncorhynchus kisutch) in the measuring chamber. The fish rested at the inner diameter of the chamber where the current speed was around 2 cm/s (1). In the swimming phase the fish left the inner part and swam against the current (9–10 cm/s, (2) and (3)). The fish later returned to the resting place (4). An opaque tube was installed in the centre of the chamber so that the fish could hide and avoid aggressive encounters
Activity bouts typically lasted less than 15 s, but in a few cases coho continued swimming for more than 1 min. Resting fish always hid in the inner part of the measuring chamber avoiding encounters with other fish. Normally the swimming activity of a fish did not affect others. On the other hand, a fish would defend its territory against intruders. In a few cases agonistic encounters were observed in which a dominant fish ventrally approached a subdominant fish, displacing and following it for several seconds. This behaviour increased the level of activity to a maximum, often disturbing the entire group.
At 235 μmol/L oxygen concentration the spontaneous activity of coho levelled off between 0.1 and 2.3 B/ind/min ( Fig. 6). Average activity was 0.93 ± 0.49 B/ind/min. At intermediate oxygen levels of 157 μmol/L the swimming activity of coho was reduced ( Fig. 6) when compared with that observed at normoxic conditions; average activity was 0.48 ± 0.42 B/ind/ min with extreme values of 0 and 1.5 B/ind/min. At the lowest oxygen level of 81 μmol/L, the swimming activity was in the range of 0–1.2 B/ind/min, averaging 0.46 ± 0.33 B/ind/min. The Kruskal–Wallis nonparametric test (α = 0.05) did not reveal a significant influence of the oxygen level on the activity of test animals. However, it is obvious from the results that the highest activity, indicated by the extreme values in Fig. 6, was lower at reduced oxygen levels (157 and 81 μmol oxygen/L).
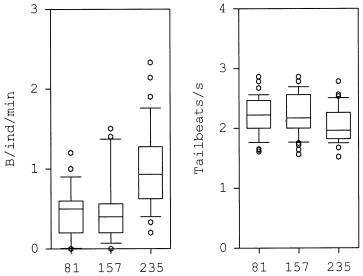
Swimming activity and speed of freely swimming coho (Oncorhynchus kisutch) at different oxygen concentrations. The graph shows the 10th, 25th, 50th, 75th, and 90th percentiles, extreme values are open circles
In general, the swimming velocity of coho did not significantly differ at the three oxygen levels tested (Kruskal–Wallis, α = 0.05). Because the fish mostly swam in the centre of the measuring channel, a certain swimming speed was required to move against the current. Therefore, it could be expected that the average speed would not change much with the oxygen level. These results, however, imply that at this oxygen range the swimming performance of coho salmon was not affected. It appears that reduced oxygen levels (81, 157 μmol/L) affected the activity pattern rather than the potential swimming speed.
In Fig. 7 the daily pattern for the respiration rate is shown together with the results of the behavioural investigations from field studies on a fish farm. Both results are shown as a relative deviation (Di)( Fig. 7) from the average for the entire observation period. The general temporal pattern in the field was similar to those found in the laboratory experiments, i.e. the respiration rates in the laboratory experiments increased at the same time when ‘splashing’ and ‘jumping’ behaviour reached a maximum in field experiments. It can be concluded that the laboratory experiments well reflected the natural behaviour. However, the behavioural inventory of coho comprises much more; ‘splashing’ and ‘jumping’ are only two examples of what could effortlessly be observed in the field.
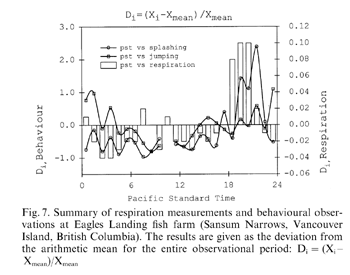
Summary of respiration measurements and behavioural observations at Eagles Landing fish farm (Sansum Narrows, Vancouver Island, British Columbia). The results are given as the deviation from the arithmetic mean for the entire observational period: Di = (Xi − Xmean)/Xmean
During the evening and night, the activity of Pacific salmon increased markedly. During the day, activity remained on a reduced level with only minor increases. However, the intensity of behaviour in the field varied far more (Ri ≤ 2.5) when compared with the respiration rate in the laboratory (Ri ≤ 0.1). In the field ‘splashing’ was far more variable than ‘jumping’ behaviour. ‘Splashing’ is a leap out of the water followed by a lateral side splash (laterad) on the water surface. ‘Jumping’ requires a controlled return of the fish head first (craniad) into the water.
In general, salmon were not evenly distributed in the net pen. Two groups of either subdominant or dominant individuals were often observed in a pen. Dominant fish generally occupied the surface area, chasing subdominant individuals that almost never had access to the water surface. Due to the above-described distribution pattern, the number of events could not be related to the number of fish in the net pen. However, ‘splashing’ was the most frequent behaviour.
Discussion
The respiration rates obtained in the experiments with coho were highly variable. Because the fish were able to swim freely in the measuring chamber the fish were not disturbed by external stimuli. The fish were unfed and exposed to a natural light cycle. The metabolic level measured in this study was the routine metabolism of spontaneously active fish ( Brett 1962). At normoxic conditions (235 μmol oxygen/L) respiration rates ranged from 4 to 15 μmol oxygen/g/h. The respiration of coho salmon was strictly related to the activity of the test fish and increased notably when fish were swimming in the measuring chamber (15 μmol oxygen/g/h). Corresponding results have been reported by Freadman (1981) and Lucas (1992) for other fish species.
The respiration of coho salmon was lowest in resting fish (4.3 μmol oxygen/g/h). Brett (1962) defined the basal metabolism as the metabolic activity in resting fish in the postabsorbtive stage in a thermoneutral environment. This metabolic level is also denoted as the standard metabolic rate. In the view of Brett (1962), it can be assumed that the lowest respiration rates obtained in this study give a reasonable estimate of the standard metabolic demand of coho. Brett (1965) reported, for 15 g immature O. nerka, a standard metabolic rate of around 3.7 μmol oxygen/g/h at 15 °C. Brett & Glass (1973) determined the standard respiration of a 5–19 g sockeye salmon (O. nerka) to be around 3 μmol oxygen/g/h. The data of Brett (1973) were later used by McLean (1993) to establish a numerical model describing respiration rates for different metabolic levels depending upon temperature and fish weight. From their study the range for the standard respiration in Pacific salmon having a weight between 20 and 40 g is 3–4 μmol oxygen/g/h at 14–17 °C temperature. In summary it appears that the estimated standard respiration in this study was somewhat higher, but still compares well with other data for the same genus.
When the oxygen concentration was lowered (53 ≥ x ≤ 157 μmol/L), the standard respiration rates (< 10th percentile) increased to 5.3 μmol oxygen/g/h. A gradual increase in respiration was also noted by Waller et al. (1997) . Oxygen deprivation causes the adaptation of several physiological processes in fish such as gill ventilation and cardiac work ( Holeton 1967 ; Randall 1982). Energy expenditure estimates for gill ventilation were in the range of 8% for standard metabolic respiration in Morone saxatilis and Pomatomus saltatrix ( Freadman 1981), 3% for routine respiration in Oreochromis niloticus ( Fernandes & Rantin 1994), and 3–5% of total oxygen consumption in Hoplias malabaricus and H. lacerdae ( Rantin et al. 1992 ). In the present study the standard metabolic rate of coho increased by about 25% at low oxygen levels. Fernandes (1994) as well as Rantin (1992) found that at reduced oxygen levels the energy demand for gill ventilation increased by 13–19%. It is conceivable that the increase in standard metabolic rate partly reflects the elevated ventilatory expense supporting the net oxygen uptake rate in an oxygen-depleted environment.
At very low oxygen levels (53 μmol oxygen/L, ≈20% oxygen saturation) a steep increase in the standard metabolic rate to almost 5.5 μmol oxygen/g/h was followed by a radical decline at 41 μmol/L oxygen concentration. This indicated a failure of basal metabolism and emphasized that such oxygen conditions were no longer tolerable. Unfortunately it remained unclear as to what processes were involved. One explanation is that coho salmon attempted to improve their oxygen uptake by a further rise in ventilatory and cardiac work but this failed, i.e. it did not improve the net oxygen uptake rate. Beamish (1964) found similar response patterns in brook trout (Salvelinus fontinalis) and other fish. However, the response observed by Beamish (1964) occurred at much higher oxygen levels and did not appear as drastic as found in the present study.
The highest oxygen uptake rates measured defined the other extreme of the routine oxygen demand of coho salmon, i.e. the upper range of the routine scope. Brett (1962) defined active metabolism as ‘the maximum rate consistent with the highest level of continued level of activity’. Brett (1964) showed that the restless behaviour of O. nerka could exceed the standard oxygen uptake (≈ 3 μmol oxygen/g/h) by six times (≈ 20 μmol oxygen/g/h). In the present study peak values were four times the standard rate, or two to three times the routine respiration rate at normoxic conditions. Maxime (1989) found a much smaller range in Atlantic salmon smolt (Salmo salar). The maximum respiration (8.6 μmol oxygen/g/h) was only slightly above the average routine respiration rate (7.1 μmol oxygen/g/h), implying that Atlantic salmon was less impetuous in the experiment.
An avoidance behaviour increasing the oxygen demand at intermediate levels (60% oxygen saturation)( Waller et al. 1997 ) was not found in the present investigation. Under long-term conditions with a short adaptation period it is likely that any initial reaction would not last long. The fish ceased any avoidance behaviour after a short time, because they were unable to find an oxygen gradient that at the same time would stimulate further search behaviour.
At lowered oxygen concentrations, the average energy expenditure for continued activity remained fairly stable until the fish were exposed to oxygen levels of less than 81 μmol oxygen/L. However, the range for activity already indicated that even at 81 and 157 μmol oxygen/L the swimming activity of the fish was already lower. Davis et al. (1963) reported that the sustained swimming speed of coho salmon decreased when oxygen levels dropped to around 190 μmol oxygen/L or 80% oxygen saturation. Davis et al. (1963) investigated swimming performance directly. In the present study swimming performance was derived from changes in the oxygen uptake rate and only few hints were available from the behavioural observations. However, the decrease in oxygen uptake parallel with the reduction of locomotory activity gives a similar picture, even if coho salmon was less sensitive to decreasing oxygen levels in the present study.
The swimming speed of the fish in this study was estimated as the tailbeat frequency shown to be linearly related to the absolute speed in distance per unit time by Bainbridge (1958), Puckett (1984), and Williams (1987). Using the relationships given by these authors, frequencies of 1 and 3 tailbeats/s may have resulted in a swimming speed of 10–25 cm/s. Because behavioural observations were made in the morning they never included the periods with the highest activity at night and swimming velocities. However, the highest respiration rates measured at normoxic conditions were close to 15 μmol oxygen/g/h. Brett (1963) reported that this oxygen uptake corresponds to a swimming speed between 3 and 4 body lengths/s in sockeye salmon (O. nerka) suggesting that the maximum swimming speed of the fish might have reached far higher levels of up to 40–50 cm/s during the night. The range in the present study embraced motionlessness, moderate swimming speeds and burst swimming. Burst activity decreased at intermediate oxygen levels; tailbeat frequencies and recorded respiration rates implied that under such conditions swimming speed was lower, probably not exceeding 2–3 body lengths/s. At the lowest oxygen levels a further reduction in swimming activity occurred; however, the fish were never inactive, and even at 41 and 53 μmol oxygen/L a small amount of oxygen was still available for spontaneous activity.
A result of this investigation shows that besides physiological adaptations, spontaneous activity adaptation plays an important role in an oxygen-depleted environment. By reallocating oxygen to vital metabolic functions coho salmon survived oxygen concentrations of 50 μmol/L (< 20% oxygen saturation) for at least 1 day. However, under fish farm conditions it is to be expected that even with low environmental oxygen coho are still exposed to agonistic behaviour of other fish because net pen design does not permit hiding to avoid encounters. Further, it is unlikely that fish held in a net pen can reduce their swimming effort. In net pens fish are typically ‘milling’. Even if this behaviour is interrupted at a low ambient oxygen level, the high density of fish will cause nonessential swimming activity and reduced resistance. The results of the present study point out that oxygen depletion need not result in the loss of fish. This, however, requires that the behaviour of the fish is considered during the design and operation of the facilities.




