An efficient method for dispersing Ds elements in the barley genome as a tool for determining gene function
Summary
To devise a method for function-based gene isolation and characterization in barley, we created a plasmid containing the maize Activator (Ac) transposase (AcTPase) gene and a negative selection gene, codA, and a plasmid containing Dissociation (Ds) inverted-repeat ends surrounding the selectable herbicide resistance gene, bar. These plasmids were used to stably transform barley (Hordeum vulgare). In vitro assays, utilizing a Ds-interrupted uidA reporter gene, were used to demonstrate high-frequency excisions of Ds when the uidA construct was introduced transiently into stably transformed, AcTPase-expressing plant tissue. Crosses were made between stably transformed plants expressing functional transposase under the transcriptional control of either the putative AcTPase promoter or the promoter and first intron from the maize ubiquitin (Ubi1) gene, and plants containing Ds-Ubi-bar. In F1 plants from these crosses, low somatic and germinal transposition frequencies were observed; however, in F2 progeny derived from individual selfed F1 plants, up to 47% of the plants showed evidence of Ds transposition. Further analyses of F3 plants showed that approximately 75% of the transposed Ds elements reinserted into linked locations and 25% into unlinked locations. Transposed Ds elements in plants lacking the AcTPase transposase gene could be reactivated by reintroducing the transposase gene through classical genetic crossing, making this system functional for targeted gene tagging and studies of gene function. During the analysis of F3 plants we observed two mutant phenotypes in which the transposed Ds elements co-segregate with the new phenotype, suggesting the additional utility of such a system for tagging genes.
Introduction
Recently extensive databases of genomic sequences and expressed sequence tags have become available for a variety of plant species. The availability of this information, while informative in its own right, leaves a multitude of unanswered questions regarding the functions of thousands of previously unknown genes. One definitive mechanism for assigning functions to genes involves the biological characterization of mutants. One means of accomplishing this type of reverse genetics is through the use of plant transposable elements (TEs) that can be used as efficient insertional mutagens. Such methods have been used for gene tagging and functional genomics in various plant species ( Bouchez and Hoefte, 1998 ; Martienssen, 1998) and are particularly well suited for large-scale reverse genetics because, in species where extensive physical maps exist, insertion points can be easily mapped by polymerase chain reaction (PCR)-based methods.
Isolation, cloning and extensive analysis of endogenous transposons from snapdragon and maize, as well as the development of transformation systems for heterologous species, have made these genetic tools accessible for use in plant species other than their natural hosts ( Balcells et al., 1991 ; Haring et al., 1991 ). The maize transposon systems Activator–Dissociation (Ac/Ds) and Enhancer–Suppressor-mutator (En/Spm) are the most commonly used transposons for tagging strategies in heterologous species. The Ac/Ds elements have been shown to transpose in many dicotyledonous species and were used successfully for gene tagging in petunia ( Chuck et al., 1993 ); Arabidopsis ( Altmann et al., 1995 ; Bancroft et al., 1993 ; James et al., 1995 ; Long et al., 1993a ; Springer et al., 1995 ); tomato ( Jones et al., 1994 ); tobacco ( Whitham et al., 1994 ); and flax ( Lawrence et al., 1995 ). Ac/Ds are the only transposons for which reports have been published of stable introduction into heterologous monocotyledonous species, e.g. rice ( Izawa et al., 1991 ; Murai et al., 1991 ; Shimamoto et al., 1993 ) and wheat ( Takumi, 1996). The preference of Ac/Ds to transpose to closely linked sites in maize ( Dooner and Belachew, 1989) as well as in heterologous dicot systems ( Jones et al., 1990 ; Osborne et al., 1991 ) makes these transposons valuable tools for targeted tagging strategies for specific genes. In such strategies, genomic insertion sites of TEs are mapped near genes of interest followed by activation of TEs by reintroduction of the transposase gene.
Two-element transposon systems are widely used for tagging in dicotyledonous species, and have several advantages over systems based on the use of a single autonomous element. In a two-element system the transposase gene (AcTPase) can be segregated away from the non-autonomous element, thereby stabilizing the non-autonomous element after its transposition to a new location, and avoiding reversions of genes mutated by transposon insertions. Linking the transposase gene to a negative selectable marker gene facilitates the identification and elimination of plants containing the transposase ( Perera et al., 1993 ) when necessary due to high transposition frequencies.
The development of a highly efficient transformation system for barley ( Lemaux et al., 1996 ; Wan and Lemaux, 1994) made it possible to test the feasibility of a two-element system in barley for gene tagging. This report shows evidence of high transposition frequencies of a Ds element in barley plants that resulted from crosses of plants containing Ds-bar with AcTPase gene-containing plants. Advanced generations from these plants were analyzed to determine the frequencies of somatic and germinal transposition and to demonstrate that the system could be used to tag barley genes.
Results
Expression of AcTPase in stably transformed barley plants
The AcTPase gene and a Ds element carrying bar were stably introduced into barley. A total of 44 independent transgenic barley lines carrying either the transposase gene, Ds-bar, or both elements, were generated. Only phenotypically normal plants with a low copy number of the intact introduced gene were used for further experiments. The expression of the stabilized AcTPase gene and the functionality of AcTPase in barley T1 and T2 immature embryos of plants carrying either AcAc or UbiAc were monitored using a transient assay for Ac/Ds activity ( McElroy et al., 1997 ). The number of blue spots, an indicator of the level of transposase expression, showed wide variability among plants from different independent transformation events, as well as among plants derived from the same transformation event, indicating that expression varied greatly from plant to plant. Plants containing intact copies of the Ds-bar unit were used for crosses with AcTPase-expressing plants.
Transactivation of Ds by Ac transposase
After crossing AcTPase-expressing plants with Ds-bar-containing plants, DNA hybridization analysis was carried out on the resulting F1 plants. Only three of a total of 144 F1 plants (2%) had bands that were not present in the parental plants (data not shown). In these three plants the observed excisions and reinsertions occurred exclusively in somatic tissue, as evidenced by the presence of non-stoichiometric bands in DNA hybridization blots and transmission of the transposed element in a non-Mendelian fashion in F2 plants.
Selfing of F1 plants carrying both the Ac transposase gene and the Ds-bar element resulted in a striking increase in transposition frequencies in the F2 generation ( Figure 1) . Progeny of 24 different F1 plants, derived from 10 independent crosses with different parents, were analyzed. F2 plants of 18 of these 24 F1 plants (75%) showed evidence of transposition ( Table 1).
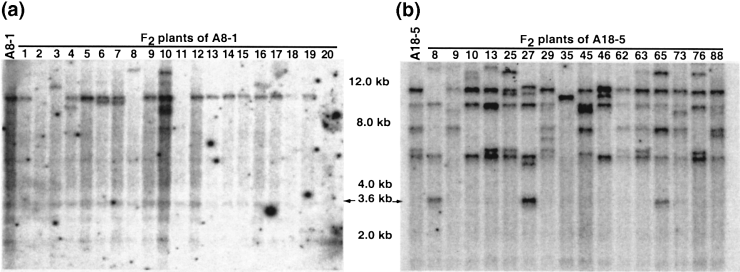
DNA hybridization pattern of Ds-bar in individual F2 plants derived from parents A8-1 (a) and A18-5 (b).
DNA of F2 plants was digested with HindIII and blots were probed with fragment DsA. Each band on the blot represents one or more Ds insertions. Lanes on the far left in (a) and (b) on the left show the integration patterns of the parental plants. DNA from F2 plants are shown in the remaining lanes. The arrows at 3.6 kbp indicate the minimum size of the Ds-Ubi-bar cassette.
| F1 plant | Promoter adriving AcTPase | No. F2 plantsanalyzed b | Average no. bluespots per embryo c | No. with Tnp d | % Tnp e |
|---|---|---|---|---|---|
| A1-1 | Ubi-1 | 40 | 1.1 | 7 | 23 |
| A1-5 | Ubi-1 | 100 | 0.9 | 16 | 20 |
| A1-8 | Ubi-1 | 40 | 0.5 | 5 | 16 |
| A8-1 | Ubi-1 | 200 | 1.4 | 57 | 38 |
| A8-5 | Ubi-1 | 100 | 1.2 | 25 | 33 |
| A12-1 | Ubi-1 | 20 | ND | 4 | 27 |
| A12-2 | Ubi-1 | 20 | ND | 3 | 20 |
| A15-1 | Ubi-1 | 20 | ND | 1 | 7 |
| A15-3 | Ubi-1 | 20 | ND | 0 | 0 |
| A20-7 | Ubi-1 | 20 | ND | 2 | 13 |
| A20-8 | Ubi-1 | 20 | 0 | 0 | 0 |
| A20-11 | Ubi-1 | 20 | 0.7 | 3 | 20 |
| A20-13 | Ubi-1 | 20 | ND | 1 | 7 |
| A2-3 | Ac | 20 | 0 | 0 | 0 |
| A2-8 | Ac | 20 | 0 | 0 | 0 |
| A3-1 | Ac | 100 | 0.2 | 0 | 0 |
| A3-5 | Ac | 20 | ND | 3 | 20 |
| A5-7 | Ac | 20 | ND | 0 | 0 |
| A5-8 | Ac | 20 | ND | 2 | 13 |
| A10-1 | Ac | 20 | 1.1 | 4 | 27 |
| A10-2 | Ac | 20 | 2.1 | 7 | 47 |
| A18-2 | Ac | 20 | 0.3 | 1 | 7 |
| A18-3 | Ac | 60 | 1.1 | 11 | 24 |
| A18-5 | Ac | 160 | 1.4 | 32 | 27 |
- a Promoter used to regulate Ac transposase expression.
- b Number of F 2 plants analyzed from specific F1 plant.
- c Average number of blue spots per bombarded embryo after Ac activity test.
- d Based on DNA hybridization analysis, number of F 2 plants showing new bands (transpositions, tnp) relative to parent.
- e Number of plants with transposition as percentage of plants carrying Ds-Ubi-bar element.
- ND, not determined.
PCR analysis was used to determine if the plants showing evidence of transposition had empty donor sites. The amplification of a specific 300 bp PCR product, indicating excision of the entire 3.6 kbp Ds-Ubi-bar cassette from the original integration site, as well as a new banding pattern following DNA hybridization analysis, is strong evidence that excision and reinsertion have occurred. The frequency of transposition in F2 plants derived from independent F1 parents varied from 0 to 47% ( Table 1). Taking into account the copy number of the Ds element in different F1 plants, and assuming all copies are equally likely to transpose, the observed transposition frequencies ranged from 0 to 38% per introduced Ds element. Transposition frequencies in sibling F2 plants, derived from F1 plants having the same parents were similar in most cases. For example, F2 plants of A1-1, A1-5 and A1-8 in Table 1 had transposition frequencies of 23, 20 and 16%, respectively. In these cases it is likely that differences in transposition frequencies between crosses were due to the level of AcTPase expression in the original parental AcTPase-containing plant.
No significant correlation was observed between transposition frequency and the particular promoter driving the AcTPase gene in F2 plants. For example, F2 plants of line A1-5, carrying AcTPase under control of the maize ubiquitin promoter, exhibited a very similar transposition frequency to F2 plants of line A18-5 where AcTPase was driven by the Ac promoter sequence. In addition, the copy number of the AcTPase gene under the control of either promoter did not appear to influence transposition frequency with either promoter, although there was a positive correlation between the level of AcTPase activity in F2 immature embryos, as demonstrated in transient assays, and the number of transpositions in F2 plants ( Table 1).
F3 immature embryos derived from selfed F2 plants that carried both AcTPase gene and Ds-bar continued to express Ac-transposase, and showed evidence of transposition of Ds-bar. The transposition frequencies were similar to those observed in the F2 generation (compare Tables 1 and 2). DNA hybridization analysis of F3 plants also revealed that about 25% of all new Ds insertions observed in the F2 generation were due to transpositions to unlinked sites, as evidenced by the independent segregation of new and old Ds integration sites (data not shown).
| F2 plant | Promoter drivingAcTPase | % Tnp inF2 generation | % Tnp inF3 generation | % F3 plants carryingnew band in F2 | Co-segregation of existing and new bands in F3 plants |
|---|---|---|---|---|---|
| A1-5-10 | Ubi-1 | 20 | 10 | 100 | Yes |
| A1-5-36 | Ubi-1 | 20 | 10 | 40 | No |
| A1-5-40 | Ubi-1 | 20 | 15 | 80 | Yes |
| A1-5-67 a | Ubi-1 | 20 | 10 | 30 | Yes |
| A1-5-67 a | Ubi-1 | 20 | 10 | 65 | No |
| A1-5-87 | Ubi-1 | 20 | 15 | 45 | Yes |
| A1-5-89 | Ubi-1 | 20 | 15 | 80 | Yes |
| A8-1-3 | Ubi-1 | 38 | 40 | 75 | Yes |
| A8-1-7 | Ubi-1 | 38 | 15 | 75 | No |
| A8-1-27 | Ubi-1 | 38 | 20 | 35 | Yes |
| A10-2-6 | Ac | 47 | 25 | 80 | Yes |
| A10-2-13 | Ac | 47 | 15 | 75 | Yes |
| A10-2-20 | Ac | 47 | 20 | 80 | Yes |
| A18-5-9 | Ac | 27 | 20 | 75 | No |
| A18-5-13 | Ac | 27 | 25 | 75 | Yes |
| A18-5-27 | Ac | 27 | 15 | 70 | Yes |
- a F 2 plant A1-5-67 has two new Ds insertion sites compared to the F1 parent.
Our scheme for the development of a tagging population containing highly dispersed Ds elements proved to be successful. DNA hybridization analysis of randomly chosen F6 plants showed that each plant has at least one new, Ds-hybridizing band ( Figure 2). Many plants show two or more changes in banding pattern compared to the original Ds integration pattern in the F1 plant. The average number of Ds elements per plant remains relatively constant.
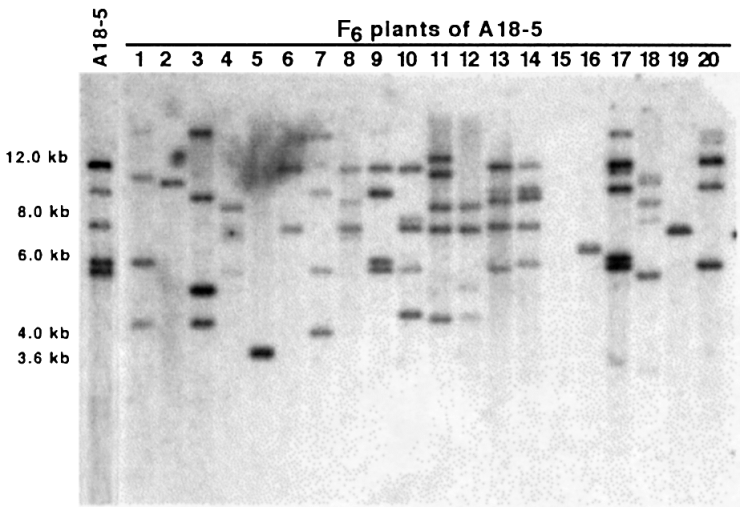
DNA hybridization pattern of Ds-bar in F6 plants of a tagging population derived from plant line A18-5.
DNA of randomly chosen F6 plants was digested with HindIII and the blots probed with fragment DsA. Bars on the left indicate the original banding pattern of the F1 parent.
In order to demonstrate that this system can be used for targeted gene tagging, we crossed plants containing a single transposed Ds element, but no AcTPase gene, with transposase-expressing plants. Resulting F1 and F2 plants showed evidence of Ds transposition, indicating that the Ds element could be reactivated in the presence of AcTPase (data not shown).
Timing of Ds transposition
Analyses of DNA hybridization patterns of F2 and F3 plants yielded information regarding the timing of transposition. For example, many of the F2 plants derived from A8-1 that showed evidence of transposition exhibited the same banding pattern for Ds, indicating an excision and reintegration of Ds in somatic tissues early in development of the F1 plant. The reintegration occurred in a sector of the plant that ultimately went through the germline, resulting in the same Ds integration pattern in many F2 plants. In most F2 plants from other independent events, however, the majority of transpositions resulted in unique banding patterns ( Figure 1b). These unique patterns, indicative of independent integration sites, could be due either to germinal transpositions in F1 plants or to somatic transpositions in F2 plants. Germinal transpositions in the F1 generation resulted in F2 plants that are heterozygous for the new Ds integration site. Selfing of these plants, which also results in a segregation of the AcTPase gene, results in an F3 generation exhibiting an expected segregation ratio of 3 : 1 for plants carrying either a particular transposed Ds element or plants without a transposed Ds.
Somatic transpositions in the F2 generation can occur early or late in plant development. Late transpositions affect only small sectors in F2 plants. In most cases these transposed elements are not transmitted through the germline and therefore were not detectable in the F3 generation, or were found to segregate the Ds element at an aberrant segregation ratio in F3 plants. Conversely, transpositions at a very early stage of embryo development are transmitted to the next generation and result in an expected 3 : 1 segregation of the new Ds integration site in the F3 generation ( Table 2).
Barley mutants generated by transposition of Ac/Ds
Two mutant phenotypes ( Figure 3) were observed when populations of F3 plants were analyzed. Sixteen of 70 F3 plants derived from A18-5-116 showed a mutant phenotype ( Figure 3a); this is close to a segregation ratio of 3 : 1, indicating that the tagged mutation might be recessive. Eight of 68 F3 plants derived from A8-1-3 exhibited a phenotype as shown in Figure 3(b). Despite the skewed ratio in the latter case, DNA hybridization analyses in both cases showed that the mutant phenotype co-segregated with a new Ds band observed in both A18-5-116 and A8-1-3. The isolated and sequenced 3′ and 5′Ds flanking genomic regions from line A18-5-116 have an identity of 82% (P = 1.2e-28) to the maize EST AW231419 (GenBank) ( Figure 4a). No significant homologies were found for the Ds flanking regions from A8-1-3 in public databases. When used in a Northern blot analysis as a probe of 350 bp length, the isolated 5′ flanking region of the transposed Ds element of line A18-5-116 hybridized to an mRNA of about 2.6 kb ( Figure 4b). Hybridization signals were detected in RNA samples from embryos, young seedlings (3 days after germination), shoots of plants at the three-leaf stage, and shoots and roots of plants at the six-leaf stage. No positive signals were detected in RNA samples of leaf tissue of 2- and 4-week-old seedlings even after overexposure of the membrane. The Ds flanking regions isolated from line A8-1-3 were not used as probes in Northern blots.
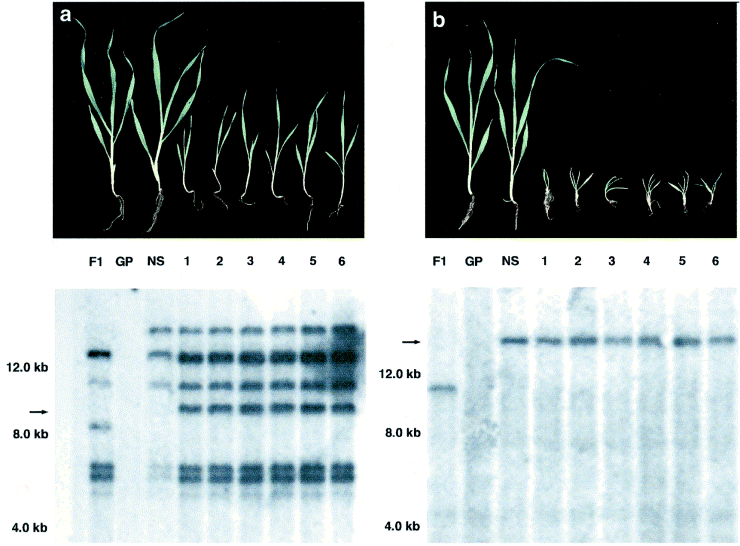
Co-segregation of transposed Ds elements with mutant phenotypes in F3 plants.
(a) A normally developed Golden Promise plant (GP), a phenotypically normal F3 negative segregant (NS) derived from A18-5-116, and six F3 plants with mutant phenotype derived from A18-5-116 are shown from left to right 3 weeks after germination. DNA hybridization analysis of these plants shows the co-segregation of one newly observed Ds insertion and its mutant phenotype in the F2 plants. Changes in the Ds banding pattern from F1 to F2 generations are indicated by arrows.
(b) As in (a), except that these plants derive from event A8-1-3.
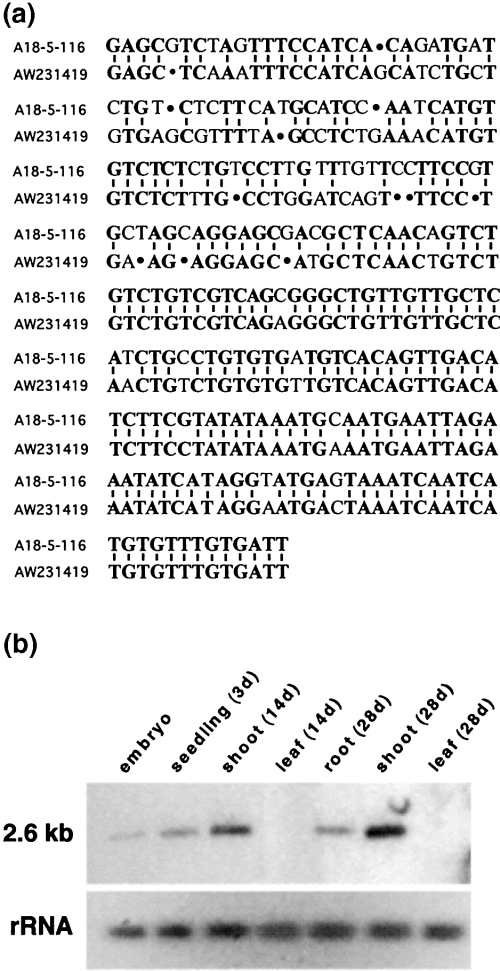
Analysis of Ds flanking genomic DNA of line A18-5-116.
(a) Comparison of the Ds flanking sequence of line A18-5-116 and maize EST AW231419.
(b) Northern blot analysis using a Ds flanking genomic region as a probe. Total RNA extracted from embryos, seedlings, shoots and leaves of 2-week-old plants and roots, shoots and leaves of 4-week-old plants was blotted and probed with a 32P-labeled 350 bp probe isolated from genomic DNA flanking a transposed Ds element that co-segregates with the mutant phenotype shown in Figure 3(a). rRNA18S served as a control for sample loading and blotting (lower panel).
Discussion
The maize transposable element Ds containing bar and the Ac transposase gene, driven by two different promoters, were stably introduced into barley in order to develop a system that would lead to the rapid generation of independent Ds insertions over the entire genome. Once mapped, such elements can be used to direct efforts at insertional mutagenesis to specific linked regions. In order to stabilize the transposed element for characterization, it is essential to eliminate the transposase source by segregation, using a negative selection system when necessary (e.g. Koprek et al., 1999 ).
Our data demonstrate that the Ac/Ds system of maize provides such a useful tool for targeted insertional mutagenesis in barley. We show that the AcTPase gene is expressed over several generations in stable transformants, and that the expression level is sufficient to transactivate Ds elements, both in transient assays using embryos stably transformed with the AcTPase gene, and in vivo in crosses between plants carrying the two genes. The rough correlation between the level of AcTPase activity in bombarded F2 embryos and the frequency of transposition in resultant F2 plants ( Table 1) make this assay a simple and valuable tool to screen for plants likely to give rise to high transposition frequencies.
In the present study, intensive analyses by DNA hybridization and PCR of successive generations of plants containing the AcTPase gene and Ds-bar proved the functionality of the described transposon system in supporting efficient transposition of the Ds element, and permitted the quantification of frequencies. In F2 plants containing both Ds-bar and the AcTPase gene, high rates of Ds excision and reinsertion were observed. The measured frequencies of Ds transposition varied over a wide range (0–47%) but were comparable to those in many Ac/Ds tagging systems in dicot species (0–87%) ( Hehl and Baker, 1990; Jones et al., 1989 ; Jones et al., 1991 ; Rommens et al., 1992 ; Scofield et al., 1992 ). For example, in this study, selfing of a single copy Ds-bar line A8-1 resulted in 38% of all F2 plants in having transpositions of Ds. In a two-element tagging system in rice based on Ac/Ds ( Izawa et al., 1997 ), transposition frequency was not accurately determined, but was sufficiently high to generate several phenotypic mutants.
Transposition frequencies remained high in the F3 generation when AcTPase-expressing and Ds-bar-containing F2 plants were selfed. Consecutive selfing of F2 plants homozygous for AcTPase derived from line A18-5 resulted in a high frequency of dispersion of Ds elements. This line is potentially very useful for insertional mutagenesis that is not directed towards a particular linked gene. Results of experiments in which transposed Ds elements were reactivated by providing AcTPase in trans indicate that high transposition frequencies are not restricted to the original site of integration of the element. Therefore, transposed and mapped Ds elements can be used for targeted tagging experiments.
Analyses of progeny from several F1 plants containing AcTPase under control of either the maize ubiquitin or the Ac promoter region did not give conclusive evidence as to which promoter results in higher total (somatic plus germinal) transposition frequencies of Ds. The relatively weak Ac promoter region ( Fridlender et al., 1996 ) gave rise in certain crosses to similar frequencies of transposition compared to crosses of lines containing AcTPase driven by the relatively strong ubiquitin promoter from maize ( Christensen and Quail, 1996). These results are consistent with observations made in tobacco, where high-level expression of AcTPase, driven by the heterologous promoters cauliflower mosaic virus (CaMV) 35S promoter, the relatively weak Agrobacterium ocs and nos promoters, or the Ac promoter region, did not result in dramatic differences in transposition frequencies ( Scofield et al., 1992 ).
In tobacco it was shown that where AcTPase was highly expressed, there was an inhibitory rather than a stimulatory effect on transposition frequency ( Scofield et al., 1993 ). The opposite observation was made with Ac/Ds tagging systems in Arabidopsis thaliana, where the strong constitutive CaMV 35S promoter increased the transposition frequency ( Grevelding et al., 1992 ; Honma et al., 1993 ; Long et al., 1993a ; Swinburne et al., 1992 ) compared to the Ac promoter region.
The transposition frequency of Ac and Ds elements in both maize and heterologous dicot systems is also affected by a dosage effect caused by the copy number of AcTPase. The natural host of Ac/Ds, Zea mays, exhibits a negative dosage effect ( McClintock, 1948; 1951), in contrast to the situation in tobacco ( Hehl and Baker, 1990; Jones et al., 1989 ; Keller et al., 1993a ) and Arabidopsis ( Bancroft and Dean, 1993a; Keller et al., 1992 ), where an increase in Ac copy number, mediated by Agrobacterium gene delivery, correlates with higher transposition frequencies. Neither a positive nor a negative correlation between copy number and transposition frequency was observed in our experiments. This is probably because with direct DNA introduction, methods increasing transgene copy numbers do not generally correlate with higher transgene expression ( Hobbs et al., 1993 ).
Higher levels of transposase expression have previously been shown to be associated with transpositions occurring earlier in development ( Balcells and Coupland, 1994; Jones et al., 1989 ; Keller et al., 1993a ; Long et al., 1993b ). This occurrence leads to larger somatic sectors which are more likely to transmit the transposed element through the germline and result in large numbers of siblings carrying the same transposition pattern. This situation was observed in some plants carrying AcTPase under the control of the ubiquitin promoter (e.g. lines A8-1 and A8-5). In contrast, in plants containing AcTPase gene driven by the putative Ac promoter (e.g. A18-3 and A 18-5), a high proportion of the transpositions appeared to have occurred during late developmental stages of the reproductive cells or directly in the germline; their progeny showed a high frequency of independent transpositions as demonstrated by distinct DNA hybridization patterns ( Table 2). Similar results, indicating a higher frequency of germline transpositions, were obtained in experiments with Arabidopsis when the putative Ac promoter was used to drive AcTPase expression ( Bancroft and Dean, 1993a). In our experiments, differentiation of somatic and germinal transpositions could be assessed only after analyzing DNA hybridization blots of two successive generations (e.g. F2 and F3). Comparison of the numbers of transposition events showed that transcriptional control of the AcTPase gene by the putative Ac promoter leads in most cases to higher germinal transposition frequencies than in plants in which AcTPase expression is driven by the maize ubiquitin promoter.
It is important for insertional mutagenesis and gene tagging strategies to characterize the preference of the Ds element to transpose to either linked or unlinked sites in heterologous systems. There is a strong tendency of Ac and Ds to transpose to genetically linked sites in maize ( Dooner and Belachew, 1989; Greenblatt, 1984) as well as in tobacco ( Dooner et al., 1991 ; Jones et al., 1990 ); tomato ( Healy et al., 1993 ; Osborne et al., 1991 ); and Arabidopsis ( Bancroft and Dean, 1993b; Keller et al., 1993b ). DNA hybridization analysis of advanced generations of transgenic barley plants carrying transposed Ds elements demonstrated that about 75% of the reinserted elements are linked to the original integration site. This frequency of reinsertion into a linked site is comparable to other systems, and is sufficiently high to allow for efficient insertion of the transposable element into genes located near the insertion site.
Further molecular characterization is needed of the mutant phenotypes observed in the F3 generation. However, in lines A18-5-116 and A8-1-3 there is a tight correlation between a specific transposed Ds element and a mutant phenotype. In addition, in line A18-5-116 there is a high sequence homology of the Ds flanking regions to an EST from maize. Results from Northern blot analysis, in which the Ds flanking DNA of line A18-5-116 was used as a probe and hybridized specifically to an RNA species of 2.6 kb, indicate that the putatively tagged gene is expressed from the embryo stage to at least the six-leaf stage of plant development. However, expression was detected in shoots and roots but not in leaves. Taken together, this is compelling evidence that this system can be used to tag genes in barley.
The characteristics of Ds transposition in transgenic barley described here demonstrate that the Ds transposon system has full functionality of a two-element transposon system, and can be used for insertional mutagenesis and transposon tagging in barley. Assuming a reasonably constant transposition frequency in subsequent generations, some of the lines described in this report (e.g. A1-5, A18-5), containing high numbers of independent Ds inserts, make feasible a systematic mutant screen for random insertional mutations. Perhaps a more efficient use of the system is to couple the efficient insertion of Ds into linked locations with the capability of placing the insertion sites on a fine structure map of barley. The existence of mapped elements scattered randomly throughout the genome will facilitate a targeted insertional mutagenesis strategy, and permit the assignment of functions to as-yet uncharacterized gene sequences in barley. Characterizations of genes from the diploid barley system will be useful in ascribing functions to genes in other cereals, based on the high level of synteny and functional similarity between barley and other cereals and grasses.
Experimental procedures
Construction of vectors
All DNA modifications were carried out according to standard protocols ( Sambrook et al., 1989 ). Plasmid pSP-Ds-Ubi-bar ( Figure 5) contains the Streptomyces hygroscopicus phosphinothricin acetyl transferase gene (bar) and the nos terminator as a 0.9 kbp ClaI–NotI restriction fragment derived from pBARGUS ( Fromm et al., 1990 ). The bar gene is under control of the Ubi1 promoter and first intron from maize, derived from plasmid pAHC27 ( Christensen and Quail, 1996) as a 2.0 kbp PstI fragment. The UbiI-bar-nos cassette is flanked by a 254 bp 5′ sequence and a 340 bp 3′ sequence from Ds, derived from pDs7 ( Wirtz et al., 1997 ) as a 0.59 kbp SalI–BamHI restriction fragment. The construction of the AcTPase containing plasmids pUC-codA-Act-AcAc and pBS-codA-Act-UbiAc, as well as plasmid pSP-WDV-Act1 <Dsbar> GUS.N has been published ( Koprek et al., 1999 ; McElroy et al., 1997 ). Plasmid pAHC20 ( Christensen and Quail, 1996) was co-transformed with plasmids pUC-codA-Act-AcAc or pBS-codA-Act-UbiAc to facilitate selection.
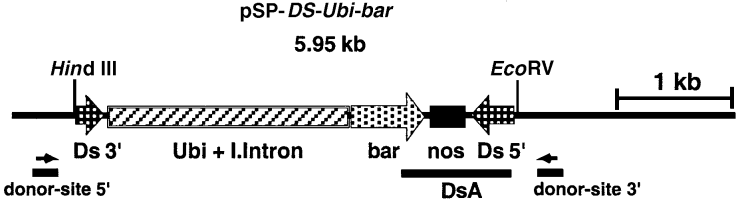
Schematic representation of plasmid pSP-Ds-Ubi-bar.
The plasmid contains the selectable marker gene bar under control of the ubiquitin promoter and first intron from maize and nos terminator flanked by the 5′ and 3′ regions of the Ds element having the AcTPase recognition sites. The probe used for DNA hybridization analysis (DsA) and the positions of primers for PCR reactions (donor site 5′ and 3′) are indicated by bars and arrows; a 1 kb size bar is included.
Plant transformation and regeneration
Immature embryos (1.5–2 mm) of barley (Hordeum vulgare L. cv. Golden Promise) were excised, cultured ( Wan and Lemaux, 1994), transformed via particle bombardment either with pSP-Ds-Ubi-bar alone or co-transformed with pAHC20 and pUC-codA-Act-AcAc or pBS-codA-Act-UbiAc ( Lemaux et al., 1996 ), and selected ( Cho et al., 1998 ) as previously described. T0 plants were regenerated from stably transformed callus lines, and phenotypically normal plants were visually selected and transferred to soil in the greenhouse following the protocol of Cho et al. (1998) .
Development of a tagging population
T2 plants carrying one or more Ds elements were crossed with plants expressing the AcTPase gene. Testing of AcTPase activity in immature embryos of AcTPase gene-containing plants was performed as described ( McElroy et al., 1997 ). Resulting F1 plants were selfed and the resulting F2 plants analyzed for transposition events. Plants containing at least one transposed Ds element and AcTPase were selfed, seeds were bulk harvested, and about 100 seeds randomly chosen, planted and again self-pollinated. This procedure was repeated over four generations (F3–F6). The Ds integration patterns in F6 plants were analyzed by DNA hybridization.
DNA hybridization analysis
Genomic DNA, isolated from two leaves when plants were at the three-leaf stage ( Cone, 1989), was digested with either PstI to determine the intact nature of the Ubi-Ac or Ac-Ac cassettes (6.0 and 4.4 kbp, respectively) or with EcoRV and HindIII to release the intact Ds-Ubi-bar cassette (3.6 kbp). To determine the copy number of Ds and to detect transposition events, DNA was digested with HindIII, which does not cut within the Ds-Ubi-bar cassette but cuts once at the 3′ end of the cassette. Excision and reinsertion of a Ds element into a new location creates a different sized HindIII fragment that can be detected after hybridization with a Ds-specific probe.
Digested DNA was electrophoretically separated on 0.8% agarose gels, transferred to Zeta-Probe membranes (BioRad, Hercules, CA, USA), hybridized with 32P-labeled probes, and washed according to the manufacturer's instructions. The position on the plasmid of the probe used in the hybridizations is indicated in Figure 5. The probe for DsA was PCR-amplified using plasmid DNA from pSP-Ds-Ubi-bar as a template. The primers were as described below, and the PCR product was purified using the QIAquick PCR purification kit (Qiagen, Chatsworth, CA, USA). Labeling of the probes was performed using the Promega (Madison, WI, USA) Prime-A-Gene labeling kit.
PCR analysis
Genomic DNA (0.5 µg) was subjected to PCR amplification in an MJ Research (Waltham, MA, USA) thermocycler (model PTC-100). PCR reactions (50 µl) contained 1 × PCR buffer (Promega), 200 µm of each dNTP, 1.5 m m MgCl2, 1 µm primer, 1% DMSO and 2.5 U Taq DNA polymerase (Promega). The primer pairs used for the AcTPase coding region were Ac5′ (5′-AAC CTA TTT GAT GTT GAG GGA TGC-3′) and Ac3′ (5′-ACC ACC AGC ACT GAA CGC AGA CTC-3′) to produce a PCR product of 852 bp. For DsA, they were bar5′ (5′-TGC ACC ATC GTC AAC CAC TA-3′) and Ds3′ (5′-AAC GTC AGT AGG GCT TAA TCT TTT-3′) to produce a 650 bp PCR fragment. For analysis of empty donor sites, primers EDS5′ (5′-CGT CAG GGC GCG TCA GCG GGT GTT-3′) and EDS3′ (5′-AAT ACG CAA ACC GCC TCT CCC CGC-3′) were used to amplify a 300 bp fragment. PCR reactions were performed with an initial denaturation at 94°C for 2 min followed by 10 cycles of a touch-down program with decreasing annealing temperatures from 65 to 60°C in increments of −0.5°C per cycle for 45 sec, an extension at 72°C for 60 sec, a denaturation at 94°C for 45 sec, subsequent 25 amplification cycles for 45 sec at 60°C, 60 sec at 72°C and 45 sec at 94°C, and a final extension at 72°C for 5 min. PCR products were analyzed by gel electrophoresis in 1.1% agarose gels.
RNA hybridization analysis
10 µg of total RNA, isolated from different plant tissues using the RNeasy kit (Qiagen), was separated in denaturing 1.0% formaldehyde-agarose gels, blotted to Zeta-Probe membranes, hybridized with a 32P-labeled probe and washed according to the manufacturer's instructions. The probe was a 350 bp fragment isolated from the Ds 5′-flanking region of a transposed Ds element that co-segregates with a mutant phenotype.
Isolation of transposon flanking regions by TAIL-PCR
TAIL-PCR was carried out as described previously ( Liu et al., 1995 ) . 100 ng of genomic DNA was used as template DNA. The nested specific primers for the downstream regions were: DsA (5′-GAAACGGTCGGGAAACTAGCTCTA-3′), DsB (5′-TGTATATCCCG TTTCCGTTCCGTT-3′), DsC (5′-ACTAACAAAATCGGTTA-TACG ATA-3′) and for the upstream regions: Ds1 (5′-AAGTTTTGA ATATATGTTTT-CATG-3′), Ds2 (5′-TTAACCCGACCGGATCGTAT CGT-3′), Ds3 (5′-GTTTCCGTC-CCGCAAGTAAATAT-3′). Success fully used arbitrary primers were, for A18-5-116: downstream AD1 (5′-NGTCVAGBATNCWAGC-3′); upstream AD3 (5′-WGTGNAGCA-NCANAGA-3′); and for A8-1-3: downstream AD2 (5′-NGT CGASWGANAWGAA-3′) and upstream AD6 (5′-VTGYCRTWG GBTASTC-3′). Specific tertiary PCR products were analyzed on 1.5% agarose gels, isolated using the QIAquick gel extraction kit (Qiagen), sequenced or used as probes for Northern blots.
Database search
Partial sequences of the 5′ and 3′ flanking regions (300–500 bp) of both events were obtained commercially. These sequences were used in blast searches for similarities in GenBank and TIGR.
Acknowledgements
The authors would like to thank Drs Uwe Wirtz and Barbara Baker for plasmid pTps, Dr Jonathan Jones for the gift of plasmid pSLJ721, and Rachel Fessenden for technical assistance. Thomas Koprek was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft (Ko 1791/1-2) and Novartis Agricultural Discovery Institute, Inc.; Dr David McElroy and Jeanine Louwerse were supported by funding from the North American Barley Genome Mapping Project and USDA NRI competitive grant #9500682. Rosalind Williams-Carrier and Peggy Lemaux were supported by the USDA Cooperative Extension Service through the University of California.