The Scent of Genetic Compatibility: Sexual Selection and the Major Histocompatibility Complex
Abstract
Individuals in some species prefer mates carrying dissimilar genes at the major histocompatibility complex (MHC), which may function to increase the MHC or overall heterozygosity of progeny. Here I review the evidence for MHC-dependent mating preferences from recent studies, including studies on the underlying olfactory mechanisms and evolutionary functions. Many studies indicate that MHC genes influence odour, and some work is beginning to examine the potential role of MHC-linked olfactory receptor genes in mating preferences. MHC-dependent mating preference increases the MHC-heterozygosity of progeny, which is suspected to confer resistance to infectious diseases. In humans, heterozygosity at MHC loci is associated with increased resistance to hepatitis and HIV infections, but experimental evidence for the heterozygote advantage hypothesis has been lacking. Here I re-analyse data from previously published experimental infection studies with mice. I show that although overdominance is rare, resistance is often dominant, suggesting that heterozygotes are often protected. A second (nonmutually exclusive) possibility is that MHC-disassortative mating preferences promotes inbreeding avoidance. This hypothesis is supported by recent evidence that MHC genes play a role in kin recognition, and that mating with close kin has rather deleterious fitness consequences. In conclusion, I discuss other ways that MHC genes might influence sexual selection. The research on MHC-mediated mating preferences is integrating the study of animal behaviour with other seemingly disparate fields, including sensory biology and immunogenetics.
Introduction – A Preference for Nonself?
Increasing evidence indicates that females can increase the viability of their offspring by selectively mating with certain males (Promislow et al. 1998). In house mice (Mus musculus domesticus), for example, females that are allowed to choose their mates produce offspring with greater viability than controls mated at random (Drickamer et al. 2000). The benefits from such mating preferences are usually attributed to females acquiring males with good genes, and yet they may also be due to increasing genetic compatibility (Trivers 1972). For example, avoiding inbreeding and distant outbreeding (interspecific hybridization) apparently function to reduce genetic incompatibilities. There are several potential examples of more finely tuned mating preferences for genetic compatibility in vertebrates (reviewed in Tregenza & Wedell 2000), and the most cited example involves the genes of the major histocompatibility complex (MHC).
MHC genes are highly polymorphic loci (class I and II MHC genes) that control immunological self/nonself recognition in vertebrates (MHC loci, known as ‘H-2’ in mice and ‘HLA’ in humans, are closely linked in mammals, but not fish and frogs; Klein 1986; Penn 2000a; Penn 2000b). Class I and II MHC genes encode cell-surface glycoproteins (MHC molecules) that present small peptide antigens to T-cells, thereby controlling all specific immune responses, both cell- and antibody-mediated. Since MHC genes play such a key role in the immune system, they influence resistance and susceptibility to a wide range of infectious and autoimmune diseases (Apanius et al. 1997), as well as controlling tissue-graft rejection. MHC genes are the most polymorphic coding loci known in vertebrates (which is why unrelated individuals rarely make compatible tissue donors), but it is still unclear how natural selection maintains these extreme polymorphisms. The leading hypotheses to explain the polymorphisms of MHC loci are selection from (1) parasites, either through overdominant selection or frequency-dependent selection, and (2) disassortative mating preferences (Apanius et al. 1997; Penn & Potts 1999).
Evidence for MHC-disassortative mating preferences has been found in house mice (Yamazaki et al. 1976; Yamazaki et al. 1988; Egid & Brown 1989; Eklund 1997; Penn & Potts 1998d), humans (Ober et al. 1997), and recently in salmon (Salmo salar) (Landry et al. 2001). The main two questions are how do individuals recognise the MHC-identity of conspecifics, and why do they have this preference? MHC-identity can be distinguished through specific odour cues (reviewed in Penn & Potts 1998c; Yamazaki et al. 1998; Eggert et al. 1999), although it is not completely clear how. MHC-disassortative mating preferences offers two (nonexclusive) fitness benefits (Grob et al. 1998; Penn & Potts 1999). First, this preference may increase the resistance of progeny to infectious diseases, either by increasing their MHC-heterozygosity (heterozygote advantage hypothesis) (Potts & Wakeland 1990) or by providing a ‘moving target’ against rapidly evolving parasites (moving target hypothesis) (Penn & Potts 1999). Second, it may function to prevent kin matings (inbreeding avoidance hypothesis) (Brown & Eklund 1994; Potts et al. 1994). Either way, MHC-dependent mating preferences can potentially increase the genetic compatibility of mates.
In this review, I examine MHC-dependent mating preferences, both the underlying chemosensory mechanisms and evolutionary functions of this behaviour. First, I review the most recent studies on MHC-dependent mating preferences in fish, mice, sheep, and humans. Second, I review studies examining how MHC genes influence odour. Third, I review evidence that MHC-dependent mating preferences function to produce disease-resistant, MHC-heterozygous offspring. MHC-heterozygotes have long been suspected to be resistant to infectious diseases; however, there has been no unambiguous experimental evidence (Apanius et al. 1997). Here I show that re-analysis of previously published experimental infection studies with mice suggests that MHC-heterozygotes are often resistant to infectious diseases. Fourth, I review evidence for the hypothesis that MHC genes play a role in inbreeding avoidance. This hypothesis is supported by recent work indicating that MHC genes play a role in kin recognition (Olsén et al. 1998; Yamazaki et al. 2000), and that avoiding kin matings has large fitness benefits (Meagher et al. 2000). In conclusion, I consider how future work might address how MHC genes influence other aspects of sexual selection, including cryptic female choice and ‘good-genes’ sexual selection.
The Evidence for Mating Preferences
Yamazaki and his colleagues first discovered evidence for MHC-disassortative mating preferences in MHC-congenic strains of male mice (i.e. strains that differ genetically only in the MHC region) (Yamazaki et al. 1976; Yamazaki et al. 1978). This preference only occurred in some strains therefore it is impossible to extrapolate the results to wild house mice. Yamazaki’s studies remained unknown to most researchers in animal behaviour, genetics, and immunology for many years, until evidence for MHC-dependent mating preferences was found in populations of wild-derived house mice.
In a study that aimed to determine the selective forces maintaining MHC polymorphisms in populations of house mice, Potts et al. (1991) found indirect evidence for MHC-based mating preferences. They found significantly fewer MHC-homozygous progeny than expected (significant deficiencies in five out of nine populations). They found no evidence for nonrandom fertilization or abortional selection in experimental matings, which left mating preferences as the most likely explanation. Females did not show a significant pattern of settling on territories with MHC-dissimilar males, nor were significant homozygote deficiencies found among the litters apparently sired only by a female’s dominant territorial male. (Experimental evidence indicates that females prefer habitat quality over a male’s quality when choosing nest sites, Rich & Hurst 1998.) Females often mated with neighbouring territorial males, and at least 52% of litters born within a dominant male’s territory contained offspring sired by another male. Among these litters, there were 41% fewer homozygous offspring than expected if females had mated only with the dominant, territorial male. To explain this disparity, Potts et al. (1991) suggested that ‘females seek extraterritorial matings with males that are relatively more MHC-disparate than their own territorial male.’ Since this study provided no direct evidence for MHC-disassortative mating preferences, it has been argued that the results may have been due to simple, non-MHC-mediated inbreeding avoidance, which requires experimental tests that control relatedness (Hughes & Hughes 1995).
Experimental laboratory tests for MHC-disassortative mating preferences, however, have shown mixed results (reviewed in Penn & Potts 1999). Some studies have found experimental evidence for MHC-dependent mating preferences in male laboratory mice (Beauchamp et al. 1988; Yamazaki et al. 1988) and also wild-derived female mice (Egid & Brown 1989; Eklund 1997). Other studies, however, found no evidence for mating preferences in male or female mice (Beauchamp et al. 1988; Eklund et al. 1991; Manning et al. 1992a; Eklund 1998). Mice reportedly prefer the odour of MHC-dissimilar individuals (at least in some strains) (Eklund et al. 1992; Ninomiya & Brown 1995); however, a recent study found none (Ehman & Scott 2001). Some sceptics have ruled out the mate choice hypothesis due to these negative results (Hughes & Hughes 1995); but these negative results are inconclusive for several reasons (see Penn & Potts 1999): laboratory studies create artificial conditions, they rely on indirect methods for assaying preferences, and furthermore, none of these studies applied power statistics to determine whether their sample sizes were sufficient to accept the null hypothesis. Taken together, though these studies indicate that MHC-dependent mating preferences are not strong, all-or-none biases, unlike tissue-graft rejection, fungal mating-types, and plant self-incompatibility systems.
The strongest experimental evidence for MHC-dependent mating preferences comes from cross-fostering studies with house mice. Yamazaki, Beauchamp and their colleagues (Beauchamp et al. 1988; Yamazaki et al. 1988) found that artificially rearing male laboratory mice with MHC-dissimilar parents (cross-fostering) reverses their MHC-dependent mating preferences, which suggested that mice learn the MHC-identity of their parents and avoid mating with familial-smelling females (‘familial imprinting’). Another laboratory study also found evidence for the imprinting hypothesis in one strain of female mice (Eklund 1997). We used a cross-fostering experiment to test whether MHC genes influence mating preferences in wild-derived female mice under more natural social conditions, and found experimental evidence for MHC-dependent mating preferences based on imprinting (Penn & Potts 1998d).
Studies with humans have found evidence for MHC-disassortative odour and mating preferences, but the evidence is mixed. First, Wedekind and his colleagues (Wedekind et al. 1995; Wedekind & Füri 1997) found evidence for MHC-disassortative odour preferences among Swiss students (also see Hedrick & Loeschcke 1996; Wedekind & Seebeck 1996). Second, Ober and others (Ober et al. 1997) found MHC-disassortative marriage patterns in Hutterites, a reproductively isolated population in North America. Third, Milinski and Wedekind (Milinski & Wedekind 2001) recently found three MHC alleles associated with preferences for perfume ingredients. They suggest that people might select perfumes to amplify their own particular MHC-mediated odour-type. On the other hand, several other studies on humans, including a study on South Amerindians by Hedrick and Black (Hedrick & Black 1997) and a recent study on Japanese couples (Ihara et al. 2000) have found no evidence for MHC-dependent marriage preferences (Pollack et al. 1982; Rosenberg et al. 1983; Jin et al. 1995). It is impossible to rule out mating preferences from these negative data because these studies had smaller sample sizes and typed fewer MHC loci than the positive finding with Hutterites (Ober et al. 1997). Yet, taken together, these studies suggest that MHC-dependent preferences in humans, like mice, are neither absolute nor particularly strong.
Population studies on other species have also yielded mixed results. A recent study found indirect evidence for MHC-disassortative mating preferences in wild salmon (Salmo salar) (Landry et al. 2001). This study involved a genotypic analysis of 262 matings from 650 offspring produced by 66 adult fish. Interestingly, there was no indication of mating preferences when the authors examined the numbers of alleles shared between individuals; the pattern only became apparent in the functional genetic sites (i.e. the amino-acid composition of different alleles at the putative antigen-binding site of a class II locus). Although the authors ruled out inbreeding avoidance as an explanation for this pattern, they did not rule out sperm selection or differential survival of heterozygous offspring. In contrast, a large study on a population of feral sheep (Ovis aries) found no evidence for MHC-dependent mating preferences (Paterson & Pemberton 1997). Perhaps dominant male sheep, unlike house mice and salmon, are able to control females’ preferences. Thus, more studies are needed to test the generality of MHC-dependent mating preferences. One problem is that weak biases in MHC-dependent mating preferences will be difficult to detect, especially in populations with high genetic diversity (Hedrick 1999; Penn & Potts 1999) and it is unlikely that MHC will provide a strong predictor of mate choice simply because the potential benefits are indirect. Still, MHC-disassortative mating preferences may be functional and provide a selective factor maintaining MHC polymorphisms even if they are weak.
Sniffing out Genetic Dissimilarity
How do individuals recognise the MHC-identity of potential mates? A tremendous amount of evidence from mice and rats indicates that MHC genes influence an individual’s odour (reviewed in Yamazaki et al. 1998; Penn & Potts 1998c; Eggert et al. 1999). Yamazaki and his colleagues (Yamazaki et al. 1979) initially found that mice can be trained to distinguish odours of MHC-congenic strains of laboratory mice. This finding has been replicated with wild-derived house mice (Penn & Potts 1998b) and laboratory rats (Rattus norvegicus) (Brown et al. 1987; Brown et al. 1989). Furthermore, trained mice can discriminate among MHC-congenics even when the strains are re-derived (F2 segregants) (Yamaguchi et al. 1981). This finding rules out several potential extraneous sources of variation, including incidental background mutations that may have accumulated (outside of the MHC region) since the strains were originally bred. There are many questions, however, that still need to be answered.
1 . Do class I and II genes or linked loci control variation in individual odour? To test whether odour differences are controlled by the highly polymorphic antigen-binding (class I and II) MHC loci, Yamazaki and his colleagues have used various mouse strains, including recombinants, targeted gene disruptions (knockouts), and mutants. They found that trained mice can distinguish odour differences due to a natural deletion of a single class I locus (Yamazaki et al. 1991) or artificially disrupting the expression of all class I loci (β2 microglobulin knockouts) (Bard et al. 2000). To test whether changes at the antigen-binding site of class I loci result in distinctive odours, Yamazaki and his colleagues used bm mutant mice (which differ genetically from their parental strain by only a few amino acids in the antigen-binding site of class I MHC molecules). Trained mice could distinguish some (Yamazaki et al. 1983a), but not all mutants (Yamazaki et al. 1990a; Yamazaki et al. 1990b).
2 . Are MHC-mediated odours salient under natural conditions? Although mice can be trained to make impressive odour discriminations, this does not necessarily indicate that MHC-mediated odours are recognizable under natural conditions, without training (some odours are only recognizable after repeated exposure) (Penn & Potts 1998c). Richard Brown and his colleagues (Brown et al. 1987; Brown et al. 1989) found that untrained laboratory rats can spontaneously distinguish odours among congenic strains of rats that differ in the MHC region (recombinants) in a habituation-discrimination assay. Using this habituation assay, we found that untrained, wild-derived mice are able to detect odour differences due to a single MHC (class I) deletion (Penn & Potts 1998b). Recently, we found that untrained mice are able to discriminate the odours among some bm mutant strains, even after controlling for background mutations that may have accumulated among these strains (Carroll et al., in press). Recent studies have also found that injecting soluble MHC class I molecules into rats alters their odour in habituation-discrimination assays (Pearse-Pratt et al. 1999; Janssen et al. 2001).
Although, class I-mediated odours are discriminable by untrained mice, the relative salience of these odours (compared to other genes in the MHC region or rest of the genome) is unclear. The only experiment to address this question concluded that ‘judged by ease of training, the genome as a whole, apart from H-2 is about a potent source of individuality as H-2 alone’ (Boyse et al. 1987). This may be an over exaggeration, however, since the comparison was made with genes on the Y-chromosome, which may be inactive. A related question is whether MHC-mediated odours are salient among individuals having a normal, genetically diverse background. Yamazaki and his colleagues (Yamazaki et al. 1994) found that trained mice can distinguish MHC genotypes among outbred, heterozygous mice, and one study found that a salmonid fish can spontaneously recognize MHC-identity among outbred conspecifics (Olsén et al. 1998). Thus, the available evidence suggests that MHC-mediated odours are discriminable among individuals having genetically heterozygous backgrounds.
3 . What is the origin and the chemical nature of MHC-mediated odours? The main problem has been determining how class I and II molecules, which are ‘designed’ for peptide presentation, can also control the production of volatile odours. Several hypotheses have been proposed to explain how MHC genes influence odour (reviewed in Penn & Potts 1998c; Yamazaki et al. 1998; Eggert et al. 1999): (1) MHC molecules may provide the odorants themselves (Singh et al. 1987); (2) MHC molecules may act as carriers for volatile odorants (the carrier hypothesis) (Pearse-Pratt et al. 1999; Singh 1999); (3) metabolites of MHC-bound peptides may provide the source of volatile odorants (the peptide hypothesis) (Singer et al. 1997); (4) MHC genes may influence odour indirectly by shaping an individual’s particular microflora composition (the microflora hypothesis) (Howard 1977; Schellinck et al. 1991; Schellinck & Brown 1992). All of these hypotheses, or some combination, may be correct (e.g. commensal microflora may play a role in metabolizing MHC-derived peptides (Penn & Potts 1998c). There is little evidence that MHC genes influence qualitative differences in odour (Eggert et al. 1996), though one study found quantitative differences in the relative concentrations of volatile carboxylic acids in the urine of MHC-congenic mouse strains (Singer et al. 1997). These acids may be metabolic by-products of MHC-bound peptides or microbial flora (or they may simply be due to MHC-linked genes or incidental background mutations that have accumulated among these strains). A recent study found that the composition of gastrointestinal microflora of mice varies among MHC-congenic strains (Toivanen et al. 2001), which supports the microflora hypothesis. However, this association may simply be due to an incidental evolutionary divergence of bacterial populations during the development of these strains. Another recent study found that the serum odour of MHC-congenic mouse strains is distinguishable by trained mice, but only if serum proteins are denatured with a protease (Yamazaki et al. 1999). This suggests that the MHC-mediated odorants occur in the blood, and are transported to the urine by a protein carrier molecule (the carrier hypothesis). Another recent study found that the odour of pregnant females (mice and humans) is influenced by the MHC genes of the developing foetus (Beauchamp et al. 2000), which also indicates that the odorants are produced endogenously. (Oddly, males were attracted to pregnant females when they carried MHC-dissimilar foetuses). Taken together, these studies indicate that MHC-mediated odorants are produced endogenously and transported by a protein carrier molecule, although commensal microflora may be necessary to amplify or make these odorants attractive.
4 . How are MHC-mediated odours detected? A recent study found that odours from MHC-disparate mice evoke different neuronal activation patterns in the main olfactory bulb of other mice (Schaefer et al. 2001), which should help explain how MHC-mediated odours are discriminated. Yamazaki et al. (1976) originally proposed that there are specific receptors for detecting MHC-mediated odorants, which is not so far-fetched since the recent discovery that the vomeronasal organ has specific pheromone receptors (Leinders-Zufall et al. 2000). An increasing number of studies have begun to examine the possibility that olfactory-like receptor genes, which are linked to MHC loci, might play a role in MHC-dependent mating preferences (Ehlers et al. 2000; Eklund et al. 2000; Ziegler et al. 2000; Younger et al. 2001). Olfactory receptors (OR) might play a role in recognizing MHC-mediated odours, or mating preferences may be due to polymorphisms in OR rather than MHC genes. Recent work indicates that MHC-linked olfactory-like receptor loci are somewhat polymorphic in humans (Ehlers et al. 2000; Eklund et al. 2000); however, it is unclear whether this diversity is sufficient to explain mating patterns or variation in odour preferences. Such research should prove interesting since OR genes are the only other loci in vertebrates – besides MHC – that show high levels of polymorphism maintained by adaptive molecular evolution (Gilad et al. 2000; Seielstad 2000).
Research on odour cues and olfactory recognition is crucial for determining how MHC genes influence mating preferences. This research, however, cannot evaluate whether MHC-dependent mating preferences actually serve any function in the wild.
Potential Functions
MHC-dependent mating preferences provide at least two (nonmutually exclusive) potential adaptive benefits (Penn & Potts 1999): they may increase the resistance of an individual’s progeny to infectious diseases (parasite-resistance hypothesis) or enable individuals to avoid kin matings (inbreeding-avoidance hypothesis). There is evidence to support both of these functional hypotheses.
MHC-Heterozygosity and Parasite Resistance
MHC-disassortative mating preferences may increase the resistance of progeny to infectious diseases by increasing their heterozygosity at MHC loci (heterozygote advantage hypothesis) (Potts & Wakeland 1990). Although MHC-heterozygotes are widely assumed to be more resistant to infectious diseases than homozygotes (Hughes & Hughes 1995), others argue that experimental evidence is lacking. In a large review on this subject, for example, Apanius et al. (1997) argued that ‘Heterozygote advantage is strongly predicted by theory but is seldom observed in epidemiological and experimental studies’ (p. 210).
Theoretically, MHC-heterozygotes are expected to be resistant to infectious diseases because they present a greater diversity of antigens to the immune system than homozygotes (Doherty & Zinkernagel 1975). Yet, increasing MHC-heterozygosity may also reduce the T-cell repertoire during the ontogeny of the immune system, i.e. there may be an optimal level of MHC-heterozygosity for overall immune function (Nowak et al. 1992; Penn & Potts 1999). If correct, this predicts that females should prefer males that have intermediate rather than maximal MHC-disparity. On the other hand, a recent computer simulation suggests that depletion of the T-cell repertoire only occurs at unrealistically high levels of MHC heterozygosity (Borghans 2000).
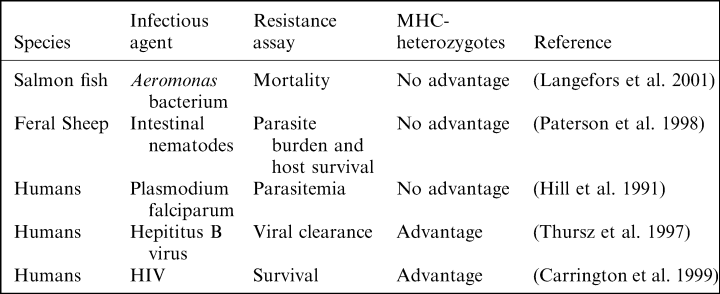
Observational evidence for the MHC-heterozygote advantage hypothesis is mixed (Table 1). Two studies on human populations suggest that MHC-heterozygotes are more resistant than homozygotes to viral infections: (1) Among individuals exposed to hepatitis B, MHC-heterozygotes are more likely to clear the virus than homozygotes (i.e. heterozygosity at one class II locus was associated with viral clearance) (Thursz et al. 1997); (2) A long-term study on HIV-positive individuals found that heterozygosity at MHC loci is associated with higher survival (Carrington et al. 1999). In contrast, three other population studies found no evidence that MHC-heterozygotes are protected: (1) In humans, some MHC alleles are associated with resistance to malaria, yet there is no evidence that heterozygotes have an advantage (Hill et al. 1991); (2) In feral sheep, MHC variation is associated with survival and resistance against a parasitic nematode, but there is no evidence for a heterozygote advantage (Paterson et al. 1998); (3) A recent study with salmon, which screened fishes from families showing high or low-resistance to a pathogenic bacterium, found resistant and susceptible alleles, but found no evidence that resistant families had greater MHC heterozygosity than susceptible ones (Langefors et al. 2001). These observational studies suggest that MHC-heterozygosity increases resistance to some (viruses), but not all infectious agents. Experimental infection studies are crucial to rule out the effects of other loci, and the possibility that heterozygotes are resistant because they carry rare, resistant MHC alleles (rather than heterozygosity increasing resistance per se).
Numerous studies with mice and chickens have experimentally challenged MHC-heterozygotes using a variety of infectious agents (Apanius et al. 1997). However, most of these studies only examined immune responsiveness or parasite clearance, which do not necessarily predict how well hosts cope with the infection. Consider, for example, the study most widely cited as experimental evidence showing MHC-heterozygote advantage. Doherty & Zinkernagel (1975) found that MHC-heterozygotes had higher in vitro T-cell responses against lymphocytic choriomeningitis virus (LCMV) than homozygotes. They interpreted this as a heterozygote advantage, despite the fact that when they infected the mice, the homozygotes survived, but all of the heterozygotes died! The authors point out that LCMV kills mice in laboratory studies due to the host’s own immunological over-responsiveness to artificial cerebral injections of the virus. They suggested that the high responsiveness of heterozygotes might be advantageous under normal conditions, but they never tested this hypothesis.
Other laboratory studies have also found that MHC-heterozygotes are susceptible to immunopathology, such as autoimmune diseases (Teuscher et al. 1990; Kimoto et al. 1993; Morel et al. 1994; Ibnou-Zekri et al. 2000). If MHC-heterozygotes are high responders to infection, then they may be more resistant to infectious diseases in the wild, especially if the costs of immunopathology are largely an artefact of laboratory conditions. For example, one study found that MHC-heterozygous mice are high immune responders to an antigen challenge, but they have shorter longevity in sterile laboratory conditions compared to homozygotes (Salazar et al. 1995). It is a mistake, however, to assume that high immunological responsiveness is equivalent to high fitness because natural selection probably favours optimal rather than maximal immune responses (Gemmill & Read 1998; Penn & Potts 1998a). Thus, it is necessary to determine the fitness consequences of infection under more natural conditions, rather than simply examining immune responsiveness or parasite clearance in the laboratory. Given this caveat, let us examine the results from other laboratory infection studies.
Experimental laboratory infection studies indicate that MHC-heterozygotes are susceptible nearly as often as they are resistant; however, most of these studies only compared the resistance of MHC-heterozygotes vs. one class of parental homozygotes (e.g. ab vs. aa) (Apanius et al. 1997). Among the infection studies on mice that compared the resistance of heterozygotes to both classes of parental homozygotes (i.e. ab vs. aa and bb), I found only two cases in which heterozygotes are more resistant to infection than both parental homozygote genotypes. When mice are infected with Toxoplasma gondii, MHC-heterozygous mice have fewer brain cysts (McLeod et al. 1989), and a survival advantage compared to either parental homozygotes (Williams et al. 1978). MHC-heterozygotes have an advantage because resistance to infection is overdominant, rather than being dominant (heterozygotes are resistant as the most resistant parental homozygote), codominant (heterozygotes are intermediate between the dominant and susceptible homozygotes), or recessive (heterozygotes are as susceptible as the most susceptible homozygote).
Based on a sample of these studies, Apanius et al. (1997) concluded that experimental infection studies show ‘codominant expression and not heterozygote advantage’, and therefore ‘the selective pressure from any single agent does not appear to confer heterozygote advantage’ (p. 191). Like many, they equated ‘heterozygote advantage’ with ‘overdominance;’ however, overdominance is just the most extreme form of heterozygote advantage. If resistance to infection is generally dominant, then heterozygotes should be more resistant than the average homozygote. In other words, heterozygotes can have an advantage even if there is no overdominance. On the other hand, if MHC-dependent resistance to infection is generally codominant, or it is recessive as often as it is dominant, then heterozygotes will have no advantage.
Among the experimental infection studies that I found with laboratory mice, MHC-dependent resistance is dominant or over-dominant in 10 of 17 studies (Table 2). Although none of these studies compared the resistance of heterozygotes with the average of the homozygotes statistically, the data from these studies suggest that MHC-heterozygotes have an advantage when resistance is dominant. For example, MHC-dependent resistance to the protozoan parasite, Trypanosoma cruzi, is dominant (Trischmann & Bloom 1982), and the data from this study indicate that MHC-heterozygous mice had a significant survival advantage over homozygotes (Fisher’s exact test, p=0.03, n=122). Note that another study on MHC-dependent resistance to Trypanosoma found codominance (Wrightsman et al. 1984) (Table 2). Interestingly, studies on MHC-dependent resistance with another Trypanosoma species also provide conflicting results (Seed & Sechelski 1995), which might be due to differences in the parasite strain, the mouse strains or haplotypes tested. Still, studies on other infectious agents suggest that MHC-heterozygotes are more resistant than homozygotes when resistance is dominant. For example, MHC-heterozygous mice showed reduced pathogenicity during bacterial infection (streptococcus-induced lesions) (Chen et al. 1992), and they have a faster clearance rate of parasitic worms, Heligmosomoides polygyrus (Behnke & Wahid 1991) and S. mansoni (Sher et al. 1984), than the average homozygote. In contrast, five out of these 17 studies indicate that resistance is under-dominant or recessive (susceptibility is dominant), suggesting that MHC-heterozygotes sometimes have a disadvantage, even though this is less common.
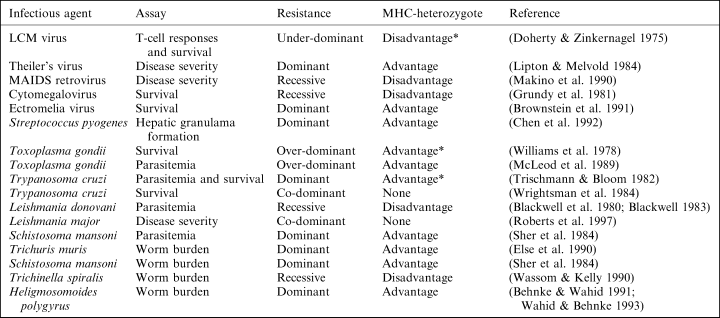
Taken together, experimental infection studies with mice suggest that MHC-dependent resistance is generally dominant rather than overdominant, and that dominance is enough to provide an advantage for heterozygotes. This ‘heterozygote advantage through dominance’ hypothesis is also supported by experimental infection studies on chickens (Sato et al. 1992). This suggests that dominance, rather than overdominance, explains why some population studies find that MHC-heterozygosity is associated with increased resistance (Thursz et al. 1997; Carrington et al. 1999). Still, determining whether resistance is sufficiently dominant to provide a general advantage for MHC-heterozygosity requires testing the resistance of multiple allelic combinations (previous studies only tested one or two) against more than one parasite, and examining host fitness, rather than simply using immunological responses or parasite clearance.
Because heterozygotes recognize more antigens than homozygotes it has been suggested that the heterozygotes should be particularly resistant to multiple infections (Hughes & Nei 1992). This is also expected because MHC alleles resistant to one infectious agent are susceptible to others, suggesting that heterozygotes may be particularly resistant to coinfections of parasites showing the opposite or reciprocal resistance profiles (Apanius et al. 1997; Penn & Potts 1999). This hypothesis assumes that MHC-dependent resistance is generally dominant, which is supported by the available evidence (Table 2). The original version of this model predicted that such coinfections would result in overdominance (Apanius et al. 1997), but this is unlikely because it requires that the coinfecting parasites have approximately equal virulence (otherwise the most virulent parasite will simply dominate the infection).
A heterozygote advantage through dominance may selectively favour disassortative mating preferences, but it is unlikely to maintain MHC polymorphisms. Contrary to what is often assumed (Takahata & Nei 1990; Hughes & Hughes 1995; Slatkin 2000), population genetic models have long indicated that overdominance cannot maintain the high levels of polymorphisms of MHC alleles (reviewed in Hedrick 1999). These models indicate that overdominance can only support limited levels of polymorphism, with only a few alleles (Lewontin et al. 1978; Spencer & Marks 1988). Takahata & Nei (1990) found that overdominant selection can maintain high levels of polymorphism; however, their model was stable only because it assumed that heterozygotes all have equal fitness. If the fitness among homozygotes or heterozygotes is asymmetrical, as in nature, then high levels of polymorphism are evolutionarily unstable because the most resistant alleles become fixed by directional selection (Mandel 1970).
The alternative explanation for how selection maintains MHC polymorphisms is frequency-dependent selection from rapidly evolving parasites adapting to host MHC alleles (the Red Queen hypothesis) (Haldane 1949; Bodmer 1972; Slade & McCallum 1992). If MHC polymorphisms are maintained by rapidly evolving parasites, then mating preferences could potentially provide a mechanism to ‘keep up’ in this molecular arms race with parasites (the moving target hypothesis) (Penn & Potts 1999). This hypothesis does not require that MHC-heterozygotes have any advantage per se. Because MHC alleles resistant to one infectious agent are susceptible to others, matings that confer disparate MHC alleles to progeny should increase their resistance of progeny to parasites that have adapted to their genotype. Like the Red Queen hypothesis for sex, this hypothesis requires that parasites are able to adapt to host genotypes (Ebert & Hamilton 1996). MHC-disassortative matings may effectively provide offspring with a disparate MHC-allele to present novel antigens and alter the T-cell repertoire (Penn & Potts 1999). Thus, MHC-dependent mating preferences may increase the resistance of progeny to parasites by increasing either MHC-heterozygosity or disparity of an individual’s progeny.
Inbreeding Avoidance
Because MHC loci are often highly polymorphic and influence highly specific odours, they provide a potential marker for relatedness, and therefore, MHC-disassortative mating preferences may help to avoid kin matings (Potts & Wakeland 1993; Brown & Eklund 1994; Penn & Potts 1999). Recent studies on MHC-mediated kin recognition provide strong evidence for the inbreeding avoidance hypothesis. MHC genes appear to be used as a kin recognition mechanism in contexts other than inbreeding avoidance, such as sibling recognition via odour cues in a fish (Salvelinus alpinus) (Olsén et al. 1998). Female mice nest communally and they appear to nest with MHC-similar females when siblings are unavailable (Manning et al. 1992b). A recent study, however, failed to find evidence for MHC-similar odour preferences among congenic strains of female mice (Ehman & Scott 2001). A recent study by Yamazaki et al. (Yamazaki et al. 2000) found that MHC genes also play a role in parent-offspring recognition: female mice are more likely to retrieve pups when they are MHC-similar, and pups are attracted to the odours of mothers that are MHC-similar. This study provides direct evidence that MHC genes play a role in kin recognition.
Is it useful to recognise kin? Until recently it was unclear whether inbreeding depression is harmful enough to favour inbreeding avoidance behaviours (Pusey & Wolf 1996). The fitness consequences of inbreeding are not so deleterious in the laboratory, but they may be more severe in natural, competitive conditions (Miller 1994). To test this hypothesis, we compared the survival and reproductive success of inbred vs. outbred wild-derived house mice living in large, competitive enclosures (Meagher et al. 2000). We found that one generation of inbreeding (full-sib matings) resulted in a 57% fitness decline in wild populations of house mice, which was a significantly greater decline than we found in our labo- ratory controls. By increasing genome-wide heterozygosity, inbreeding avoidance apparently helps to shelter recessive, deleterious mutations and increase resistance to infectious diseases (Coltman et al. 1999; O’Brien 2000). Studies have found it easier to detect fitness benefits for inbreeding avoidance (increased genome-wide heterozygosity) than for MHC-heterozygosity (Potts et al. 1994; O’Brien 2000), which suggests that inbreeding avoidance offers the greatest potential benefits for MHC-disassortative mating preferences (at least for species at risk of inbreeding).
Conclusions – Future Directions
Although evidence for MHC-disassortative mating preferences has been found in fish, mice, and humans, more work is needed to determine the strength and generality of this preference. More research is also needed to determine how MHC genes influence odour, and whether such mating preferences are functional. The available evidence suggests that MHC-disassortative mating preferences enhance the resistance of an individual’s progeny to infectious diseases by increasing their MHC-heterozygosity and disparity. Yet, the main benefits of MHC-disassortative mating preferences may be to reduce inbreeding. Therefore, by increasing either MHC or overall heterozygosity, MHC-disassortative mating preferences should increase the genetic compatibility of mates. Future research in this area might consider other ways how MHC genes might play a role in sexual selection.
For example, besides influencing odour-based mate choice, MHC genes may play a role in postcopulatory or ‘cryptic’ female choice to increase the genetic compatibility of progeny (Wedekind 1994; Apanius et al. 1997; Penn & Potts 1999). Many studies have found that human couples sharing MHC alleles have a higher rate of recurrent spontaneous abortion (Ober 1995), and some studies in rodents also suggest that females reject MHC-similar sperm or zygotes (see Rülicke et al. 1998). The evidence has been inconsistent, but one study suggests a potential explanation: female mice produce more MHC-heterozygous offspring when they are fighting an infection (Rülicke et al. 1998). This intriguing finding needs to be replicated, however, since the effect was only significant due to the control mice producing an unusually high proportion of homozygous offspring.
Besides increasing genetic compatibility, MHC genes may also play a role in ‘good genes’ sexual selection. Since MHC genes are highly polymorphic and influence resistance to many infectious diseases, females who prefer disease-resistant males are likely to confer resistant alleles to their progeny (Hamilton & Zuk 1982). For example, female mice and rats prefer the scent of healthy vs. infected males (reviewed in Penn & Potts 1998a), which may enable females to confer resistant MHC alleles to their progeny. A study on sexual selection in pheasants (Phasianus colchicus) found that females prefer males with larger spurs, and this sexually selected trait is associated with a particular MHC allele, suggesting that large spurs may advertise a male’s genetic resistance (von Schantz et al. 1989). A recent study found that antler development and body mass in male white-tailed deer (Odocoileus virginianus) is associated with certain MHC combinations (Ditchkoff et al. 2001). Males with the largest antlers were heterozygous for functionally disparate alleles, suggesting a heterozygote (or disparate allele) advantage; however, females do not obtain genetic benefits by mating with males that are resistant due to a heterozygous advantage (Irwin & Taylor 2000). Thus, it remains to be determined whether males with attractive displays carry resistant MHC alleles. If most of the variation in resistance is among individuals carrying different alleles, rather than between homozygous and heterozygous genotypes, then it would seem that the evolutionarily stable strategy would be to mate with the most resistant male (Hamilton & Zuk 1982).
Research on MHC genes and behaviour is establishing interdisciplinary connections between ethology and many other seemingly unrelated fields. For example, sexual selection and kin recognition research is becoming more integrated with immunogenetics, sensory biology, chemical ecology (Penn & Potts 1998a; Yamazaki et al. 1998; Eggert et al. 1999; Pearse-Pratt et al. 1999), immunology, infectious diseases, and population genetics (Potts & Wakeland 1993; Penn & Potts 1999). Research on MHC and mate choice in humans is helping to integrate the biological and social sciences, and finally, if MHC genes are involved in abortional selection, then such a finding will integrate animal behaviour with reproductive immunology and medicine (Haig 1993; Haig 1997).
Acknowledgements
I am very grateful to the editor, Michael Taborsky, and two anonymous reviewers for their thoughtful comments. I thank Lara Carroll and Erin McClelland, and especially my colleague and wife, Sarah Zala, who offered comments on an earlier draft. I thank Wayne Potts and the Department of Biology, University of Utah for support and use of facilities. This material is based upon work supported by the National Science Foundation under Grant no. 9904609 and by the National Institute of Health under Grant No. GM39578.