Functional, c-myc-tagged Cf-9 resistance gene products are plasma-membrane localized and glycosylated
Summary
The Cf-9 resistance gene from tomato confers resistance to races of the fungal pathogen Cladosporium fulvum that express the corresponding avirulence gene, Avr9. Avr9 encodes a secreted peptide. To investigate Cf-9 function, we tagged the Cf-9 protein with a triple myc epitope at either the amino- or carboxy-terminus of the mature protein. Tobacco plants carrying these constructs activate a defence response to Avr9 peptide. The Cf-9 sequence predicts a protein of 94 kDa, with 22 glycosylation sites. Using c-myc antibodies, c-myc : Cf-9 protein was detected as a unique band with a molecular size of 160 kDa. The band shifted to approximately 105 kDa after glucosidase treatment, indicating that Cf-9 protein is highly glycosylated. Plasma membranes were isolated using two-phase partitioning, and c-myc : Cf-9 was enriched in these fractions, indicating that Cf-9 is a plasma membrane protein. This was confirmed by silver-enhanced immunogold labelling of tobacco protoplasts carrying the amino-terminal c-myc tag; a higher labelling density was observed on the surface of protoplasts derived from c-myc : Cf-9 tobacco compared to untransformed control. The presence of Cf-9 in the plasma membrane is consistent with its role in conferring recognition of the extracellular Avr9 peptide.
Introduction
Plants are constantly subject to attack by pathogens. Disease resistance is often controlled by plant resistance (R) and pathogen avirulence (Avr) genes. It has been postulated that R gene products are receptors for the matching Avr product ( Staskawicz et al. 1995 ), although direct interaction has been demonstrated only for Pto and AvrPto ( Scofield et al. 1996 ; Tang et al. 1996 ). R gene products fall into four main structural classes ( Hammond-Kosack & Jones 1997), one of which is exemplified by the tomato Cf-9 gene that confers resistance to races of Cladosporium fulvum expressing the complementary Avr9 gene. The Cf-9 sequence predicts an extracytoplasmic membrane-anchored glycoprotein with 27 leucine-rich repeats ( Jones et al. 1994 ). This structure is consistent with a receptor function but provides no obvious mechanism for subsequent signal transduction. The short putative cytoplasmic domain terminates with the amino-acid sequence KKRY. In mammals and yeast, the KKxx sequence motif at the C-terminus of membrane-anchored proteins acts as a signal for retrieval from the Golgi apparatus to the endoplasmic reticulum (ER) ( Teasdale & Jackson 1996) or for retention in the ER ( Pagny et al. 1999 ). If Cf-9 were ER-localized, it is hard to envisage how it could carry out a receptor function. Thus it is important to establish whether Cf-9 protein is in the plasma membrane or the ER.
Epitope tagging permits detection of a protein and is especially suitable when the gene product to be analysed is encoded by one member of a gene family. We used this technique to study Cf-9 subcellular localization. Here we show that in transgenic tobacco plants that express either of two epitope tagged-Cf-9 constructs, Cf-9 co-fractionates with plasma-membrane markers in aqueous two-phase partitioning. Also, in antibody detection of protoplast cell surface proteins, immunogold labelling was increased on protoplasts derived from c-myc : Cf-9-tagged tobacco in which the epitope tag was at the N-terminus of Cf-9. Since tobacco has proved an excellent system to study Cf-9 function in cell cultures ( Piedras et al. 1998 ; Romeis et al. 1999 ), this permits the establishment of systems to study the earliest events in Avr9-dependent Cf-9 signal transduction.
Results
Two c-myc-tagged Cf-9 alleles confer Avr9 recognition in tobacco
A triple c-myc sequence was inserted in frame in Cf-9, either behind the putative signal peptide cleavage site (domain B) near the N-terminus of the predicted extracellular part of the protein, or in the putative cytoplasmic part of the protein (domain G) (see Experimental procedures). We introduced three c-myc sequences (EQKLISEEDL) in tandem to improve sensitivity for the detection of the chimeric protein with the anti-myc antibodies. These constructs were fused to the 35S promoter and were cloned into an Agrobacterium binary plasmid. The resulting clones, SLJ9161 and SLJ9171, are represented in Fig. 1. Tobacco plants were transformed, and at least 10 independent kanamycin-resistant plants were analysed for each construct. Transgenic tobacco expressing the tomato Cf-9 gene can be used to study Cf-9- and Avr9-dependent responses ( Hammond-Kosack et al. 1998 ; Piedras et al. 1998 ; Romeis et al. 1999 ).
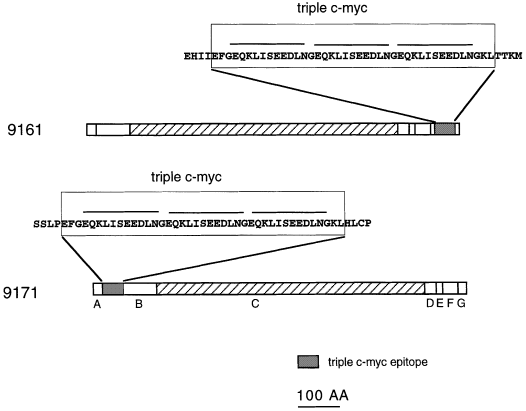
Structure of the predicted c-myc : Cf-9.
The Cf-9 sequence has been divided into seven domains (A–G) as described by Jones et al. (1994) . The triple c-myc was inserted in the middle of domain G in 9161, or after the second amino acid for the mature Cf-9 protein after removal of signal peptide in 9171. The predicted sequence is shown in the magnified blocks, and the sequence that codifies for the myc epitope is over-lined.
To assess whether these transgenic plants still retain Cf-9 function, IF(Avr9+) and IF(Avr9–) was injected into the leaves. Most transformants exhibited either a grey necrosis or chlorosis in response to the IF(Avr9+) challenges, and no macroscopic response to the IF(Avr9–) challenges ( Fig. 2a), indicating that these plants were still able to initiate the Avr9-dependent defence response. The response was stronger in plants expressing c-myc : Cf-9 with the tag in domain B (9171 plants) than plants with the tag in domain G (9161 plants).
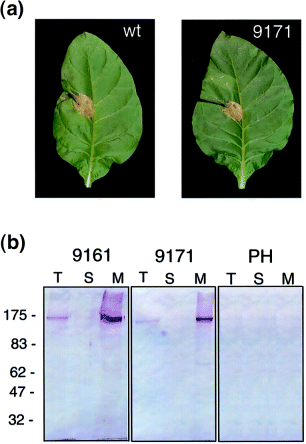
Function and expression of c-myc : Cf-9.
(a) Avr9-dependent necrosis in transgenic c-myc : Cf-9. Tobacco leaves from control tobacco plants or 9171 plants were injected with IF(Avr9+) (left) and IF(Avr9–) (right). Photographs were taken 72 h after injection.
(b) Detection of c-myc : Cf-9. Equal amounts of protein (50 μg for 9161, 200 μg for 9171 and the untransformed line Petite Havana, respectively) from total (T), soluble (S) and microsomal (M) fractions were loaded in SDS–PAGE gel and blotted with anti-C-myc antibodies.
Expression of c-myc-tagged Cf-9 protein
The expression of c-myc-tagged protein was analysed by Western blots with the anti-myc antibodies in total protein extracts from 9161 and 9171 tobacco plants. A strong cross-reacting band was detected in plants transformed with either plasmid ( Fig. 2b, lanes marked as T), whereas signal was not observed in the control extracts obtained from non-transformed plants ( Fig. 2b, PH panel). The protein band observed in both 9161 and 9171 microsomes showed an estimated molecular mass of 160 kDa. The signal was stronger in plants with the tag in the G domain (9161 plants). The fact that 9161 plants showed higher c-myc : Cf-9 protein levels but were less responsive to Avr9 infiltration suggests either that the insertion of the triple c-myc in the G domain affects the functionality of Cf-9, or that the B domain tag is more prone to proteolysis.
c-myc-tagged Cf-9 is glycosylated
The Cf-9-predicted protein has a molecular mass of 94 kDa and contains 22 putative glycosylation sites distributed throughout its sequence. The protein recognized by the c-myc antibodies shows a molecular mass of 160 kDa, higher than predicted from the Cf-9 amino-acid sequence, even considering the 5 kDa added by the inserted triple c-myc. This difference between the observed and estimated molecular masses led us to test whether the potential glycosylation sites in Cf-9 are used. Solubilized microsomal fractions from c-myc : Cf-9 plants were treated with PNGase F. This enzyme is a glycoamidase that cleaves the bond between the asparagine residue of the protein and the N-acetylglucosamine residue that joins the carbohydrate to the protein, liberating nearly all N-linked oligosaccharides from glycoproteins. After incubation of c-myc : Cf-9 microsomes with PNGase F, a shift in the mobility of the protein was observed. Within 5 min, two smaller bands, of 155 and 105 kDa, were detected ( Fig. 3). As the enzymatic reaction proceeded, the 105 kDa band increased in intensity concomitantly with the 155 kDa band becoming fainter. The 105 kDa size is close to the molecular mass of 100 kDa predicted for the c-myc : Cf-9 amino-acid sequence, indicating that Cf-9 is highly glycosylated.
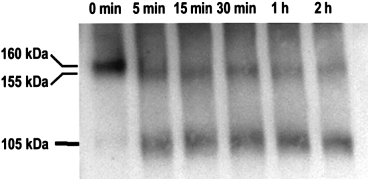
c-myc : Cf-9 is glycosylated.
c-myc : Cf-9 microsomes were incubated with PNGaseF (see Experimental procedures) and aliquots of the reaction mixture were taken at the times indicated and subjected to Western blot analysis with the anti-c-myc antibodies.
c-myc-tagged Cf-9 is in the plasma membrane fraction
Domain F in Cf-9 is predicted to be a transmembrane domain, but this does not establish whether Cf-9 is a plasma membrane or an ER protein. Separation by ultracentrifugation of soluble and membrane-associated proteins confirms that c-myc : Cf-9 is associated with membranes; the c-myc : Cf-9 was detected in the microsomal pellet fraction and signal was not detected in the soluble fraction ( Fig. 2b) for both plants 9161 and 9171.
The presence of the KKRY sequence in the Cf-9 C-terminus prompted us to investigate whether Cf-9 is a plasma-membrane protein. We attempted to separate plasma membranes from ER using sucrose gradients, but clear resolution between ER and plasma membranes was not achieved (data not shown). Therefore we purified plasma membranes using the two-phase partitioning method of Kjellbom & Larsson (1984). Using this technique, plasma membranes are separated from other membranes based upon differences in their surface charge. The plasma-membrane vesicles obtained with this method are predominantly sealed and oriented right-side-out. The partitioning in dextran-polyethylene was repeated three times, generating U3 (upper phase re-extracted three times and enriched in plasma membrane) and L3 (lower phase re-extracted three times). Both fractions, U3 and L3, as well as the microsomal membranes, were assayed for the presence of H+-ATPase (plasma membrane marker), NADPH-cytochrome c reductase (ER marker), cytochrome c oxidase (mitochondrial marker), and chlorophyll (chloroplast marker). The results are summarized in Table 1. The values represent the specific activity compared with the microsomal preparation. The plasma membranes obtained from plants with tags in both positions were enriched seven-fold in vanadate-sensitive H+-ATPase, were chlorophyll-free and were also very pure with respect to contamination by mitochondria. However, a relatively high specific activity of cytochrome c reductase was found in the plasma membrane preparations. This NADPH-cyt c reductase activity is probably not due to contamination by ER, but is a true activity of the plasma membrane ( Askerlund et al. 1991 ; Kjellbom & Larsson 1984). Antibodies raised against the Arabidopsis plasma membrane aquaporin PIP1a were used as a second marker for plasma-membrane proteins ( Kammerloher et al. 1994 ). Western blots using those antibodies confirmed the data obtained using H+-ATPase activity. This aquaporin was enriched in our plasma membrane preparations. The same fractions were analysed for c-myc : Cf-9 protein using the myc antibodies. Enrichment for c-myc : Cf-9 was always observed in plasma membrane fractions ( Fig. 4).
| Compounds and marker activities in upper and lower phases | ||||
|---|---|---|---|---|
| Fraction | Vanadate-sensitive H+-ATPase | NADPH-cyt c reductase | Cyt c oxidase | Chlorophyll |
| 9161 | ||||
| U3 | 6.9 | 0.9 | 0.2 | nd |
| L3 | 1.3 | 0.7 | 1.2 | 1.2 |
| 9171 | ||||
| U3 | 6.8 | 1.3 | 0.1 | nd |
| L3 | 0.5 | 1.0 | 1.5 | 2.8 |
- Two-phase partitioning was carried out for 9161 and 9171 plants, as described in Experimental procedures. The final upper and lower phases after repartitioning three times were designated as U3 and L3, respectively. Chlorophyll and enzymatic activities were determined for the microsomal, U3 and L3 fractions using the same amount of protein. The specific values obtained were normalized to the microsomal values.
- nd, Not determined.
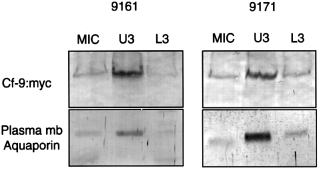
Western blotting of membrane fractions obtained by two-phase partitioning.
The same amount of protein was loaded for microsomal (MIC), plasma-membrane fraction (U3) and lower fraction (L3). After SDS–PAGE and Western blots the filters were analysed with either c-myc or aquaporin antibodies. For 9161 extracts, 30 and 6 μg were loaded in the gel to be analysed by c-myc or aquaporin antibodies, respectively. For 9171 extracts the values were 90 and 18 μg for c-myc and aquaporin, respectively.
Immunolocalization of c-myc : Cf-9 in protoplast plasma membranes
As an additional test of whether Cf-9 is plasma-membrane localized, protoplasts were obtained from suspension cultures derived from 9171 plants. In these plants c-myc is in the B domain and therefore should be extracellular. Protoplasts from these plants, as well as from control plants, were incubated with myc antibodies and with secondary gold-coupled antibodies. The gold particles on the surface of protoplasts were visualized after silver enhancement. A clear difference between 9171 and untransformed protoplasts was reproducibly obtained in many separate experiments ( Fig. 5). Although some antibody binds non-specifically to untransformed protoplasts, transformed cells show substantially enhanced specific binding of c-myc antibodies to the protoplast surface. These data provide independent verification of the plasma membrane localization of the c-myc : Cf-9, and confirm its anticipated orientation in the membrane.
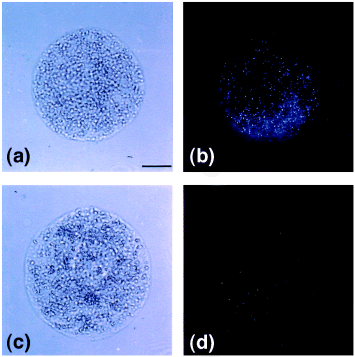
Detection of c-myc : Cf-9 in tobacco protoplasts.
Tobacco protoplasts were obtained from 9171 tobacco suspension cultures (a,b) and control cultures (c,d). The protoplasts were photographed (a,c), or incubated with c-myc antibodies and gold-coupled secondary antibodies and visualized by silver enhancement as described in Experimental procedures (b,d).
Discussion
The tomato Cf-9 gene sequence predicts a mainly extracytoplasmic protein with numerous LRRs and a very short C-terminal cytoplasmic domain without an obvious signalling domain ( Hammond-Kosack & Jones 1997). It is not known how Cf proteins signal upon elicitation, but the process probably involves interactions with other proteins. Comparative analysis of all the Cf gene LRR domains reveals extensive conservation in domain C3 (the membrane-proximal four LRRs) ( Thomas et al. 1998 ). If different Cf genes share the same resistance pathway, these conserved membrane-proximal LRRs could be the region responsible for interaction with other proteins to activate defences after Avr binding.
We generated two different tobacco lines that express triple c-myc-tagged Cf-9 from the 35S promoter. The resulting c-myc : Cf-9 proteins, tagged either at the N-terminus or near the C-terminus of the mature protein, were detectable in crude extracts and were localized in the membrane fractions. More interestingly, plants expressing each of these constructs still conferred recognition of the Avr9 peptide. This is important as it suggests that the crucial protein–protein interactions in Cf-protein function are more likely to be mediated through extracellular amino acids conserved between Cf-2 and Cf-9 ( Dixon et al. 1996 ) than through the non-conserved cytoplasmic domain.
However, insertion of the triple myc tag into the short cytoplasmic domain was correlated with slightly weaker Avr9 responsiveness than the amino-terminal location of the tag. This weaker Avr9 responsiveness suggests that this cytoplasmic domain also contributes to Cf-9 function.
The Cf-9 sequence carries putative glycosylation sites, and consistent with this Cf-9 is a glycoprotein. The treatment of microsomal fractions containing c-myc : Cf-9 with the enzyme PNGase F, which removes the N-linked oligosaccharides from glycoproteins, converted the 160 kDa cross-reacting band to a 105 kDa protein consistent with the expected molecular size for the myc-tagged Cf-9. The fact that two different bands appear during the deglycosylation reaction (155 and 105 kDa, respectively) might reflect the presence of different sites in the c-myc : Cf-9 protein with different sensitivities to the PNGase enzymatic activity.
Although a plasma-membrane localization has been proposed for Cf-9 protein, no data testing this theory have previously been reported. Cf-9 has the sequence KKRY at the C-terminus, and the KKxx motif has been identified at the C-terminus of many type I integral membrane proteins that reside in the ER ( Pagny et al. 1999 ; Teasdale & Jackson 1996). Cf-9 has a putative signal peptide, and the fact that c-myc : Cf-9 was not detected in the soluble fraction indicates that this sequence indeed introduces the protein into the secretory pathway. Despite the presence of the domain KKxx, using two-phase partitioning, and immunolocalization in protoplasts, we found that c-myc : Cf-9 is only enriched in the plasma-membrane fraction (U3), and is nearly undetectable in the corresponding ER fraction (L3). Furthermore, the enrichment observed for c-myc : Cf-9 correlated perfectly with the enrichment obtained for the plasma-membrane aquaporin marker and the vanadate-sensitive ATPase activity. The values obtained for these markers provide evidence for the quality of the fractionation. Taken alone, the immunogold labelling does not prove that all c-myc : Cf-9 is at the plasma membrane, but considered in conjunction with the two-phase partitioning data, it is likely that most of the c-myc : Cf-9 protein is plasma-membrane localized in these transgenic lines.
It is unlikely that the localization of c-myc : Cf-9 in the plasma membrane is due to saturation of the secretion system by high-level expression from the 35S promoter. In that case one would expect most of the c-myc : Cf-9 protein to be found in the corresponding ER fraction (L3). Also, in analysis of Cf-9 promoter fusions to the β-glucuronidase (GUS) gene in transgenic tobacco and tomato, we found that although overall expression levels were low, strong GUS expression is detected in certain cell types in the vascular system (M. Torres and J.D.G Jones, unpublished data). Thus, although overall expression levels of Cf-9 appear to be lower than 35S fusions in gel blots of leaf RNA, in the cells that do express Cf-9, mRNA levels may be not dissimilar from 35S.
A possible role for the KKxx domain could be to facilitate the proper assembly of complexes in the ER before accumulation of these complexes at their final destination ( Teasdale & Jackson 1996). The domain KKxx would retain Cf-9 in the ER until it makes a complex with other proteins, as a result of which the domain would be sterically masked and the complex would then exit the ER and go through the secretion pathway. As Cf-9 does not have an obvious signalling domain, formation of complexes with other proteins could provide this function. In principle, the insertion of 41 amino acids containing the triple myc tag could compromise this masking and enhance ER retrieval; however, the c-myc : Cf-9 with the tag in G domain (9161) was still primarily located in the plasma membrane. It is noteworthy that the Cf-2 protein is of a broadly similar structure to Cf-9, but does not carry the KKxx motif at the C-terminus ( Dixon et al. 1996 ).
RPM1, an Arabidopsis gene that confers recognition of an Avr protein that is pumped into the plant cell by a Pseudomonas type III secretion system, is associated with the plasma membrane and turns over upon elicitation ( Boyes et al. 1998 ). Thus even R proteins that would be predicted to be cytoplasmic can be plasma-membrane associated. The tomato Pto gene has a putative myristoylation site, again suggesting association with the plasma membrane ( Martin et al. 1993 ). With the data in this paper showing that c-myc : Cf-9 is in the plasma membrane, it appears that this compartment plays a crucial role in initiating R gene-dependent signalling.
Cf-9 is required for resistance of tomato to races of C. fulvum carrying Avr9. However, a high-affinity binding site for Avr9 in tomato membranes has been reported even in tomato genotypes that lack Cf-9 ( Kooman-Gersmann et al. 1996 ). This Avr9-binding protein is widespread in Solanaceous species. The fact that c-myc : Cf-9 is located in the plasma membrane, and that Avr9 peptide is secreted to the apoplastic space by the fungus, is consistent with an important role for Cf-9 in Avr9 recognition. At present the molecular details of this recognition event are unknown. It is plausible that Cf-9 may recognize a conformational change in the Avr9 receptor, in which case c-myc : Cf-9 may be a useful tool to investigate the Avr9-recognition complex. We are currently establishing tobacco suspension cultures of Cf-9 myc-tagged plants to facilitate the investigation of the earliest Avr9-dependent changes in the Cf-9 complex.
Experimental procedures
Vector construction
The triple c-myc coding sequence was amplified by PCR from the plasmid CB2339 (a gift of Kim Arndt) to create EcoRI and HindIII cloning sites. The primers used were 5′ ATG GAA TTC GGT GAA CAA AAG TTG 3′ (creating an EcoRI site 5′) and 5′ ATG AAG CTT TCC GTT CAA GTC TTC TTC T 3′ (creating a HindIII site 3′). The amplified fragment was cloned in KS Bluescript after digestion with EcoRI and HindIII restriction enzymes. The EcoRI/HindIII fragment was inserted in two different positions in a Cf-9 clone from which the internal EcoRI and HindIII restriction sites were removed (SLJ8501).
Cf-9 clones with the myc inserted were created as follows. For clone SLJ9161, EcoRI and HindIII restriction sites were created in the G domain by oligomutagenesis (5′ TTT CAT TTT CGT AGT AAG CTT CAT GAA TTC AAT TAT GTG TTC CAA 3′). The underlined restriction sites were used to clone the HindIII–EcoRI fragment containing the triple c-myc. A ClaI restriction site was created by oligomutagenesis at the translational initiation ATG of Cf-9 to allow cloning of the resulting c-myc : Cf-9 behind the 35S promoter. The 35S promoter (as an EcoR–ClaI fragment from the clone SLJ4K1; Jones et al. 1992 ), and the final Cf-9 with the myc tag in the G domain (as a ClaI–BamHI fragment) were cloned in the binary vector SLJ7291 digested with EcoRI and BamHI to generate the vector SLJ9161. Clone SLJ9171 was constructed as for SLJ9161 but using the primer (5′ TTC GGG GCA CAA ATG AAG CTT CAT GAA TTC AGG CAA GGA TGA GGA 3′) to create the cloning sites EcoRI and HindIII in the B domain-encoding DNA of Cf-9.
Plant transformation and growth conditions
The binary clones SLJ9161 and SLJ9171 were mobilized into Agrobacterium tumefaciens LBA4404, and introduced into tobacco cultivar Petite Havana (Nicotiana tabacum) by the method of Horsch et al. (1985) . At least 10 independent transformants of each construct were selected for further analysis. To select the subset of primary tobacco transformants carrying single T-DNA integration sites, T2 progeny seed was plated on Murashige & Skoog (1962) medium containing 300 μg ml−1 kanamycin sulphate, and the ratio of kanamycin-resistant to sensitive seedlings was determined. Plants were grown as described in Hammond-Kosack et al. (1998) .
Generation of suspension cultures
Cultures were generated from leaf pieces taken from 9171 tobacco plants as described by Piedras et al. (1998) .
Treatment of tobacco plants
IFs from Petit Havana tobacco plants, either non-transgenic or expressing the Avr9 peptide (SLJ6201 A) ( Hammond-Kosack et al. 1994 ), grown under the same conditions, were extracted after infiltration with distilled water as described by De Wit & Spikman (1982) and frozen at −20°C until use. The IF from Avr9-expressing plants was designated IF(Avr9+) and the IF from control plants IF(Avr9–). The interveinal regions of fully expanded leaves were injected with both IFs.
Isolation and purification of plasma-membrane vesicles
Leaves were cut into pieces and placed in a homogenizer fitted with razor blades and homogenized in lysis buffer (25 m m Tris–HCl pH 7.5, 0.5 m sucrose, 3 m m EDTA, 0.5 m m 4-(2-aminoethyl)-benzenesulfonyl fluoride, AEBSF) four times for 15 sec. The homogenate was filtered through four layers of miracloth and centrifuged at 6000 g for 10 min. The supernatant was recovered and centrifuged at 100 000 g for 1 h to pellet the microsomal membranes. Microsomes were resuspended in 5 m m potassium phosphate buffer, 0.33 m sucrose, 3 m m KCl, 1 m m AEBSF. Plasma-membrane vesicles were prepared by aqueous two-phase partitioning ( Kjellbom & Larsson 1984) with a final composition of 6.4% (w/w) dextran T500, 6.4% PEG 3350, 330 m m sucrose, 5 m m potassium phosphate pH 7.8, 3 m m KCl. Aliquots of microsomes were dissolved in the phase mixture to a final weight of 28 g. Partitioning was accelerated by 10 min centrifugation at 2000 g in a swinging-bucket rotor. The resulting lower and upper phases were repartitioned twice with fresh phases to produce U3 and L3. Those were then diluted, centrifuged at 120 000 g for 1 h, and resuspended in 5 m m potassium phosphate buffer, 0.33 m sucrose, 3 m m KCl, 1 m m AEBSF. All manipulations were conducted at 4°C.
Deglycosylation experiment
c-myc : Cf-9-containing microsomes were solubilized with 0.1% (octylphenoxy)-polyethoxyethanol (Nonidet P-40) before treatment with the peptide : N-glycosidase F (PNGaseF) (New England Biolabs, Hutchin, UK). The solubilized microsomal fraction (1 mg total protein) was centrifuged at 100 000 g for 1 h at 4°C, and the supernatant was incubated at 100°C for 10 min in denaturing buffer (0.5% SDS, 1% β-mercaptoehanol). The deglycosylation reaction was carried out at 37°C after the addition of 500 units of PNGaseF. Aliquots were taken at different times and the reaction was stopped by addition of loading buffer and boiling. The proteins were subjected to SDS–PAGE and Western blotting with the myc antibodies as described bellow.
Enzymatic activities
H+-ATPase, cytochrome c oxidase (EC 1.9.3.1) and NADPH-cytochrome c reductase (EC 1.6.2.5) were determined according to Schaller & De Wit (1995).
Protein determination
All protein concentrations were determined by BCA protein assay kit (Pierce, Chester, UK) using 1% (v/v) Triton X100. BSA was used as a standard.
SDS–PAGE and immunoblotting
Samples were mixed with loading buffer (with a final concentration of 100 m m SDS and 200 m m DTT) and incubated for 15 min at 60°C. After electrophoresis ( Laemmli 1970), proteins were transferred to nitrocellulose (Amersham, Little Chalfont, UK) by wet electroblotting (Mini-Protean II system; Bio-Rad, Hemel Hempsted, UK). Transfer buffer consisted of 20% (v/v) methanol, 38.64 m m glycine, 47.89 Tris, 1.28 m m SDS.
Western blots were blocked for at least 1 h in PBS-T (137 m m NaCl, 2.7 m m KCl, 4.3 m m Na2HPO4, 1.4 m m KH2PO4, 0.1% Tween 20 pH 7.3) with 5% (w/v) dried milk. Antibodies were diluted in PBS-T at the following concentrations: antiaquaporin (1 : 10 000) raised against the Arabidopsis plasma membrane aquaporin PIP1a ( Kammerloher et al. 1994 ), anti c-myc (1 : 2000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), goat anti-rabbit IgG (1 : 2000) (Sigma, Dorset, UK). Primary and secondary antibody incubations were for 1 h at room temperature and were followed by one wash for 15 min, and two washes for 5 min in PBS-T. Secondary antibodies were detected using alkaline phosphatase or peroxidase (Sigma, Dorset, UK).
Protoplast preparation
Suspension cultures were incubated at 27°C for 3 h in protoplasting medium (0.4 m mannitol, 10 m m ascorbate, 1 m m CaCl2, 0.1% (w/v) Onozuka Cellulase RS, 0.05% (w/v) Onozuka Macerozyme R10, 0.05% (w/v) pectolyase Y23 (Seishin, Tokyo, Japan), 1% (w/v) BSA. Homogenate was filtered through a nylon mesh (80 μm pore size) and the protoplasts were washed three times in 0.55 m mannitol supplemented with 1 m m CaCl2 by low-speed centrifugation (70 g; Labofuge 400 e, Heraeus, Hanau, Germany) and resuspension of the pellet.
Fixation, immunolabelling and silver enhancement
c-myc : Cf-9 protoplasts and protoplasts from untransformed plants were mildly prefixed for 12 h at 4°C (0.3% paraformaldehyde, 0.05% glutardialdehyde, 10 m m phosphate buffer pH 7, 0.47 m mannitol, 1 m m CaCl2) before being post-fixed with 0.05% (w/v) OsO4 for 20 min at room temperature. After washing for 10 min in 25 m m phosphate buffer pH 7, the protopasts were incubated for 30 min in PBS pH 7.4 supplemented with 1% BSA and 0.1% BSAc (Aurion, Wageningen, the Netherlands) as a blocking medium. Protoplasts were then incubated with primary and secondary gold coupled antibodies, respectively (GAM5; BioCell, Cardiff, UK) diluted in PBS pH 7.4 for 1 h at room temperature. Between the two incubations the protoplasts were washed three times with fresh PBS. To allow the visualization of 5 nm gold particles on the protoplast surface, protoplasts were washed twice with distilled water and subjected to silver enhancement for 5 min in the dark (Silverenhancement Kit, BioCell). Samples were then mounted on glass slides and observed and photographed with a Axivert 35 inverted microscope (Carl Zeiss, Oberkochen, Germany), equipped with a plan-neofluar 63×/1,25 oil Ph3 antiflex objective, using epipolarization or bright-field mode. Particle counting and determination of the labelling densities were according to Diekmann et al. (1994) .
Acknowledgements
We thank Kim Arndt (Cold Spring Harbor Laboratory) for providing the triple c-myc, Anton R. Schäffner (Ludwig-Maximilians University, Munich) for supplying the antibodies raised against the plasma-membrane aquaporin from Arabidopsis, Saijun Tang for making available some Cf-9 constructs, and Kate Harrison for transforming tobacco and generating suspension cultures. We also thank Matthew Smoker and Sara Perkins for culture and plant maintenance. We are also grateful to Kim Hammond-Kosack, Martin Parniske and Tina Romeis for helpful discussion. This work was supported by the Gatsby Charitable Foundation and the CAST project (BIO4-96-0101) of the EEC Framework IV program.