IMB1, a bromodomain protein induced during seed imbibition, regulates ABA- and phyA-mediated responses of germination in Arabidopsis
Summary
We report the characterization of a plant gene encoding a member of the BET subgroup of bromodomain proteins, a novel class of putative transcription factors. Imbibition-inducible 1 (IMB1) appears to be a nuclear protein as suggested by subcellular localization in onion epidermal cells using an IMB1–yellow fluorescent protein (YFP) fusion protein. In Arabidopsis thaliana, IMB1 is expressed at very low levels in dry seeds, but is markedly induced during seed imbibition. In addition, IMB1 transcript levels are down regulated during germination. Seeds of a loss-of-function mutant allele, imb1, show impaired cotyledon greening during germination in abscisic acid (ABA) and express higher levels of ABI5 protein than the wild type. Moreover, imb1 seeds are deficient in the phytochrome A (phyA)-mediated very-low-fluence response of germination. Microarray analysis revealed that genes included in different functional categories, such as cell-wall metabolism or plastid function, are repressed in imbibed imb1 seeds. Mutant imb1 plants appear normal, indicating that IMB1 is involved in regulating a specific developmental stage. Taken together, these results show that IMB1 plays a role in the promotion of seed germination by both negatively and positively regulating the ABA and phyA transduction pathways, respectively. In imbibed seeds, IMB1 modulates the transcription of a battery of genes, providing clues on its mode of action.
Introduction
It is becoming clear that the control of plant chromatin modification and remodeling by transcriptional regulators plays a crucial role in gene control and development (Li et al., 2002). As in animals and yeast, the combination, both in kind and number, of histone covalent modifications (including acetylation, phosphorylation, methylation, and ubiquitination) may provide a histone code (Jenuwein and Allis, 2001) whose translation dictates a particular cellular response.
The bromodomain is an acetylated-lysine-binding motif conserved in a diverse array of proteins that associates with acetylated histones and is thought to act as a chromatin-targeting module (Winston and Allis, 1999). The bromodomain-containing BET proteins represent a novel yet poorly understood class of transcriptional regulators, which share an additional unique extra terminal (ET) domain. The latter is a protein–protein interaction motif (Matangkasombut et al., 2000) and consists of three separate regions, only one of which, the N-terminal ET (NET) domain, is conserved in all BET proteins. Unlike BET proteins from animals and yeast, which contain two bromodomains, plant BET proteins appear to carry only one. By associating with chromatin, these proteins might function in either opening chromatin or initiating transcription, and it has been proposed that they interact with acetylated histones via their bromodomain(s), whereas the NET domain serves as an interface to localize different proteins or complexes to chromatin (Florence and Faller, 2001). Characterized members of the BET family include human RING3 (now designated Brd2), murine MCAP (now designated Brd4), Drosophila Fsh, and yeast Bdf1 and Bdf2. Several of these proteins have been shown to affect the transcription of a wide range of genes (Denis et al., 2000; Huang and Dawid, 1990; Lygerou et al., 1994). So far, BET proteins have been implicated in functions such as homeosis, cell-cycle control, or oncogenesis.
Seed germination is a complex adaptive trait of higher plants controlled by a number of environmental signals, such as light, moisture, and temperature, and by the co-action of two internal growth regulators: gibberellin (GA) and abscisic acid (ABA). ABA is known to inhibit, whereas GA promotes germination. The central role of these phytohormones in seed germination has been best elucidated by genetic studies of GA-deficient mutants, which fail to germinate in the absence of exogenous GA, and of ABA-insensitive mutants. Among the identified loci involved in ABA responses, three are known to primarily affect seed sensitivity to the hormone, ABI3–ABI5. Whereas the physiological role of ABI4 remains less clear, ABI5 has been recently shown to act downstream of ABI3 in the ABA transduction pathway to arrest growth after emergence of the radicle from the seed coat (Lopez-Molina et al., 2002). This developmental arrest has been proposed to represent a checkpoint during which the seedling monitors the water status of the environment and maximizes its survival by preventing premature development under unfavorable conditions (Lopez-Molina et al., 2001).
The light environment of seeds is perceived by phytochromes. In fact, the control of seed germination by red light (R) and far-red light (FR) is a widely documented phytochrome-mediated response (Casal and Sánchez, 1998). Of the five Arabidopsis phytochromes A–E (phyA–phyE), phyA and phyB play a well-established role in the photoregulation of seed germination (Shinomura et al., 1996). The classical, photoreversible low-fluence response (LFR) is mediated by phyB, whose activation by R is reversed by FR. On the other hand, induction of germination by FR is mediated by phyA via the very low-fluence response (VLFR). Whereas phyB is present in dry seeds, phyA is synthesized only several hours after imbibition (Sharrock and Clack, 2002). Continuous FR inhibits germination in many plant species (high-irradiance response), but not in Arabidopsis (Botto et al., 1996). Recently, R/FR-reversible seed germination has been reported to be additionally under the control of phyE, which also appears to be required for germination in continuous FR (Hennig et al., 2002). Besides seed germination, phytochromes are known to control flowering time and several aspects of Arabidopsis seedling development including hypocotyl elongation, leaf expansion, and chloroplast differentiation.
Around 10 genes encoding putative BET transcription factors have been identified from the Arabidopsis genome (Florence and Faller, 2001), suggesting that these proteins may serve an important function in plants. However, to date, none of these genes has been characterized. Here, we report the characterization of a plant gene encoding a BET protein, imbibition-inducible 1 (IMB1), and show that it plays a role in ABA- and phyA-mediated responses of seed germination in Arabidopsis.
Results
Imbibition-inducible 1 (IMB1) is a BET protein
To investigate the role of BET proteins in plants, we identified and isolated a gene putatively encoding a bromodomain-containing protein, At2g34900, annotated in the Arabidopsis Genome Initiative database as ‘RING3-like protein’. The cDNA was obtained by RT-PCR, cloned into a binary vector, and sequenced. Comparison with the GenBank sequence revealed that the gene, which we later named IMB1, contains nine exons and eight introns, with the 8th exon being 42 bp shorter than predicted by the conceptual translation in the database.
The IMB1 gene encodes a protein of 386 amino acids with one 89-amino-acid bromodomain and an N-terminal, 64-amino-acid NET domain (Figure 1a) that is unique to the bromodomain subfamily of BET proteins. The amino acid sequences of the two domains are closely related to those of other putative BET transcription factors identified in the Arabidopsis genome (Florence and Faller, 2001). Moreover, both the bromodomain (Figure 1b) and the NET domain (Figure 1c) of IMB1 also show homology to their counterparts in other previously characterized BET proteins, such as human RING3 (Denis et al., 2000), murine MCAP (Dey et al., 2000), Drosophila Fsh (Haynes et al., 1989), and yeast Bdf1 (Lygerou et al., 1994).
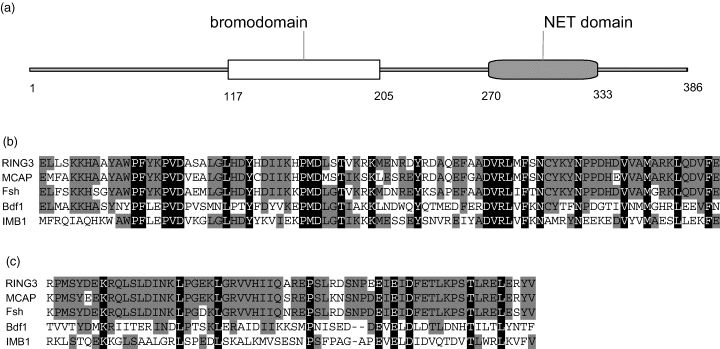
IMB1 is a BET protein.
(a) Schematic representation of the 386-amino-acid IMB1 protein, showing the positioning of the bromodomain and the NET domain.
(b) Amino acid sequence alignment comparing the second bromodomain of human RING3, murine MCAP, Drosophila Fsh, and yeast Bdf1 with the bromodomain of Arabidopsis IMB1. Identical amino acid residues are highlighted in black; gray indicates conservative substitutions.
(c) Amino acid sequence alignment of the NET domain of BET proteins RING3, MCAP, Fsh, Bdf1, and IMB1. Identical amino acid residues are shown in black and conserved residues in gray.
Nuclear localization of IMB1
The IMB1 amino acid sequence contains a C-terminal putative bipartite nuclear localization signal. In addition, bromodomain-containing proteins have been found to be associated with chromatin. Therefore, the prediction was that IMB1 would function in the nucleus. To verify the subcellular localization of IMB1, onion epidermal cells were transiently transformed with an IMB1–YFP fusion protein under the control of the cauliflower mosaic virus (CaMV) 35S promoter. In contrast to the control YFP protein, which was distributed throughout the cytoplasm and the nucleus (Figure 2a), the IMB1–YFP fusion protein was localized exclusively in the nuclei of onion cells (Figure 2b). These results strongly suggest that IMB1 is indeed a nuclear protein.
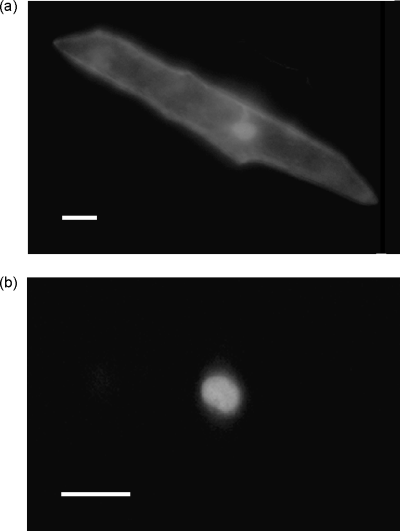
IMB1 localizes to the nucleus of onion cells.
Representative images of the subcellular localization of the IMB1–YFP fusion protein in transiently transformed onion epidermal cells. YFP fluorescence was visualized 16–20 h after bombardment with plasmids expressing the fusion gene transcribed from a 35S promoter. Scale bar, 50 µm.
(a) A control cell expressing YFP alone.
(b) A cell expressing the IMB1–YFP fusion protein.
Expression pattern of IMB1
Northern blot analysis was used to investigate the expression of IMB1 in different tissues and at different stages of the life cycle in Arabidopsis thaliana. The IMB1 transcript was barely detectable in stems, leaves, siliques, and dry seeds, but was present at considerable levels in roots and flowers (Figure 3a). Furthermore, we assessed steady-state levels of the IMB1 transcript at different time points during seed imbibition, the process of water uptake by dry seeds, which is considered to trigger germination (Figure 3b,d). We also investigated the expression pattern of IMB1 at different stages of seed germination (Figure 3b,c).
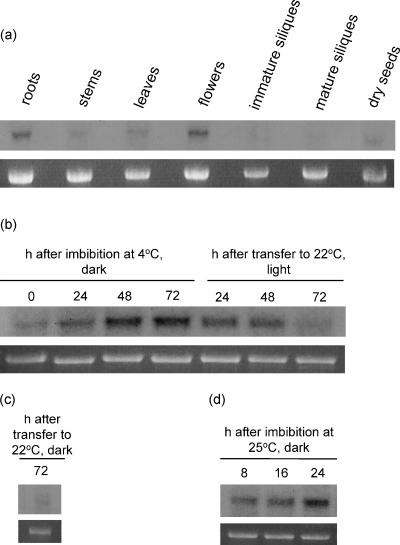
IMB1 is expressed in flowers, roots and imbibed seeds.
Northern blot analysis of the IMB1 transcript in wild-type A. thaliana. Each lane contained 7 µg of total RNA. Ethidium-bromide-stained 25S RNA is shown as a loading control.
(a) IMB1 expression levels in different plant tissues.
(b) Induction of IMB1 expression during seed imbibition in darkness at 4°C, and its down regulation during germination in light at 22°C.
(c) IMB1 transcript levels in seeds allowed to germinate 72 h in darkness at 22°C after imbibition in darkness at 4°C for 72 h.
(d) Induction of IMB1 expression during seed imbibition in darkness at 25°C.
The IMB1 transcript was barely detectable in dry seeds, but its levels increased markedly during seed imbibition at 4°C in darkness (stratification). Consequently, IMB1 was expressed at high levels after 72 h of stratification (Figure 3b). When the seeds were transferred to 22°C in light, germination (radicle emergence from the seed coat) occurred rapidly and was accompanied by a steep decline in the levels of IMB1 transcript. Indeed, the transcript was barely detectable 72 h after stratification (Figure 3b), at which time cotyledon expansion and greening had already occurred (data not shown). If the seeds were transferred to 22°C but kept in the dark, germinated seedlings exhibited as expected elongated hypocotyls and etiolated cotyledons (data not shown) and the IMB1 transcript was also barely detectable after 72 h (Figure 3c); expression levels were therefore comparable to those observed when the seeds were allowed to germinate in light for the same period of time (Figure 3b). This indicates that the down regulation of IMB1 expression during germination is light-independent. In addition, if seed imbibition occurred in darkness but at 25°C, IMB1 was similarly induced except that the rise in transcript levels occurred more rapidly than at 4°C, with the gene being highly expressed after 24 h (Figure 3d). This indicates that the observed up regulation of IMB1 transcript levels during seed imbibition is temperature independent.
Molecular characterization of an IMB1 knockout
To gain insight into the function of IMB1, we screened a Ds-transposant collection (Parinov et al., 1999; Sundaresan et al., 1995) for transposon insertions within the IMB1 gene. To this end, the genomic sequence was used to screen a database containing the sequences adjacent to the insertion sites within the transposant collection (Parinov et al., 1999). One line (SET6311) with an insertion in IMB1, hereafter referred to as imb1, was identified. Sequencing of PCR fragments obtained using primers flanking the insertion site and a 5′-end Ds primer confirmed that the transposon was inserted in the first intron of IMB1, 239 bp downstream of the ATG start codon, in the orientation indicated in Figure 4(a). Moreover, comparison of PCR results using the same primers allowed the identification of imb1 lines homozygous for the transposon insertion (data not shown).
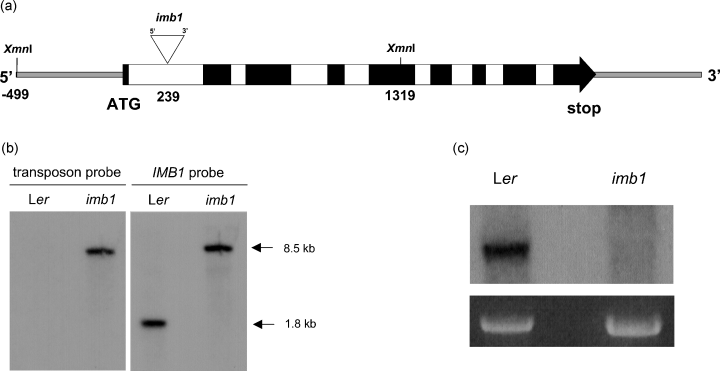
Molecular characterization of imb1.
(a) Schematic representation of the IMB1 gene, showing the site of insertion and orientation of the Ds element in the imb1 mutant (not to scale). Exons (black), introns (white), non-coding regions (thin, gray-filled line), XmnI restriction sites, and the Ds insertion site (▿) are shown. The Ds element is inserted 239 bp from the ATG start codon as indicated.
(b) Southern blot analysis of Ler and imb1 genomic DNA digested with XmnI, using a transposon or an IMB1 probe.
(c) Northern blot analysis of the IMB1 transcript in Ler and imb1 seeds imbibed in darkness at 4°C for 72 h. Each lane contained 7 µg of total RNA. Ethidium-bromide-stained 25S RNA is shown as a loading control.
Southern blot analysis of XmnI-digested Landsberg erecta (Ler) and imb1 genomic DNA using a hybridization probe for the Ds insertion revealed, in the imb1 lane, a single band of the expected size (the Ds element is approximately 6.7 kb), which was absent in DNA from Ler (Figure 4b, transposon probe). This indicated that the mutant line contained a single transposon insertion. Hybridization of the same membrane with a probe derived from IMB1 identified the same band (approximately 8.5 kb) observed with the transposon probe in the imb1 lane and, as predicted from the XmnI restriction map, an approximately 1.8-kb band in the Ler lane (Figure 4b, IMB1 probe). This confirmed that the gene was indeed interrupted in the imb1 mutant, and that the lines isolated using the PCR strategy were, in fact, homozygous for the insertion.
Northern blot analysis using an IMB1 probe detected a single band of approximately 1.2 kb in total RNA from Ler seeds imbibed for 72 h at 4°C (Figure 4c). This corresponds approximately to the size of the isolated IMB1 cDNA. No band could be detected in RNA from homozygous imb1 imbibed seeds (Figure 4c), suggesting that imb1 is a true null mutant.
Phenotypic analysis of the imb1 mutant
Adult imb1 knockout plants appeared normal, and their general morphology was indistinguishable from wild-type Ler plants (data not shown). As the IMB1 transcript was detected at considerable levels in roots and flowers (see Figure 3a), particular attention was paid to these organs. Root morphology, flower morphology, and flowering time of mutant and wild-type plants did not show appreciable differences (data not shown). On the other hand, the characteristic seed expression pattern of IMB1 suggested that the gene might be involved in the regulation of seed dormancy and/or germination. We therefore compared several seed germination responses in Ler and imb1 plants.
imb1 seeds are hypersensitive to ABA-mediated inhibition of cotyledon greening
Disruption of the IMB1 gene did not affect seed dormancy or the timing of germination, and neither the cold nor the light requirement for seed germination was affected (data not shown). Likewise, when germination rates were scored under conditions of osmotic stress (imposed by 0.45 m mannitol), no difference was apparent between wild-type and mutant seeds (data not shown). In addition, IMB1 does not appear to control gibberellin-regulated germination responses, as sensitivity to neither GA3 nor paclobutrazol (an inhibitor of gibberellin biosynthesis) was affected in the knockout mutant (data not shown). By contrast, although no appreciable differences were observed when radicle emergence from the seed coat in different ABA concentrations was scored (Figure 5a), imb1 seeds showed an ABA-hypersensitive response to the expansion and greening of cotyledons (Figure 5b,c). After stratification and 14 days in 1 µm ABA, around 15% of the Ler seedlings exhibited expanded green cotyledons, whereas only approximately 3% of imb1 seedlings had turned green (Figure 5b,c). At lower ABA concentrations (0.5 µm), rates of cotyledon greening were greater than 80% and indistinguishable between the two genotypes, whereas both Ler and imb1 seedlings were incapable of greening in the presence of 1.5 µm ABA (data not shown).
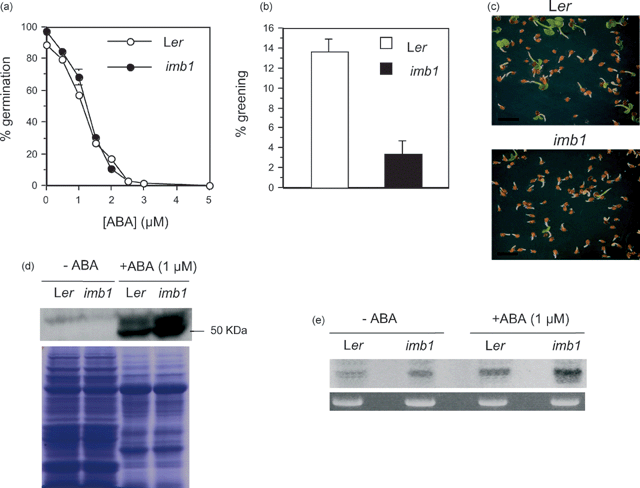
imb1 seeds are hypersensitive to ABA inhibition of cotyledon expansion and greening.
(a) Germination (scored 7 days after stratification) of Ler (○) and imb1 (●) seeds in different ABA concentrations. Percentages are averages of triplicates ± SE.
(b) Greening of expanded cotyledons (scored 14 days after stratification) of Ler (□) and imb1 (▪) seeds in 1 µm ABA. Percentages are averages of triplicates ± SE.
(c) Representative images of 14-day-old Ler and imb1 seedlings grown in the presence of 1 µm ABA. Scale bar, 2 mm.
(d) Western blot analysis using antibodies raised against ABI5 in 14-day-old Ler and imb1 seedlings grown in the presence or absence of 1 µm ABA. Coomassie-stained protein gel is shown as a loading control.
(e) Northern blot analysis of the ABI5 transcript in 14-day-old Ler and imb1 seedlings grown in the presence or absence of 1 µm ABA. Each lane contained 7 µg of total RNA. Ethidium-bromide-stained 25S RNA is shown as a loading control.
Although imb1 seedlings show ABA hypersensitivity, the IMB1 seed expression pattern was unchanged by ABA; no differences were observed when IMB1 transcript levels of wild-type seeds imbibed and germinated for 0, 1, 2, and 4 days in 5 µm ABA were analyzed by Northern blotting (data not shown).
The ABI5 protein is required for an ABA-dependent developmental arrest, which occurs after radicle emergence and prior to the onset of autotrophic growth (Lopez-Molina et al., 2001). This knowledge prompted us to investigate whether the ABI5 protein would be present at higher levels in imb1 seeds, which are hypersensitive to the ABA inhibition of cotyledon expansion and greening during the germination process (Figure 5b,c). Western blot analysis revealed that indeed ABI5 was up regulated in imb1 seedlings grown in 1 µm ABA; as expected, the protein was not detectable in control seedlings grown in the absence of ABA (Figure 5d). Matching results were obtained by Northern blotting, in which the ABI5 transcript was detected at nearly threefold higher levels in imb1 seeds germinated in ABA when compared to the wild-type counterpart (Figure 5e). Similar analysis of ABI3, which acts upstream of ABI5 to execute the growth arrest of embryos germinating in ABA (Lopez-Molina et al., 2002), revealed no appreciable differences in the levels of the protein between wild-type and mutant seedlings, with the ABI3 transcript being undetectable in Northern blots (data not shown).
imb1 seeds show deficient phyA-mediated induction of germination
We next investigated whether IMB1 would be involved in the photoregulation of seed germination. In Arabidopsis, light-induced germination is controlled primarily by phyA and phyB (Shinomura et al., 1996). Promotion of germination by FR pulses constitutes a VLFR and is mediated by phyA (Botto et al., 1996), which is synthesized during seed imbibition (Sharrock and Clack, 2002). By contrast, phyB is present in freshly imbibed seeds (Sharrock and Clack, 2002) and mediates the R/FR-reversible LFR (Casal et al., 1998). In the present study, seed germination rates in darkness after exposure to pulses of FR and/or R were scored. Seeds were irradiated with FR for 30 min immediately after imbibition to minimize the levels of active phyB and then stratified for 3 days before any light treatment.
Exposure of Ler and imb1 seeds to a pulse of R induced high germination rates (approximately 90–95%), with no significant difference being observed between the two genotypes (Figure 6). By contrast, an FR pulse, which is incapable of triggering germination of the phyA null mutant (Shinomura et al., 1996), led to decreased germination rates. Importantly, imb1 seeds germinated at considerably lower rates (approximately 35%) than the wild type (approximately 65%), indicating reduced phyA function in the imb1 mutant (Figure 6). Irradiation with R followed by FR resulted in germination rates comparable to those observed after FR alone (data not shown), whereas the lowest germination frequencies (approximately 20%) were recorded after no light treatment (Figure 6). In addition, no differences between Ler and imb1 germination rates or hypocotyls lengths were registered after exposure to continuous FR for 3 days (data not shown). In summary, light experiment results demonstrate that the imb1 mutant shows deficient phyA-mediated VLFR of germination.
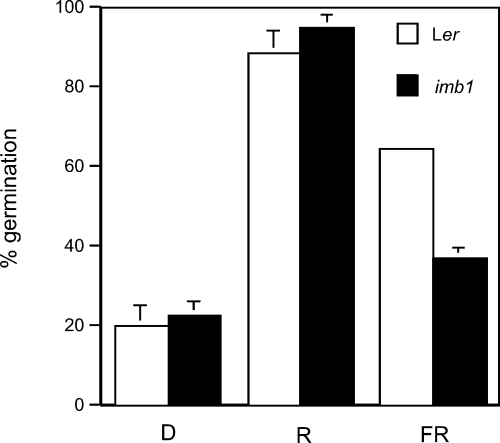
imb1 shows deficient phyA-mediated VLFR of seed germination.
Germination of Ler (□) and imb1 (▪) seeds in the dark (D) or after a 10-min pulse of R or FR (20 µmol photons m−2 sec−1). After sowing, seeds were irradiated with FR for 30 min and incubated for 72 h in darkness at 4°C before the indicated light treatments. Germination rates were scored after further incubation for 5 days in darkness at 25°C. Percentages are averages of triplicates ± SE.
Complementation of the imb1 phenotypes
To test for complementation, the imb1 mutant was transformed with the full-length wild-type IMB1 cDNA expressed under the control of a 35S promoter. The empty vector was used as a negative control, and three independent transgenic lines were characterized. After an FR pulse, all three transgenic lines showed almost complete (approximately 85%) restoration of Ler germination levels (Figure 7a). Furthermore, unlike the control transgenic line, a fraction of the seedlings (approximately 20%) from the complemented mutant lines was able to green in 1 µm ABA (Figure 7b). The finding that IMB1 was expressed at high levels transiently in imbibed seeds had already pointed to a germination-specific phenotype later observed in the knockout mutant. Taken together, results from complementation analysis show that the loss of IMB1 function is indeed responsible for the imb1 mutant phenotypes.
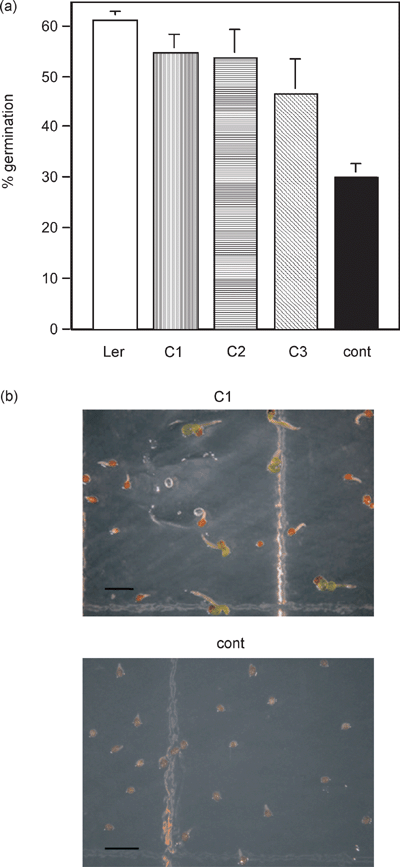
Complementation of imb1 mutant phenotypes by the IMB1 gene.
(a) Germination of Ler and three independent imb1 transgenic lines (C1, C2, and C3) carrying the wild-type IMB1 cDNA under the control of a 35S promoter or the empty vector (cont). After sowing, seeds were irradiated with FR for 30 min and incubated for 72 h in darkness at 4°C before exposure to a FR pulse. Germination rates were scored after further incubation for 5 days in darkness at 25°C. Percentages are averages of triplicates ± SE.
(b) Representative images of 28-day-old seedlings of a complemented (C1) and the empty vector (cont) transgenic lines grown in the presence of 1 µm ABA. Scale bar, 2 mm.
Microarray analysis
In an attempt to gain clues on the molecular mechanisms by which the putative transcription factor IMB1 might be regulating germination responses, genomic-scale expression-profiling experiments were performed. Using commercially available Arabidopsis Affymetrix gene chips, transcript levels in Ler and imb1 seeds imbibed for 72 h at 4°C in darkness were compared to determine which genes showed the greatest change in expression in the imb1 mutant relative to the wild type. Two independent extractions for each genotype were performed, generating four comparisons. Among approximately 24 000 different Arabidopsis genes represented in the arrays, 29 were found to be repressed at least twofold in the imb1 mutant in all four comparisons (Table 1), whereas applying the same criteria only five genes were found to be induced (Table 2). Down regulation of five of the 29 genes (At2g34900, AJ278354, At4g30270, At2g05790, and At2g37640) was confirmed by Northern blot analysis (data not shown). When less stringent criteria were applied and genes induced or repressed at least twofold in three out of the four comparisons were selected (see Tables S1 and S2), 139 genes were found to be down regulated whereas only six were found to be up regulated in the imb1 mutant. Results from the microarray analysis point to a role of IMB1 in modulating transcript levels of genes with diverse functions.
| Locus | Product | Description | FC |
|---|---|---|---|
| At3g47320 | Putative protein | Several A. thaliana hypothetical proteins | >50 |
| At2g34900b | IMB1 | Putative BET transcription factor | 36.8 |
| At2g05380 | Unknown protein | Glycine-rich protein | 15.2 |
| At4g22756 | Unknown protein | Similar to A. thaliana sterol 4-α-methyl oxidase (GI:16973471) | 13.2 |
| At1g06350 | Putative δ 9 desaturase | Fatty acid desaturase family protein | 8.1 |
| AJ278354a,b | ndhD/psaC | NADH dehydrogenase subunit4/photosystem I 9-kDa protein | 7.3 |
| AP000423a | ndhE | NADH dehydrogenase ND4L | 6.5 |
| At4g30270b | Xyloglucan endotransglycosylase | Identical to endo-xyloglucan transferase (GI:944810) | 5.3 |
| At3g49660 | Putative G-protein β-subunit | Similar to S. pombeβ-transducin WD repeat protein | 5.0 |
| X99278a | ndhG | NADH dehydrogenase ND6 | 4.8 |
| At1g71870 | Hypothetical protein | 4.7 | |
| At1g08540 | Putative plastid RNA polymerase σ subunit | Similar to A. thaliana SigB (GI:2443357) | 4.6 |
| AY122394a | ndhF | NADH dehydrogenase ND5 | 4.2 |
| At1g68560 | Glycosyl hydrolase family 31 | Identical to α-xylosidase precursor (GI:4163997) | 4.2 |
| At2g41650 | Unknown protein | 4.0 | |
| At4g39330 | Cinnamyl-alcohol dehydrogenase CAD1 | 3.9 | |
| At1g01610 | Hypothetical protein | 3.9 | |
| At3g03780 | Putative methionine synthase | Similar to A. thaliana cobalamine-independent methionine synthase (GI:2738248) | 3.6 |
| At2g21260 | Putative mannose-6P reductase | Similar to A. graveolens NADPH-dependent mannose-6P reductase (GI:1835701) | 3.5 |
| At4g21910 | Putative protein | 3.4 | |
| At2g05790b | Glycosyl hydrolase family 17 | Putative β-1,3-glucanase | 3.4 |
| At2g37640b | Putative expansin | Identical to A. thalianaα-expansin 3 precursor (GI:20138162) | 3.4 |
| At1g63680 | Putative UDP-glutamate ligase | Similar to E. coli murE (GI:127538) | 3.3 |
| AY007473a | psbJ | Photosystem II component | 3.2 |
| At3g18960 | Hypothetical protein | 3.2 | |
| At5g41600 | Putative protein | Contains similarity to 24-kDa seed maturation protein | 3.1 |
| At1g68400 | Putative transmembrane protein kinase | Similar to P. integrifolia receptor kinase (GI:498278) | 3.1 |
| At1g68530 | Fatty acid condensing enzyme (CUT1) | Identical to very-long-chain fatty acid condensing enzyme (GI:5001733) | 3.1 |
| At1g71100 | Putative ribose 5-phosphate isomerase | Similar to M. musculus ribose 5-phosphate isomerase (GI:6677767) | 3.0 |
- FC – Fold change values representing average ratios of hybridization signals (wild type/imb1).
- Total RNA extracted from seeds imbibed for 72 h at 4oC in darkness.
- a Plastid-encoded genes.
- b Down-regulation confirmed by Northern blot analysis.
| Locus | Product | Description | FC |
|---|---|---|---|
| At1g58025 | Hypothetical protein | Contains similarity to M. musculus cell proliferation related protein (GI:9931486) | >50 |
| At3g27473 | Hypothetical protein | Similar to A. thaliana hypothetical protein (GI:6996256) | 36.8 |
| At1g42705 | Tam3-like transposon protein | Similar to GI:100489 from A. majus | 24.3 |
| At1g67865 | Unknown protein | 13.2 | |
| At3g23085 | Putative Ac-like transposase | Similar to Z. mays ORF2 (GI:22491) | 7.3 |
- FC – Fold change values representing average ratios of hybridization signals (imb1/wild type).
- Total RNA extracted from seeds imbibed for 72 h at 4oC in darkness.
Among the 29 genes showing the most reduced levels in imb1 versus the wild type (Table 1), approximately 25% encode proteins with unidentified function. Not surprisingly, IMB1 was one of the most down regulated genes, supporting the reliability of the data. A considerable fraction (about 20%) of the genes repressed in the imb1 mutant, such as genes encoding a putative expansin or β-glucanases, is likely to be implicated in cell-wall metabolism. Interestingly, five of the 29 down-regulated genes are encoded by the plastid genome. One encodes a component of photosystem II, whereas all the other four appear to be ndh genes, which encode different subunits of the thylakoid NADH dehydrogenase complex. Consistent with this, a nuclear-encoded putative subunit of a plastid RNA polymerase was also significantly less expressed in imb1. Other genes down regulated in the mutant include two genes involved in lipid metabolism and a putative ribose 5-phosphate isomerase.
Using less stringent criteria, an additional set of 110 genes was found to be repressed in imb1 (see Tables S1 and S2). Of these, 10 are also plastid-encoded genes (mostly components of photosystem II and another subunit of the thylakoid NADH dehydrogenase complex). Furthermore, many other genes involved in cell-wall metabolism were identified, such as two additional putative expansins and one xyloglucan endotransglycosylase, a β-xylosidase, an endo-β-1,4-glucanase, and a pectate lyase.
Of the five genes found to be induced (Table 2), the one showing by far the most increased levels in the mutant encodes an Arabidopsis protein similar to the murine BET protein MCAP (Dey et al., 2000). Moreover, of the remaining four genes selected, two encode proteins putatively involved in the regulation of transposable elements, whereas the function of the other two remains unidentified. When the less stringent criteria were considered, only one additional gene was selected as being up regulated in the mutant (see Tables S1 and S2), indicating that IMB1 acts predominantly as a transcriptional activator.
Discussion
Amino acid sequence homology of the bromodomain and the NET domain of IMB1 with those of BET proteins both characterized in other organisms and predicted in the Arabidopsis database indicates that IMB1 belongs to the BET subgroup of the bromodomain superfamily. Although still poorly understood, these proteins were proposed to represent a novel family of transcriptional regulators with broad specificity (Florence and Faller, 2001). Consistent with this, the present study indicates that IMB1 is a nuclear protein involved in the modulation of the transcript levels of an array of different genes in Arabidopsis.
The IMB1 gene displays a characteristic seed expression pattern. The transcript is barely present in dry seeds, but is highly induced during seed imbibition in a temperature-independent fashion. Furthermore, IMB1 transcript levels show a rapid, light-independent decline as the seeds germinate. Therefore, although the transcript is also detectable in roots and flowers, IMB1 is only expressed at a high level transiently following seed imbibition. This expression pattern, centered on a specific physiological state (after water uptake by the seed) at a particular developmental stage (the earliest stages of seed germination), points to a role for IMB1 in the regulation of seed germination. Using a reverse genetics approach, we identified an Arabidopsis mutant line with a transposon insertion in IMB1. Homozygous knockout imb1 plants appear normal, and no root or flower phenotype was observed in spite of IMB1's expression in these organs. This absence of phenotype may be a consequence of the expression of other redundant BET proteins in these tissues. However, imb1 seeds show reduced FR-induced germination when compared to the wild type and are hypersensitive to ABA inhibition of cotyledon greening. Thus, as suggested by the gene's expression pattern, phenotypic analysis of a knockout line indicates that IMB1 is implicated in the control of a particular developmental phase of the Arabidopsis life cycle.
A recent study reported a very similar seed expression pattern for RGL2, an Arabidopsis gene encoding a negative regulator of GA-mediated induction of germination (Lee et al., 2002). However, the IMB1 gene does not appear to be involved in the control of GA responses, as sensitivity to the hormone was not altered in the imb1 mutant and no differences between wild-type and imb1 seeds were observed when germination rates in paclobutrazol, an inhibitor of GA biosynthesis, were scored.
On the other hand, IMB1 appears to affect embryonic ABA signaling. When imb1 seeds germinate in relatively low-ABA concentrations (1 µm), the expansion and greening of cotyledons are impaired compared to the wild type. Although blocking the emergence of the radicle from the seed coat at high concentrations, ABA is known to arrest seedling development after radicle emergence and prevent cotyledon greening at relatively low concentrations. This growth arrest was shown to be mediated by both the ABI5 and the ABI3 proteins, with the latter acting upstream of the former (Lopez-Molina et al., 2002). In agreement with this, imb1 seedlings grown in 1 µm ABA show higher levels of ABI5 protein than the Ler wild type. However, no differences are apparent in ABI3 levels, suggesting that IMB1 acts downstream of ABI3 and upstream of ABI5 in the ABA transduction pathway. The fact that a corresponding ABI5 up regulation in the mutant was observed at the transcript level suggests that IMB1 is involved in the negative regulation of ABI5 expression in the presence of ABA.
Mutant imb1 seeds show normal germination rates when submitted to a pulse of R. However, when the seeds are exposed to an FR pulse, they germinate at considerably lower rates than wild-type seeds. In these experiments, freshly imbibed seeds were irradiated for 30 min with FR and exposed to the light pulse only 3 days after imbibition. As FR inactivates phyB while activating phyA, and the latter phytochrome is only synthesized several hours after imbibition (Sharrock and Clack, 2002), the FR-pulse assay measures the phyA-mediated VLFR (Shinomura et al., 1996). The imb1 mutant is hence specifically impaired in the phyA-mediated induction of seed germination.
Both imb1 mutant phenotypes – hypersensitivity to ABA and reduced VLFR – indicate that IMB1 is implicated in promoting seed germination. This effect is exerted by both negatively and positively regulating the ABA and phyA transduction pathways, respectively. The overlapping role of light and phytohormones in a number of developmental processes has raised the possibility that plant hormones are involved in the sequence of events triggered by phytochromes (Chory et al., 1996). Whether or not light and ABA act independently to affect plant development, it is clear that IMB1 affects both pathways. In agreement with this pleiotropic effect, the nuclear IMB1 protein is a putative transcriptional regulator with broad specificity.
Indeed, microarray analysis shows that the transcription of several genes is significantly altered in the imb1 mutant. The vast majority of these genes is repressed in mutant seeds, indicating that IMB1 acts predominantly as a transcriptional activator. The predicted function of some of the genes whose expression is altered in imb1 provides clues on the mechanisms of action of IMB1.
For example, it is interesting to note that around 20% of the genes showing a significantly lower accumulation of transcript in the imb1 mutant relative to the wild type are involved in cell-wall metabolism. These include an expansin, a β-1,3-glucanase, and a xyloglucan endotransglycosylase. Expansins are known to be involved in cell-wall loosening (Cosgrove, 2000), and up regulation of a tomato expansin gene has been found to be associated with endosperm weakening during seed germination (Chen and Bradford, 2000). Also in germinating tomato seeds, both a β-1,3-glucanase (Wu et al., 2001) and a xyloglucan endotransglycosylase (Chen et al., 2002) are specifically expressed in the endosperm prior to radicle emergence. The promotional effect of IMB1 on germination may therefore include the activation of genes that play an important role in facilitating the breakdown of the mechanical barrier imposed by the endosperm and testa layers surrounding the embryo.
The development of fully functional chloroplasts depends on the coordinate expression of nuclear and chloroplast genes in response to both developmental and environmental signals (Brown et al., 2001). The IMB1 protein also induces the expression of plastid-encoded genes. In fact, five such genes are significantly repressed in the imb1 mutant; one encodes a photosystem II component, psb J, and four encode different subunits of the thylakoid NADH dehydrogenase complex involved in photosynthetic electron transport. Importantly, a gene putatively encoding the σ subunit of a plastid RNA polymerase is also down regulated in imb1. This strongly suggests that transcription of the five plastid genes is being coordinately regulated by this nuclear-encoded RNA polymerase component, providing an indication on the mechanism by which IMB1 regulates chloroplast gene expression. Furthermore, the finding that IMB1 affects chloroplast function suggests a link to both mutant phenotypes of imb1. First, the mutant shows reduced phyA function and phytochromes are well known to control chloroplast differentiation. Second, imb1 seedlings are impaired in their ability to green in the presence of ABA.
Quite surprisingly, knockout of the IMB1 gene considerably induces the expression of an Arabidopsis protein containing similarity to MCAP (Dey et al., 2000), a homolog of IMB1 in mice. Moreover, a Tam3-like transposon protein and a putative Ac-like transposase are also up regulated in the mutant, suggesting that IMB1 may be involved in silencing of transpositional activity.
When the stringency of the criteria for analysis of the microarray data is relaxed, many more genes are found to be down regulated in imb1 (110 additional genes). Analysis of this data set supports the above discussion in that more plastid-encoded genes (e.g. ndhB, genes encoding many components of photosystem II) and genes involved in cell-wall metabolism (e.g. additional expansins, β-glucanases, a xyloglucan endotransglycosylase) are present. By contrast, only one additional gene is found to be up regulated, further supporting the conclusion that IMB1 acts mainly as a transcriptional activator.
Function of the IMB1 protein per se is not essential for seed germination, as the imb1 mutant germinates promptly and develops chloroplasts under standard, optimized germination conditions. In fact, the imb1 phenotype appears to be restricted to germination in relatively low concentrations of ABA and after a FR pulse. The subtlety of the phenotype could be explained by functional redundancy with other Arabidopsis BET proteins. On the other hand, IMB1 may play an important physiological role under stress conditions that do not favor seed germination. However, disruption of the IMB1 gene did not affect germination under experimentally imposed unfavorable conditions (e.g. germination in darkness or high osmolarity).
Insight into the precise mode of action of IMB1 and the possible function of other bromodomain-containing BET proteins holds significant promise for understanding the role of chromatin remodeling in seed germination and early seedling development of higher plants.
Experimental procedures
Plant materials and growth conditions
Seeds from A. thaliana (L.) Heyhn, ecotype Ler, were surface-sterilized, sown in Petri dishes containing Murashige and Skoog (MS) salts (JRH Biosciences, Lenexa, KS, USA), 0.8% agar, and 1% sucrose. Seeds were stratified for 3 days at 4°C in the dark (to break dormancy), before being placed in a growth chamber (22°C, 16-h photoperiod). After 2–3 weeks, plants were transferred to soil in individual pots.
The imb1 Ds-insertion line (derived from the Ler ecotype) was obtained from a previously described Ds-tagging population (Parinov et al., 1999; Sundaresan et al., 1995) and grown as described above. To confirm the exact location of the Ds insertion and determine its orientation, DNA fragments were amplified from genomic DNA (isolated using the Plant DNA Mini Kit; Qiagen, Chatsworth, CA, USA) with primers flanking the Ds insertion, SET-F (5′-AGCAAAGCGTTAGCTGC-3′) and SET-R (5′-AAGAAAGTAGCAGTGCC-3′), and primer DS5-3 (5′-CGGTCGGTACGGGATTTTCC-3′), which anneals at the 5′ end of Ds. The PCR product obtained using SET-F and DS5-3 was then sequenced. The same primers were used to identify homozygous lines for the transposon insertion; genomic DNA from individual plants was used for two PCR reactions – one using SET-F/SET-R and the other SET-F/DS5-3 – and the results were compared. Phenotypic analysis was performed on imb1 homozygous lines backcrossed once with the Ler wild type.
Germination assays
Seed germination is a complex process influenced by multiple factors, such as storage conditions and exposure to light or cold. Germination experiments must therefore be carefully controlled. In this study, Ler and imb1 plants were sown and grown simultaneously under identical conditions. Mature seeds were harvested at the same time from dehydrated siliques and stored in the dark at 4°C. All germination assays were performed with seeds from comparable lots stored for 2–6 months. Batches of 80–160 seeds were sown in triplicate in Petri dishes containing MS salts and 0.8% agar. Average germination percentages were calculated with SEs of the triplicates.
For ABA sensitivity tests, seeds were surface-sterilized and sown on MS salts and 0.8% agar in the presence of the ABA (mixed isomers, A1049; Sigma, St Louis, MO, USA) concentrations indicated in Figure 5. Dishes were incubated in darkness at 4°C prior to transfer to a growth chamber (22°C, 16-h photoperiod). Germination, defined as the obvious protrusion of the radicle through the seed coat, was scored 7 days after transfer to the growth chamber. Greening of expanded cotyledons was scored 14 days after transfer to 22°C.
For light experiments, seeds were surface-sterilized and sown on MS salts and 0.8% agar in a green-safelight chamber and immediately irradiated for 30 min with FR. A 3-day dark treatment at 4°C was followed by exposure to FR or R for 10 min. FR and R were supplied by LED light sources (Quantum Devices, Barnveld, WI, USA) filtered through Plexiglass (WestLake Plastics, Lenni, PA, USA). The fluence rate for both FR and R was 20 µmol photons m−2 sec−1. After the light treatment, seeds were incubated in darkness for 5 days and germination (defined as protrusion of the radicle from the seed coat) was scored. Germination percentages were calculated from the total number of seeds that germinated after a further 5-day incubation in a growth chamber (22°C, 16-h photoperiod).
Northern, Southern, and Western blot analyses
Total RNA was extracted from different tissues of 35-day-old plants using the RNeasy Plant Mini Kit (Qiagen, Chatsworth, CA, USA). RNA from seeds was extracted according to Vicient and Delseny (1999). For Northern blot analysis, 7-µg samples of total RNA were size-fractionated on a formaldehyde-agarose gel and subsequently transferred to a nylon membrane (Duralon, Stratagene, La Jolla, CA, USA) according to standard procedures (Ausubel et al., 1994). After hybridization in 50% formamide, 5% dextran sulfate, 5× SSC, and 0.5% SDS at 42°C with random prime-labeled fragments (Megaprime DNA Labeling System RPN 1604, Amersham Biosciences, Piscataway, NJ, USA), membranes were washed three times with 0.2× SSC and 0.1% SDS at 55°C. As a probe for IMB1, the DNA fragment obtained by PCR amplification of genomic DNA using primers SET-F and SET-R was used. As a probe for ABI5, the DNA fragment obtained by PCR amplification of genomic DNA using primers 5′-CTAGAGAAACGAAGTTGACG-3′ and 5′-CAGCCTTCACCAAGAAATCC-3′ was used.
For Southern blot analysis, plant genomic DNA was extracted as described by Reiter et al. (1992) and digested with XmnI, before being size-fractionated through an agarose gel and transferred to a nylon membrane according to standard procedures (Ausubel et al., 1994). After hybridization in 5% dextran sulfate, 5× SSC, and 0.5% SDS at 65°C with random prime-labeled fragments, membranes were washed as described for Northern blot analysis. As a probe for the transposon insertion, a PCR fragment amplified from the β-glucuronidase (GUS) gene of the Ds insertion with primers 5′-CCATTTGAAGCCGATGTCCACGCCG-3′ and 5′-CCAGTTGCAACCACCTGTTGATCCGC-3′ was used. The same fragment used in Northern blot analyses was used as an IMB1 probe.
Western blot analysis of ABI5 and ABI3 was performed as described by Lopez-Molina et al. (2001).
Isolation of IMB1 cDNA and complementation analysis
First-strand IMB1 cDNA was obtained from total RNA of flower tissue using the ProStar First-Strand RT-PCR Kit (Stratagene, La Jolla, CA, USA) and primers 5′-CCGCTCGAGATGTCTGTACATGTCAA-3′ and 5′-GGTTAATTAATCAAGCTTTCTTAGCTC3′, which added a XhoI and a PacI restriction site, respectively. The same primers were used for PCR amplification of the first-strand cDNA, performed with Tgo DNA polymerase (Roche, Indianapolis, IN, USA). The resulting DNA fragment was digested with XhoI and PacI, ligated into XhoI–PacI-digested binary vector pBA002 (Kost et al., 1998), and sequenced. Comparison with the hypothetical mRNA sequence (At2g34900) in GenBank revealed that the 8th exon was 42 bp shorter than predicted in the annotation.
The empty pBA002 vector and the complementation construct containing the XhoI–PacI fragment described above were introduced into Agrobacterium strain GV3101 and used to transform homozygous imb1 plants by vacuum infiltration (Clough and Bent, 1998). T1 transformants were selected on BASTA-containing medium, grown to maturity, and selfed. Phenotypic analysis of T2 seeds stored for 30–50 days was performed by scoring germination after a FR pulse and after 28 days in 1 µm ABA as described above.
Subcellular localization of a YFP–IMB1 fusion protein in onion cells
The YFP coding sequence (Miyawaki et al., 1997) was cloned into the MluI and SpeI sites of pBA002 to generate pBA–YFP, and the IMB1 cDNA was fused in-frame to the 5′ end of the YFP sequence. To this end, the IMB1-coding region was amplified using primers 5′-GGTTAATTAAATGTCTGTACATGTCAA-3′ and 5′-TCCCCCGGGAAGCTTTCTTAGCTCTTTT-3′, which added a PacI restriction site and a XmaI site while deleting the stop codon, respectively. The resulting fragment was introduced into pBA-YFP, which alone was used as a control. Onion epidermal cells were bombarded with 1.6-mm-diameter gold particles coated with the constructs using a helium-driven biolistic particle delivery system (PDS-1000/He, BIO-RAD, Hercules, CA, USA). Epidermal cells were kept at room temperature in darkness for 16–20 h, before YFP fluorescence was visualized using an Axioskop microscope (Carl Zeiss, Thornwood, NY, USA).
Microarray experiments
For microarray analysis, total RNA from Ler and imb1 seeds imbibed for 72 h at 4°C in darkness was extracted following the method of Vicient and Delseny (1999) and further purified using the RNeasy Plant Mini Kit (Qiagen, Chatsworth, CA, USA). Two independent extractions for each genotype were performed. From each sample, 5–6 µg of total RNA were used for cDNA synthesis, labeling, hybridization, and scanning of the DNA chips carried out at the Gene Array Resource Center of The Rockefeller University according to the manufacturer's instructions (Affymetrix, Santa Clara, USA). Processing of expression levels (raw data) and comparative analysis between experiments were performed using Affymetrix MicroarraySuite 5.0 and Microsoft Excel software. Genes that were up or down regulated at least twofold in relation to the wild type in all four comparisons between the chip experiments (two for imb1, two for Ler) were selected as being misregulated. The average of the four values of fold change was then calculated. In addition, genes induced or repressed at least twofold in the imb1 mutant in three out of the four comparisons (provided that the gene was also found to be up- or down regulated, respectively, in the fourth comparison) are presented as Tables S1 and S2. This microarray analysis conforms to the MIAME recommendations.
Upon request, all biological and chemical materials not commercially available described in this publication will be provided for non-profit research purposes.
Acknowledgements
We gratefully acknowledge V. Sundaresan for providing seeds of the Ds insertion line SET6311. We thank L.-F. Huang for construction of the pBA–YFP vector; S. Mongrand and V. Garreton for assistance with Western blot analysis of ABI5 and ABI3; and P. Hare, N. Krishnamurthy, G. Jedd, J.P. Sánchez, and J. Ouyang for many stimulating discussions. P. Duque was the recipient of a post-doctoral fellowship (PRAXISXXI/BPD/20190/99) from the Portuguese FCT (EU III framework program and MCES). This work was supported by NIH grant GM 44640 to N.-H. Chua.
Supplementary Material
The following material is available from http://www.blackwellpublishing.com/products/journals/suppmat/TPJ/TPJ1848/TPJ1848sm.htm




