Identification of functional lox sites in the plastid genome
Summary
Our objective was to test whether or not cyclization recombination (CRE), the P1 phage site-specific recombinase, induces genome rearrangements in plastids. Testing was carried out in tobacco plants in which a DNA sequence, located between two inversely oriented locus of X-over of P1 (loxP) sites, underwent repeated cycles of inversions as a means of monitoring CRE activity. We report here that CRE mediates deletions between loxP sites and plastid DNA sequences in the 3′rps12 gene leader (lox-rps12) or in the psbA promoter core (lox-psbA). We also observed deletions between two directly oriented lox-psbA sites, but not between lox-rps12 sites. Deletion via duplicated rRNA operon promoter (Prrn) sequences was also frequent in CRE-active plants. However, CRE-mediated recombination is probably not directly involved, as no recombination junction between loxP and Prrn could be observed. Tobacco plants carrying deleted genomes as a minor fraction of the plastid genome population were fertile and phenotypically normal, suggesting that the absence of deleted genome segments was compensated by gene expression from wild-type copies. The deleted plastid genomes disappeared in the seed progeny lacking CRE. Observed plastid genome rearrangements are specific to engineered plastid genomes, which contain at least one loxP site or duplicated psbA promoter sequences. The wild-type plastid genome is expected to be stable, even if CRE is present in the plastid.
Introduction
Expression of the P1 phage CRE recombinase (38.5 kDa) is sufficient to cause recombination between two loxP sites, a 34-bp region consisting of two 13-bp inverted repeats separated by an 8-bp asymmetric spacer sequence. If the loxP sites are in the same orientation, recombination results in deletion of the intervening DNA. If the loxP sites are in opposite orientation, recombination results in inversion of the sequence flanked by the two loxP sites (Adams et al., 1992; Guo et al., 1997; Van Duyne, 2001). The CRE-loxP site-specific recombination system has been explored for integration and excision of foreign DNA, activation of gene expression, and creation of hybrid chromosomes in mammalian cells, yeast, and plants (Hare and Chua, 2002; Hohn et al., 2001; Metzger and Chambon, 2001; Ow, 2002). The CRE-loxP system was also adapted for the removal of marker genes from the plastid genome, and is an attractive target for engineering because it provides readily obtainable high protein levels, the feasibility of expressing multiple proteins from polycistronic mRNAs, and gene containment through the lack of pollen transmission (Bock, 2001; Maliga, 2003; Staub, 2002).
The CRE-loxP marker elimination scheme in plastids, which was developed independently by two laboratories (Corneille et al., 2001; Hajdukiewicz et al., 2001), involves flanking the marker gene with two directly oriented loxP sites. When uniform transformation of all plastid genome copies is achieved, removal of the marker gene is triggered by introduction of a cre gene into the nucleus. The cre gene encodes a CRE recombinase, which is targeted to plastids. Although the desired marker-free plants could be readily obtained, unpredicted plastid genome rearrangements were also detected. The genome rearrangements included deletions of plastid genome segments via short, directly repeated plastid DNA sequences (Corneille et al., 2001; Hajdukiewicz et al., 2001) and recombination between a loxP site and a recombination ‘hot-spot’ (Hajdukiewicz et al., 2001).
Our objective was to test whether or not CRE, when expressed in plastids, induces plastid genome rearrangements. Testing was carried out in plants in which a DNA sequence, located between two inversely oriented loxP sites, underwent repeated cycles of inversions as the means of monitoring CRE activity. We report here CRE-mediated deletion of plastid DNA sequences between a wild-type loxP site and two plastid DNA sequences (plastid lox sites). One plastid lox site is located in the rps12 leader (lox-rps12) and was earlier identified as a ‘recombination hot-spot’. The second plastid DNA sequence is localized within the psbA promoter core (lox-psbA). Deletion products have also been confirmed between two directly oriented lox-psbA sites. Deletion via directly repeated rRNA operon promoter (Prrn) sequences was also frequent in CRE-active plants. However, CRE-mediated recombination was not directly involved, as no recombination junction between loxP and Prrn could be observed. CRE activity yielded a series of plastid genome deletion derivatives, which were a minor fraction of the plastid genome population. Tobacco plants carrying the deleted plastid genomes were fertile and phenotypically normal suggesting that the absence of essential genes was compensated by expression of genes from wild-type genome copies. The deleted genome copies disappeared in the seed progeny lacking cre.
Results
Experimental design
To test for CRE-induced genome rearrangements, plants were created in which a DNA sequence, located between two inversely oriented loxP sites, underwent repeated cycles of inversions. The DNA sequence undergoing inversions was the neo coding region, encoding neomycin phosphotransferase (NPTII) that confers kanamycin resistance. The neo coding region in the transformation vector (pSAC38) is in an inverted orientation relative to the promoter (>neo<, > and < symbolize loxP sites) (Figure 1a). Introduction of CRE into pSAC38-transformed plastids was to trigger inversion of the >neo< coding region enabling transcription of the neo sense strand by the Prrn promoter. The system was designed to allow monitoring of CRE activity by repeated cycles of >neo< inversions between two inversely oriented loxP sites, which maintains a plastid genome population with both neo orientations. Incorporation of the >neo< transgene and selectable marker aadA in the inverted repeats increases the plastid genome size from 155.9 to 160.7 kb.
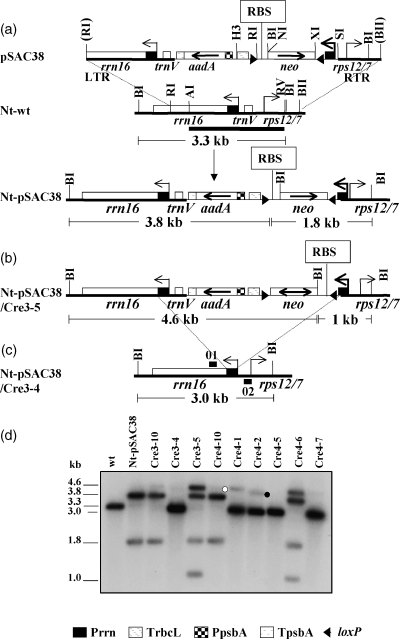
CRE-mediated plastid genome rearrangements in Nt-pSAC38 plants.
(a) Map of the plastid targeting region of transformation vector pSAC38; the cognate region of wild-type plastid genome (Nt-wt); and map of the plastid genome after transformation with plasmid pSAC38 (Nt-pSAC38). Positions of plastid genes rrn16, trnV and 3′rps12/rps7, aadA, and >neo< transgenes, left targeting region (LTR) and right targeting region (RTR), relevant restriction sites (AI, ApaI; BI, BamHI; BII, BglII; RI, EcoRI; RV, EcoRV; H3, HindIII; NI, NcoI; SI, SacI; and XI, XbaI) and loxP sites (filled triangles) are marked.
(b) Map of Nt-pSAC38 plastid genome with >neo< gene expressed after transformation with plastid-targeted nuclear cre (line Nt-pSAC38/Cre3-5).
(c) Map of Nt-pSAC38 plastid genome with deletion of the trnV-aadA-neo segment by recombination via repeated Prrn sequences. Deletion was observed after Agrobacterium-mediated transformation with a nuclear-encoded, plastid-targeted cre. This structure was present in lines Cre3-4, Cre4-1, Cre4-2, Cre4-5, and Cre4-7 shown in (d).
(d) DNA gel blot analysis confirms CRE activity by detecting mixed populations of expressed and non-expressed >neo< copies in lines Cre3-5 and Cre4-6. Shown are also wild-type (wt) control, parental control without CRE activity (Nt-pSAC38) and an Agrobacterium-transformed plant in which CRE is not expressed (Cre3-10; Cre4-10). Map of deletions in lines Cre3-4, Cre4-1, Cre4-2 Cre4-5, and Cre4-7 is shown in (c). Total leaf cellular DNA was digested with the BamHI restriction endonuclease and probed with the targeting region (P1, 1.9-kb ApaI-EcoRV fragment, heavy bar in (a). Fragment sizes detected by the P1 probe are marked in (a–c). Fragments marked with open and filled circles in the Cre4-1 and Cre4-2 lines are CRE-mediated deletion products (see Figure 6b).
Transformation of wild-type tobacco leaves with plasmid pSAC38 yielded two independently transformed lines: Nt-pSAC38-10C and Nt-pSAC38-9A. All experiments were carried out with the Nt-pSAC38-10C line, which will be referred to as line Nt-pSAC38.
Monitoring CRE activity by NPTII expression
The cre gene was introduced into the nuclear genome of Nt-pSAC38 plants to trigger inversion of the >neo< gene. To ensure plastid targeting of the CRE recombinase, the cre coding region was translationally fused with the Rubisco small subunit (SSU) transit peptide: one with 22 (Cre3 gene) and one with five (Cre4 gene) amino acids of the mature SSU N-terminus. The cre genes were expressed in P2′ promoter and Tnos terminator cassettes. In the Agrobacterium binary vectors, the nuclear cre gene is linked to the gentamycin resistance marker that allows for tracking of cre.
Co-cultivation of Nt-pSAC38 leaves with Agrobacterium resulted in nine independently transformed lines. DNA gel blot analysis confirmed CRE activity in two clones (Cre3-5 and Cre4-6) shown by a mixed population of expressed (4.6- and 1.0-kb fragments) and non-expressed (3.8- and 1.8-kb fragments) >neo< genes (Figure 1d). In two clones, Cre3-10 and Cre4-10, only two hybridizing fragments (3.8 and 1.8 kb) were present. This indicates that cre, although introduced (tested by PCR; data not shown), was not expressed. These clones have been excluded from further studies. In five clones, Cre3-4, Cre4-1, Cre4-2, Cre4-5, and Cre4-7, a smaller than wild-type fragment was found (3.0 kb instead of 3.3 kb). The plastid genome of these clones lacks the trnV-aadA-neo region (Figure 1c), which was deleted by recombination between the native rRNA operon promoter and its derivative (Prrn) driving expression of the >neo< gene, as confirmed by sequencing PCR amplification products. Deletion of plastid DNA sequences between Prrn repeats in CRE-active plants has been reported earlier (Corneille et al., 2001; Hajdukiewicz et al., 2001).
The plastid-targeted cre was also introduced into the Nt-pSAC38 plants by pollination, using the previously characterized strong activator lines Cre1-3 and Cre1-100 (Corneille et al., 2001). DNA gel blot analysis in the segregating seed progeny identified seedlings in which >neo< was present in both orientations (seedlings 2, 3, 27, and 29; Figure 2). An additional minor 3.0-kb fragment is also visible in seedling 3. This fragment is the product of trnV-aadA-neo deletion.
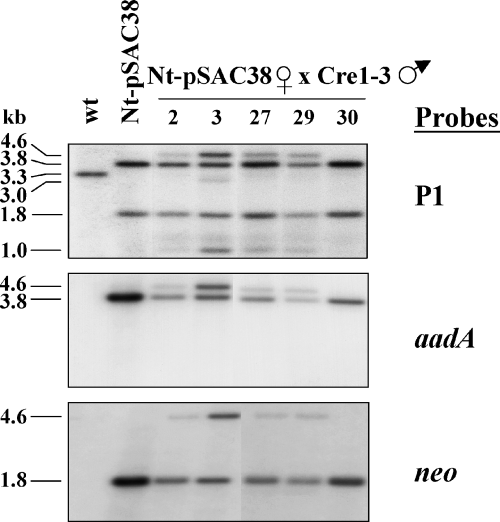
DNA gel blot analysis to test for CRE-mediated >neo< inversion in the seed progeny of Nt-pSAC38 plants (maternal parent) and Cre1-3 activator line (pollen parent).
Total leaf cellular DNA was digested with the BamHI restriction endonuclease and hybridized with the plastid targeting region (P1), aadA and neo coding region probes. For explanation of fragment sizes, see Figure 1(a–c).
Immunoblot analysis of leaf samples was carried out to test for NPTII accumulation in CRE-activated plastids. Levels of NPTII were about 1% of the total soluble cellular protein, about half of the amount found in Nt-pHK31 positive-control plants (Maliga et al., 2002) (Figure 3). Thus, incorporation of a lox site in the 5′ UTR is compatible with significant levels of NPTII expression.
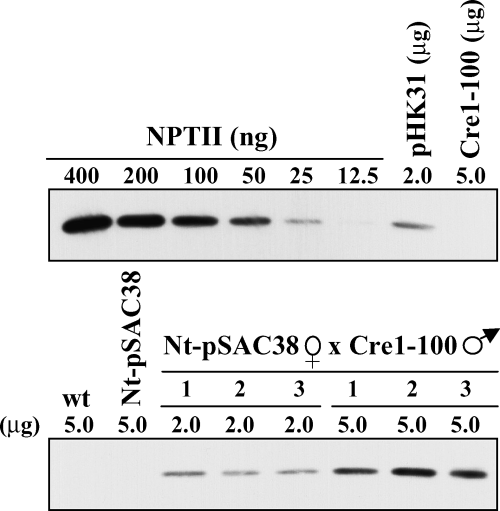
Immunoblot analysis for NPTII accumulation in leaves of CRE-activated Nt-pSAC38 plants.
Quantification of NPTII is by comparison with commercial NPTII dilution series. Controls are non-transformed tobacco (wt), Nt-pSAC38, Nt-pHK31, and Cre1-100 plants.
Testing for CRE-mediated recombination between loxP and plastid sequences
CRE-mediated recombination between loxP sites and pseudo-loxP sites have been observed in yeast (Sauer, 1992, 1996) and mammalian (Thyagarajan et al., 2000) genomes. Characteristic for CRE-mediated recombination is the junction sequence that consists of a perfect 13-bp palindromic CRE loxP binding site, a recombination junction within the spacer, and the host genome sequence with some similarity to the 13-bp loxP palindrome (Sauer, 1992). Unpredicted deletions have also been observed in the plastid genome of plants expressing CRE (Corneille et al., 2001; Hajdukiewicz et al., 2001). To determine if deletions in the plastid genome were mediated by CRE, we have sequenced PCR-amplified recombination junctions. To facilitate communication of the results, we have individually labeled sequences involved in recombination: four transgenic loxP sites: loxP1 and loxP2 in inverted repeat A (IRA), and loxP3 and loxP4 in IRB; three lox-psbA sites: lox-psbA1 in the psbA gene promoter, lox-psbA2 and lox-psbA3 driving aadA in IRA and IRB, respectively; and two lox-rps12 sites located in the rps12 leader (lox-rps12-1, IRA; lox-rps12-2, IRB) (Figure 4c).
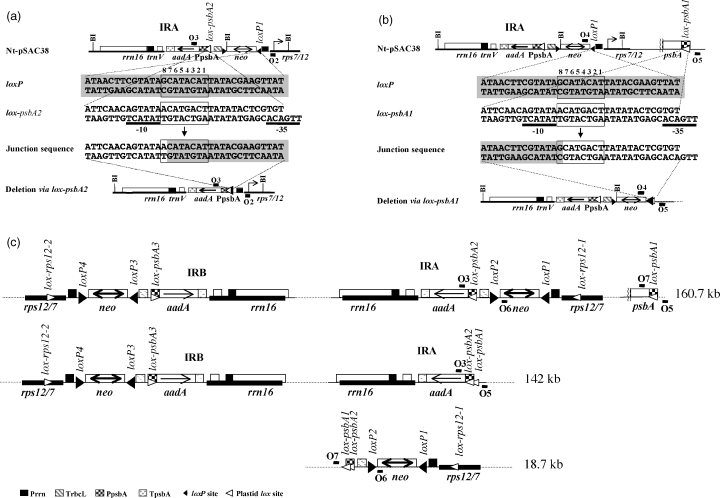
CRE-mediated recombination between loxP1 and lox-psbA and between two lox-psbA sequences.
(a) Recombination between loxP1 and lox-psbA2 sequences.
(b) Recombination between loxP1 and lox-psbA1 sequences.
(c) Recombination between lox-psbA1 and lox-psbA2 sequences. loxP and lox-psbA sites are marked with filled and open triangles, respectively. Genes in the maps are labeled as in Figure 1. Primers used for PCR amplification are also marked.
A recombination junction between the psbA2 promoter and the loxP1 sequence was identified by sequencing the PCR fragment generated by primers O2 and O3 (Figure 4a). Note that the sequences of IRA and IRB are identical, thus identical PCR products are generated from both repeats. In cases such as this, we shall discuss only data for IRA. The recombination junction consists of the psbA promoter sequence (lox-psbA2), most of the loxP1 spacer, and 13 bp of the loxP1 CRE-binding site (Figure 4a).
Encouraged by finding CRE-mediated recombination between loxP1 and lox-psbA2, we also tested recombination between loxP1 and the native psbA promoter (lox-psbA1). Sequencing of the PCR products (primers O4 and O5) confirmed CRE-mediated recombination between loxP1 and the native psbA promoter (lox-psbA1) (Figure 4b) indicating a 17.3-kb deletion in the plastid genome. This genome segment encodes a total of 10 genes. Deletions were also tested between the directly oriented lox-psbA2 site in IRA and the lox-psbA1 site at its native location in the large unique region (Figure 4c). Sequencing of PCR-amplified products confirmed deletion of sequences between lox-psbA2 and lox-psbA1 sequences (primers O3 and O5; 18.7-kb deletion yielding a 142-kb plastid genome) and lox-psbA1 and lox-psbA2 sequences (primers O6 and O7; 142-kb deletion yielding a 18.7-kb plastid genome); the two deletions combined encompass the entire 160.7-kb transplastome.
We also inspected potential recombination junctions between the loxP1 site and the previously described recombination ‘hot-spot’ in the 3′rps12 leader sequence (Hajdukiewicz et al., 2001). PCR amplification using primers O4 and O8 yielded a fragment with a characteristic CRE-mediated junction sequence shown in Figure 5. Sequencing of PCR-amplified products from three different plants yielded an identical junction site. Therefore, the former ‘hot-spot’ sequence was renamed lox-rps12-1.
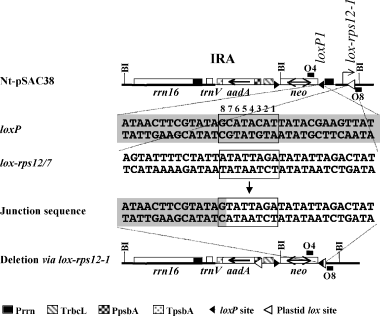
CRE-mediated recombination between loxP1 and lox-rps12-1 sequences.
loxP sites and lox-rps12-1 sequences are marked with filled and open triangles, respectively. Genes in the maps are labeled as in Figure 1. Primers used for PCR amplification are also marked.
Plastid deletion products, in the absence of direct selection, accumulate in readily detectable amounts only if non-essential genes have been excised. Fortunately, some of the deletion products, between loxP1 and lox-psbA2 or lox-rps12-1 and loxP1, are in this category. Figure 6 shows a Southern blot verifying CRE-mediated deletion between the loxP1 and lox-psbA2 (Cre4-2 line) sites and lox-rps12-1 and loxP1 (Cre4-1 line) sites. Interestingly, these deletion products were enriched in plastids in which the trnV-aadA-neo region has been deleted by homologous recombination via Prrn sequences.
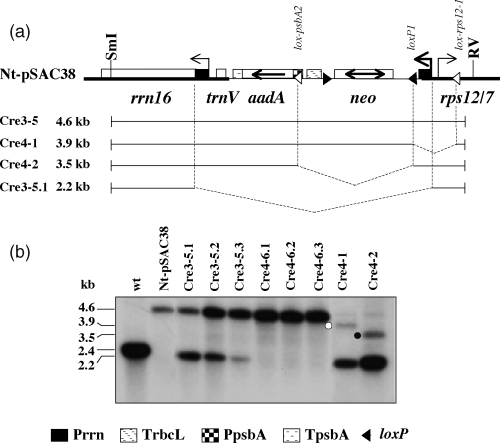
Southern analysis confirms CRE-mediated deletion between the loxP1 and lox-psbA2 (Cre4-2 line) and lox-rps12-1 and loxP1 (Cre4-1) sites.
We also show examples for deletion of the trnV-aadA-neo region in the Cre3-5 seed progeny (Cre3-5.1, Cre3-5.2, and Cre3-5.3), and for uniform transplastomes in the Cre4-6 seed progeny (Cre4-6.1, Cre4-6.2, and Cre4-6.3).
(a) Map of Nt-pSAC38 plastid genome and CRE-mediated deletion derivatives. For explanation, see caption to Figure 1.
(b) Southern blot obtained after digestion of total cellular DNA with the SmaI (SmI) and EcoRV (RV) restriction endonucleases and probing with the plastid targeting sequence (ApaI-EcoRV fragment, Figure 1a). The SmaI-EcoRV fragment includes the entire transgenic region. The 3.5-kb loxP1 and lox-psbA2 fragment in the Cre4-2 line (filled circle) and the 3.9-kb lox-rps12-1 and loxP1 deletion product in the Cre4-1 line (open circle) are marked. Same deletion products after digestion with a different restriction enzyme (BamHI) are marked in Figure 1(d). The 2.2-kb fragment is the product of deletion between the Prrn promoter driving the neo gene and the native rrn promoter sequences.
We have observed deletion of a plastid genome segment by recombination between directly repeated Prrn promoters in Nt-pSAC38 plants containing an active CRE (Figure 1c). Deletion of plastid DNA sequences between directly repeated Prrn promoters has been reported earlier (Corneille et al., 2001; Hajdukiewicz et al., 2001). To determine if these deletions were mediated by CRE, potential recombination junctions were PCR-amplified between the loxP sites in combination with all Prrn promoter sequences (one native rrn promoter and one neo transgene Prrn promoter in each repeat). No evidence was found for CRE-mediated recombination between loxP sites and Prrn sequences.
Testing recombination between loxP and plastid lox sequences identified by computer search
Computer searches were performed to identify sequences that may recombine with the loxP sites in the tobacco plastid genome, using the findpatterns algorithms of the Wisconsin software package. Candidate sequences with similarity to the 34-bp loxP sequence were: three sequences with a 22-bp match; five sequences with a 21-bp match; and 23 sequences with a 20-bp match. The 31 sites were experimentally tested by PCR for recombination with an appropriately oriented loxP site. No recombination junctions were found between any of the plastid sequences and a loxP site (data not shown).
A search was also performed to identify the plastid sites with the best match to structurally important loxP nucleotides (query sequence: ATnACnnCnTATAnnnTAnnnTATAnGnnGTnAT) (Guo et al., 1997; Hoess et al., 1982, 1986; Thyagarajan et al., 2000). Out of the seven best matches with the structurally important nucleotides, two were already identified in the group of 31 sequences tested above. No recombination junctions were found between the remaining five best matches and the nearest properly oriented loxP site (data not shown).
Testing CRE-mediated deletion in the absence of perpetual DNA inversions
We also tested CRE-mediated deletions between loxP and plastid lox sites in the absence of perpetual >neo< inversions. The test plants carried a loxP site and a lox-psbA site in each of the inverted repeats (Figure 7). The plants are seed progeny of >codA> deletion derivatives and segregated for the nuclear cre (Corneille et al., 2001). Out of the nine seedlings shown in Figure 7, eight contain cre. PCR amplification products indicating recombination between loxP1 and lox-psbA1 and between lox-psbA1 and lox-psbA2 were obtained in each of the eight cre-containing seedlings, whereas the recombination products were absent in the seedling lacking cre.
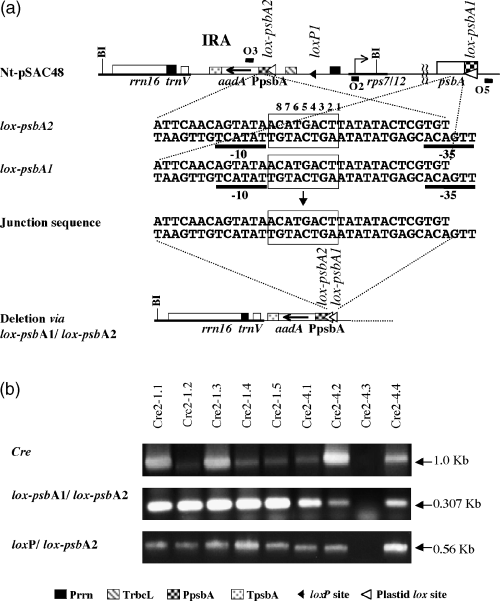
CRE-mediated recombination in Nt-pSAC48 >codA> deletion derivatives.
(a) Plastid map showing recombination between lox-psbA1 and lox-psbA2 sequences and junction sequence. Genes in the maps are labeled as in Figure 1. Primers used for PCR amplification are also marked.
(b) cre, lox-psbA1/lox-psbA2, and loxP1/lox-psbA2 PCR amplification products.
Discussion
CRE activity yields large deletions in the plastid genome
We report here CRE-mediated recombination between loxP and plastid DNA sequences that yielded large deletions in the 160.7-kb transgenic tobacco plastid genome. The test system, a >neo< gene flanked by two inverted loxP sites, allowed continuous monitoring of CRE activity ensuring that cre is not silenced.
We found two plastid sequences lox-psbA and lox-rps12 that are recognized by CRE as substrates since characteristic CRE-mediated recombination junctions between the plastid lox sites and loxP sites were found. Recombination was also observed between two directly oriented lox-psbA sites duplicated in the transformed plastid genome. CRE-mediated recombination via lox-psbA is reported in this study. Hajdukiewicz et al. (2001) previously reported recombination between loxP and sequences in the 3′rps12 5′ UTR. Recombination junctions in three independent clones were located throughout a 34-bp region. Therefore, Hajdukiewicz et al. (2001) called this region a ‘recombination hot-spot’. In our study, the loxP sequence is in an opposite orientation relative to the loxP site described by Hajdukiewicz et al. (2001), and the same recombination junction was identified in three PCR-amplified lox-rps12 recombination products (Figure 5). The recombination junctions mapped to one of the three sites identified earlier. The same rps12 sequence yielding deletions with loxP in either orientation may be explained by its degeneracy relative to the loxP consensus (17 matches out of 34 nucleotides).
Figure 8 depicts CRE-mediated recombination products of the master (monomeric) plastid genome, which are inferred from sequencing recombination junctions. Shown in the center is the Nt-pSAC38-transformed plastid genome. Two circles generated by recombination between the lox-psbA1 and lox-psbA2 sites are shown in the upper right corner. We also show deletions caused by recombination between lox-psbA1/loxP1 (lower right), loxP1/lox-psbA2 (or loxP4/lox-psbA3) (lower left) and lox-rps12-1/loxP1 (or lox-rps12-2/loxP4) sites (top left). Deletions are likely to involve both copies (IRA and IRB) of the repeated region, as the sequence of the repeats is base-by-base identical (Ohyama et al., 1986; Shinozaki et al., 1986), and transformation of the inverted repeat region involves both copies (Svab et al., 1990). Plastid genes are known to exist as a multimeric series (Deng et al., 1989; Kolodner and Tewari, 1979; Lilly et al., 2001); deletion derivatives of monomeric and head-to-tail multimeric genome copies yield identical PCR products.

CRE-mediated deletion derivatives (size inside circle) of the transformed plastid genome (160.7 kb). Recombination events yielding deletion derivatives are boxed. Abbreviations: A1, lox-psbA1; A2, lox-psbA2; A3, lox-psbA3; P1 through P4, loxP1 to loxP4; 12-1, lox-rps12-1; 12-2, lox-rps12-2.
We considered the possibility that the observed PCR products may have formed by template switching during the PCR reaction (Bradley and Hillis, 1997). We believe, this is not the case as: (i) deletion products involving lox sites occur only in CRE-active plants; (ii) the site of recombination involving loxP sequences is in the spacer region, and it is specific to CRE; and (iii) products are also detectable by DNA gel blot analysis.
It should be noted that the observed plastid genome rearrangements are specific to engineered plastid genomes because wild-type plastid genome does not contain loxP sites or duplicated psbA promoter sequences. Furthermore, no CRE-mediated recombination was found between the lox-psbA1 and lox-rps12-1 sites present in wild-type plastid genomes.
Plastid lox sites may not be identified by similarity to loxP
There are many degenerate loxP-like sequences in the plastid genome, which are not substrates for CRE-mediated recombination. Interestingly, the two plastid sites that are CRE substrates were not among the 36 best matches identified by the computer search. The lox-psbA sequence (as well as the lox-rps12 sequence) has only 17 matches out of the 34 loxP nucleotides. It is likely therefore that the overlap of lox-psbA with the conserved psbA promoter core is important for this site being a substrate for CRE, possibly through the RNA polymerase making accessible this site for interaction with the recombinase. The lox-psbA site is conserved in the plastids of monocots and dicots (Figure 9). Thus, CRE-mediated insertion at the lox-psbA site may facilitate plastid transformation in recalcitrant species.
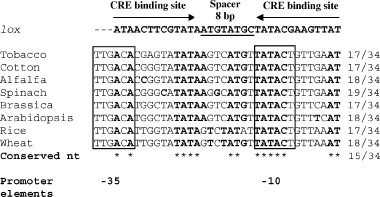
Conservation of lox-psbA sequence in the psbA core promoter. The conserved −35 and −10 core promoter elements are boxed. On top is shown the loxP sequence. Nucleotides that match the loxP sequence are in bold. Number of matching loxP nucleotides out of the 34 is listed on the right. Asterisks at bottom mark loxP nucleotides present in all species.
Elevated homologous recombination in plastids containing CRE
Frequent deletion products in CRE-active plants were obtained by homologous recombination between two directly oriented Prrn sequences. This deletion product has been described in Agrobacterium-transformed plants (Corneille et al., 2001; Hajdukiewicz et al., 2001). We did not observe these deletion products in young seedlings in our earlier study (Corneille et al., 2001). However, such deletion products were present in some of the older seedlings in the present study in which CRE was expressed from a cre gene obtained by an independent insertion event. Thus, formation of the deletion products is not specific to Agrobacterium-transformed cells and may also be observed in seedlings dependent on age and CRE expression levels. Deletions could be the result of CRE-mediated recombination via plastid lox sites, or mediated by the plastid's homologous recombination machinery. To distinguish between the two possibilities, PCR amplification of deletion products was attempted between loxP sites and Prrn sequences. No such deletion product could be amplified. Therefore, we believe that deletions between Prrn sequences are mediated by the plastid's homologous recombination machinery. Interestingly, in the absence of CRE, such deletion products have never been seen, and the transformed plastid genomes are generally stable. Thus, enhanced homologous recombination is likely to be the result of CRE interacting with the plastid recombination machinery through its known DNA-binding and DNA-bending properties (Gopaul et al., 1998; Guo et al., 1997; Van Duyne, 2001).
Large deletions in the polyploid plastid genetic system have no long-term penalty
Expression of CRE in both plants and animals lead to deleterious, unpredicted mutations in the nuclear genome, causing phenotypic abnormalities, sterility, and loss of ability to regenerate plants (Coppoolse et al., 2003; Loonstra et al., 2001; Schmidt et al., 2000). In the present study, we report CRE-mediated deletion of large plastid genome segments. Mutations in non-essential tobacco plastid genes, such as genes encoding photosynthetic function, can be readily obtained even if they cause a mutant phenotype (Allison et al., 1996; Kanevski and Maliga, 1994) reviewed by Bock and Hippler (2002). Unlike mutations induced by CRE in the nuclear genome, large deletions of the plastid genome did not lead to an obvious phenotype. Lack of a readily detectable phenotype can be explained by the deleted genome copies being only a minor fraction of the plastid genome population, and by compensation for the lacking function by genes expressed from wild-type genome copies. Deleted plastid genome copies lacking essential genes are rapidly eliminated in the absence of selection pressure (Drescher et al., 2000; Shikanai et al., 2001; Svab and Maliga, 1993), or a mechanism, such as CRE action, that continuously re-creates the mutation.
Experimental procedures
Vector construction
The inverted neo gene was contained in a SacI-HindIII fragment. The plastid rRNA operon (rrn) promoter derivative was contained in a SacI-XbaI fragment obtained by PCR, using oligonucleotides 5′-GGGGAGCTCGCTCCCCCGCCGTCGTTCAATG-3′ and 5′-GGGAATTCATAACTTCGTATAGCATACATTATACGAAGTTATGCTCCCAGAAATATAGCCA-3′ as primers and plasmid pZS176 (progenitor of plasmid pZS197) (Svab and Maliga, 1993) as a template. The promoter fragment PrrnloxI contained a loxP site at the 3′ end adjacent to the XbaI site. The neo coding region was contained in an XbaI (at the 3′ end) and NcoI fragment. The ribosome binding site from plasmid pZS176 was contained in an NcoI-EcoRI fragment. The TrbcLloxI is the rbcL 3′ UTR contained in an EcoRI-HindIII fragment obtained by PCR, using oligonucleotides 5′-GGGGAATTCATAACTTCGTATAGCATACATTATACGAAGTTATAGACATTAGC-3′ and 5′-GGGAAGCTTGCTAGATTTTGTATTTCAAATCTTG-3′ as primers and plasmid pMSK48 (Khan and Maliga, 1999) as template. TrbcLloxI contained a loxP site adjacent to the EcoRI site in indirect orientation relative to the loxP site in the neo 5′ UTR. The chimeric PrrnloxI:neo:TrbcLloxI gene was introduced into the tobacco plastid transformation vector pPRV111B (Zoubenko et al., 1994) as a SacI-HindIII fragment to obtain plasmid pSAC38.
Two plastid-targeted nuclear cre genes were tested. The cre genes in Agrobacterium binary vectors pKO30 and pKO31 encode the CRE recombinase. Two chimeric cre genes were prepared, one with five (Cre4 gene; plasmid pKO31) and the other with 22 (Cre3 gene; plasmid pKO30) amino acids of the mature pea Rubisco SSU translationally fused with CRE at the N-terminus (Timko et al., 1985). Both cre genes were described by Corneille et al. (2001). The plastid-targeted nuclear cre genes were introduced as EcoRI-HindIII fragments into the pPZP222 Agrobacterium binary vector (Hajdukiewicz et al., 1994) to obtain plasmids pKO30 and pKO31 with 22 and five amino acids of the mature Rubisco SSU, respectively.
Plant transformation
Plastid transformation using the biolistic protocol, selection of transplastomic tobacco lines (RMOP medium, 500 mg l−1 spectinomycin dihydrochloride) and characterization of the transplastomic lines by DNA gel blot analysis was performed as described by Svab and Maliga (1993). Transformation with Agrobacterium vectors pKO30 or pKO31 and regeneration of transformed tobacco plants have also been reported by Hajdukiewicz et al. (1994). Briefly, nuclear gene transformants were selected by gentamycin resistance on RMOP shoot regeneration medium containing 100 mg l−1 gentamycin and 500 mg l−1 carbenicillin. Gentamycin resistance of the shoots was confirmed by rooting on plant maintenance (RM) medium containing 100 mg l−1 gentamycin. Kanamycin resistance level of cre-transformed plants was tested on RMOP plant regeneration medium containing 50 mg l−1 kanamycin. DNA gel blot analysis was carried out on BamHI digested total leaf cellular DNA and probed with the targeting region (1.9-kb ApaI-EcoRV fragment containing the rrn16 gene), the aadA and neo coding regions were isolated as NcoI-XbaI fragments (Svab and Maliga, 1993).
PCR amplification of plastid DNA and sequencing of recombination junctions
DNA template for PCR amplification was obtained from isolated chloroplasts (Guedeney et al., 1996). Plastid genome segments were identified using the following oligonucleotides: primer O1, 5′-CCGCCAGCGTTCATCCTGAGC-3′; primer O2, 5′-GAGATGTAACTCCAGTTCC-3′; primer O3, 5′-CGCTCGATGACGCCAACTACC-3′; primer O4, 5′-TGAAGAGCTTGGCGGCGAAT-3′; primer O5, 5′-GCAACCCACTAGCATATCGAA-3′; primer O6, 5′-GCATCAGAGCAGCCGATTGT-3′; primer O7, 5′-CAGAAGTTGCCGTCAATAAGG-3′; primer O8, 5′-TGAAAGAGGTTGACCTCCTTG-3′. PCR amplification was carried out with the AmpliTaq polymerase (Applied Biosystems, Foster City, CA, USA). The PCR program was: 1 cycle, 94°C, 1 min; 30 cycles, 94°C, 30 sec; 55°C, 45 sec; 72°C, 1.5 min; 1 cycle, 72°C, 7 min. DNA template for sequencing was isolated from gels and purified on Qiaquick gel extraction columns (Qiagen, Valencia, CA, USA). DNA template was sequenced with the USB (Cleveland, OH, USA) T7 Sequenase versus 2.0 DNA sequencing kit, according to the manufacturer's protocol.
Immunoblot analysis
Total soluble leaf protein was extracted from plants grown in sterile culture. About 200 mg of leaf was homogenized in 1 ml of buffer containing 50 mm Hepes/KOH (pH 7.5), 1 mm EDTA, 10 mm potassium acetate, 5 mm magnesium acetate, 10 mm dithiothreitol and 2 mm phenylmethanesulfonyl fluoride. Protein concentration was determined by the Bradford Protein Assay reagent kit (Bio-Rad, Hercules, CA, USA). NPTII was detected by immunoblot analysis (Carrer et al., 1993) and quantified by densitometry (DensoSpot program of Alpha Imager 2000; Alpha Innotech, San Leandro, CA, USA), using a dilution series of commercial NPTII as control (5Prime→3Prime Inc., Boulder, CO, USA). Positive controls were leaf extracts of Nt-pHK31 plants (Maliga et al., 2002).
Acknowledgements
We thank Tony Cashmore for the pea Rubisco small subunit cDNA clone. This research was supported by a Rutgers F&A Special Project Grant.




