The biosynthesis of the branched-chain sugar d-apiose in plants: functional cloning and characterization of a UDP-d-apiose/UDP-d-xylose synthase from Arabidopsis
Summary
d-Apiose is a plant-specific branched-chain monosaccharide found in rhamnogalacturonan II (RG-II), apiogalacturonan, and several apioglycosides. Within RG-II, d-apiose serves as the binding site for borate, which leads to the formation of cross-links within the wall. Biochemical studies in duckweed and parsley have established that uridine 5′-diphospho-d-apiose (UDP-d-apiose) is formed from UDP-d-glucuronate by decarboxylation and re-arrangement of the carbon skeleton, leading to ring contraction and branch formation. The enzyme catalyzing this reaction also forms UDP-d-xylose by decarboxylation of UDP-d-glucuronate, and has therefore been named UDP-d-apiose/UDP-d-xylose synthase. Using a bioinformatics approach, we identified a candidate gene (AXS1) for this enzyme in Arabidopsis and functionally expressed its cDNA in Escherichia coli. The recombinant enzyme catalyzed the conversion of UDP-d-glucuronate to a mixture of UDP-d-apiose and UDP-d-xylose with a turnover number of 0.3 min-1. AXS1 required NAD+ for enzymatic activity, and was strongly inhibited by UDP-d-galacturonate. It was highly expressed in all plant organs consistent with a function in synthesizing an essential cell wall precursor. Database searches indicated the presence of closely related sequences in a variety of crop plants. The cloning of the AXS1 gene will help to investigate the biosynthesis of RG-II, and permit insights into the mechanism by which d-apiose and other branched monosaccharides are formed.
Introduction
d-Apiose (3-C-hydroxymethyl-d-erythrose) is the only plant cell wall monosaccharide with a branched carbon skeleton (Duff, 1965; Grisebach, 1980). In the cell walls of most of the higher plants, d-apiose is only present in the pectic polysaccharide rhamnogalacturonan-II (RG-II), where it serves as an attachment point for two highly complex side chains to the homogalacturonan backbone (Stevenson et al., 1988). The two vicinal hydroxyl groups in the furanose ring of d-apiose are ideally positioned to form a cyclic diester with borate, a micronutrient specifically needed by higher plants. It has recently been demonstrated that one of the two d-apiose residues in RG-II forms this type of borate diester in vivo, and that the two remaining hydroxyl groups of RG-II-bound borate react with an apiose residue in another RG-II molecule to form a borate tetraester (Ishii et al., 1999; O’Neill et al., 1996; see Figure 1). Interestingly, the stability and/or formation rate of this borate cross-link depends critically on the structural integrity of the complex side chain that is part of the borate-esterified d-apiose residue. Changes as small as the replacement of the l-fucose residue within RG-II by the structurally similar monosaccharide l-galactose in Arabidopsis mur1 reduce cross-link formation to about 53% of wild type (O’Neill et al., 2001), and loss of a disaccharide attached to this fucose residue in the nolac-H18 mutant of Nicotiana plumbaginifolia leads to an even more severe reduction of borate-mediated cross-linking (ca. 44% of wild type; Iwai et al., 2002). The significance of the borate cross-link between apiose residues is underscored by the dwarfed growth habit of mur1 plants (Reiter et al., 1993), and loss of cell–cell adhesion in the nolac-H18 mutant that is defective in organogenesis, and can only be maintained as a highly friable callus (Iwai et al., 2002).
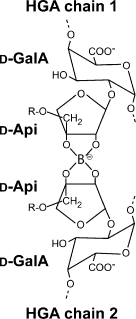
d-Apiose is involved in the formation of a borate cross-link between two different homogalacturonan chains in vivo.
The position of a borate moiety esterified to two d-apiose residues is shown (O’Neill et al., 1996). The d-apiose moieties are linked to d-galacturonate residues that are part of the homogalacturonan backbone of RG-II. ‘R’ refers to complex side chains attached to the branched C-3′ hydroxymethyl groups of the apiose moieties.
Biochemical studies on the synthesis of d-apiose have focused on the duckweed Lemna minor, where this monosaccharide is an abundant component of the unusual pectic polysaccharide apiogalacturonan (Golovchenko et al., 2002; Longland et al., 1989). d-Apiose biosynthesis has also been studied in parsley, which contains substantial amounts of d-apiose as a sugar moiety in apiin, an abundant flavonoid glycoconjugate (Hahlbrock et al., 1976). It has been established in both systems that uridine 5′-diphospho-d-apiose (UDP-d-apiose) is formed from UDP-d-glucuronate by a single enzymatic reaction that leads to the decarboxylation of the substrate followed by re-arrangement of the carbon skeleton and ring contraction (Baron et al., 1972; Gebb et al., 1975; Grisebach, 1980; Kindel and Watson, 1973; Mendicino and Abou-Issa, 1974; Sandermann and Grisebach, 1970; Sandermann et al., 1968; Wellmann and Grisebach, 1971). The initial steps of this reaction are believed to be identical to the synthesis of UDP-d-xylose from UDP-d-glucuronate, which is initiated by an NAD(P)+-dependent oxidation of C-4 followed by decarboxylation and reduction to restore the original configuration (Ankel and Feingold, 1965, 1966). The mechanism of the re-arrangement leading to the formation of UDP-d-apiose is unknown, but in analogy to the mechanism of streptose biosynthesis (Bruton and Horner, 1966; Candy and Baddiley, 1965), it may involve aldol cleavage between C-2 and C-3 followed by aldol condensation between C-2 and C-4 to yield the contracted ring structure of UDP-d-apiose (Figure 2). In this scenario, the aldehyde group at C-3 would be converted to a hydroxymethyl group by the transiently reduced NAD(P)H co-factor of the enzyme. In both duckweed and parsley, highly purified preparations of the UDP-d-apiose-synthesizing activity were found to convert part of the UDP-d-glucuronate substrate into UDP-d-xylose. Although contamination of the UDP-d-apiose synthase preparation by a separate UDP-d-xylose synthase activity could not be strictly ruled out, the co-purification of these two activities suggested that both reactions were catalyzed by the same enzyme, which was termed UDP-d-apiose/UDP-d-xylose synthase.
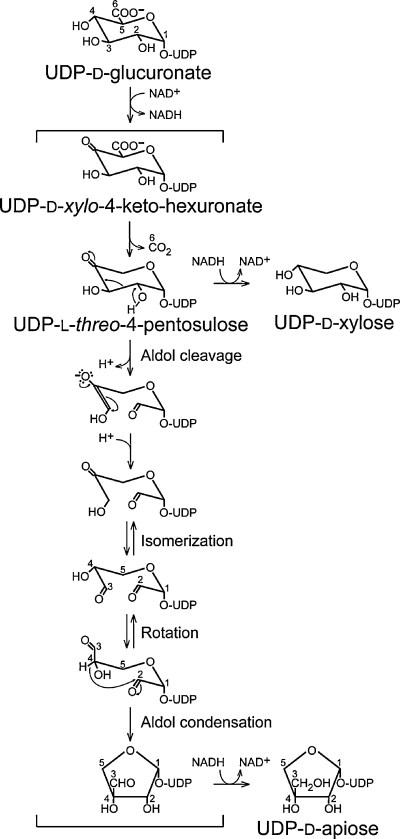
Proposed mechanism for UDP-d-apiose/UDP-d-xylose synthase.
UDP-d-apiose and UDP-d-xylose are formed from the common intermediate UDP-l-threo-4-pentosulose. While UDP-d-xylose may be formed directly by stereospecific reduction of this compound, the formation of UDP-d-apiose most likely involves aldol cleavage between C-2 and C-3 followed by aldol condensation between C-2 and C-4. The resulting aldehyde intermediate is then reduced to UDP-d-apiose. The numbering system indicates the origin of carbon atoms relative to d-glucuronate.
We are interested in the identification and characterization of coding regions for nucleotide sugar interconversion enzymes in higher plants, which represent the first stage of cell wall synthesis. The formation of UDP-d-apiose is of particular significance as this branched-chain pentose is primarily found in RG-II, where it anchors the two large side chains of this polysaccharide to the polygalacturonate backbone and mediates the formation of an important cross-link within the wall. Accordingly, changes in UDP-d-apiose availability are likely to affect the structure of RG-II without influencing the synthesis of other cell wall polymers. Here, we report the molecular cloning and characterization of UDP-d-apiose/UDP-d-xylose synthase from Arabidopsis, which represents the first instance where the coding region for an enzyme in the synthesis of a branched sugar has been obtained.
Results
Identification of a candidate gene for UDP-d-apiose/UDP-d-xylose synthase
As UDP-d-apiose/UDP-d-xylose synthase and UDP-d-glucuronate decarboxylase (synonymous with UDP-d-xylose synthase; EC 4.1.1.35) both use UDP-d-glucuronate as a substrate and catalyze identical reactions in the formation of UDP-d-xylose, we hypothesized that UDP-d-apiose/UDP-d-xylose synthases would show sequence similarities to UDP-d-glucuronate decarboxylases, for which genes have recently been cloned from the pathogenic fungus Cryptococcus neoformans (Bar-Peled et al., 2001), rat (Moriarity et al., 2002), pea (Kobayashi et al., 2002), and Arabidopsis (Harper and Bar-Peled, 2002). By searching the Arabidopsis genome sequence with these coding regions, we identified two genes named AXS1 (At2g27860) and AXS2 (At1g08200), encoding proteins with 29% amino acid sequence identity to the UDP-d-xylose synthase UXS3 from Arabidopsis (Figure 3). Both AXS1 and AXS2 have predicted sizes of 43.6 kDa, which closely match that of UDP-d-apiose/UDP-d-xylose synthase from parsley (44 kDa based on SDS–PAGE; Matern and Grisebach, 1977). For this reason, we considered AXS1 and AXS2 promising candidate genes for UDP-d-apiose/UDP-d-xylose synthases in Arabidopsis.

Comparison of AXS1 to other nucleotide sugar interconversion enzymes.
The deduced amino acid sequence of AXS1 is aligned with the UDP-d-glucuronate dehydrogenase domain of the ArnA protein from E. coli, and the UXS3 isoform of UDP-d-glucuronate decarboxylase (UDP-d-xylose synthase) from Arabidopsis. The conserved GxxGxxG and YxxxK motifs involved in the binding of the NAD+ co-factor and catalysis, respectively, are indicated above the alignment.
AXS1 and AXS2 both have eight introns and are located on chromosome 2 and chromosome 1, respectively. Both genes are predicted to encode 389 amino acid proteins belonging to the NAD+-dependent epimerase/dehydratase protein family. A highly conserved GxxGxxG motif (Wierenga et al., 1986) involved in binding of NAD+, is located at positions Gly24–Gly30 (Figure 3). Also, a conserved YxxxK sequence believed to function in catalysis (Thoden et al., 1997) is located at positions Tyr185–Lys189 in both the proteins (Figure 3). Hydropathy plots on the derived amino acid sequences (Kyte and Doolittle, 1982) did not predict any transmembrane domains or signal peptides, suggesting that AXS1 and AXS2 are soluble cytoplasmic proteins (data not shown). This is in agreement with the properties of UDP-d-apiose/UDP-d-xylose synthases from parsley and duckweed (Matern and Grisebach, 1977; Mendicino and Abou-Issa, 1974) and the soluble nature of recombinant AXS1 expressed in Escherichia coli (see below). The deduced amino acid sequence of AXS1 shares 96% sequence identity to AXS2 and 84% identity to a putative protein in rice (accession number BAB85329). tblastn searches of dbEST with the derived protein sequence of AXS1 identified homologs in Lotus japonicus, Brassica campestris, poplar, iceplant, cotton, alfalfa, potato, soybean, tomato, sorghum, maize, and wheat (data not shown). Outside the plant kingdom, AXS1 and AXS2 are most similar to the UDP-d-glucuronate dehydrogenase domain of ArnA (34% identity, see Figure 3), an enzyme involved in the biosynthesis of UDP-4-amino-4-deoxy-l-arabinose. This nucleotide sugar is a precursor in the biosynthesis of lipopolysaccharides in E. coli (Breazeale et al., 2002).
Expression of AXS1 in E. coli
To determine the function of the AXS1 gene product, a full-length cDNA was isolated and the coding region was cloned into the pET28b expression vector, utilizing a C-terminal histidine tag. Total protein from crude extracts of induced E. coli BL21 (DE3) were analyzed by SDS–PAGE, indicating the presence of a protein of approximately 46 kDa that was not observed in crude extracts of E. coli transformed with the empty vector (Figure 4a). This size is in agreement with the molecular mass predicted from the amino acid sequence of the recombinant AXS1 protein, including a histidine tag (45.8 kDa). To determine whether an antibody raised against UDP-d-apiose/UDP-d-xylose synthase from parsley (Gardiner et al., 1980) recognized the recombinant protein, crude extracts were analyzed by immunoblotting. Results from this experiment showed that the antiserum recognized the 46-kDa protein, but did not react with protein from E. coli transformed with the empty vector (Figure 4b). This supported our hypothesis that AXS1 encodes a UDP-d-apiose/UDP-d-xylose synthase.

Expression of AXS1 in E. coli.
(a) SDS–PAGE of crude protein extracts from an empty vector transformant (pET28b, lane 1), a transformant expressing AXS1 (pET28b-AXS1, lane 2), and Ni-NTA purified AXS1 (lane 3).
(b) Western blotting using antiserum towards a parsley UDP-d-apiose/UDP-d-xylose synthase. Crude protein extracts from an empty vector transformant (lane 1) and a transformant expressing AXS1 (lane 2) were separated by SDS–PAGE, and transferred to a nitrocellulose membrane prior to immunological detection.
AXS1 has UDP-d-apiose/UDP-d-xylose synthase activity
To assay AXS1 for nucleotide sugar interconversion activities, crude extracts from E. coli were incubated with UDP-d-[14C]-glucuronate, and reaction products were analyzed by thin layer chromatography (TLC) after hydrolysis to monosaccharides. Incubation with extracts containing the recombinant AXS1 protein resulted in two products, with Rf values typical of d-apiose and d-xylose, respectively, as determined by authentic standards (Figure 5a, lane 3). Extracts from E. coli transformed with the empty vector did not convert the UDP-d-[14C]-glucuronate substrate into any detectable products (Figure 5a, lane 1).
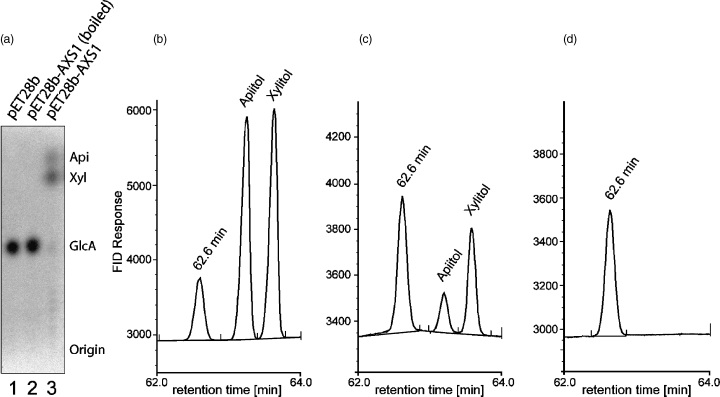
UDP-d-apiose/UDP-d-xylose synthase activity of AXS1.
(a) Autoradiogram of a thin layer chromatogram of the 14C-labeled reaction products from assays of UDP-d-apiose/UDP-d-xylose synthase activities using crude extracts from E. coli (15 μg total protein) and UDP-d-[14C]-glucuronate as substrate. Lane 1: empty vector transformant; lane 2: boiled extract from an AXS1-expressing strain; lane 3: non-treated extract from an AXS1-expressing strain. Nucleotide sugars were hydrolyzed to monosaccharides prior to TLC analysis.
(b–d) GLC of alditol acetates of apiose and xylose from assays of UDP-d-apiose/UDP-d-xylose synthase activity of purified AXS1 using unlabeled UDP-d-glucuronate as substrate. Mixture of authentic apiose and xylose (b), assay of AXS1 (c), and assay of boiled AXS1 (d). Apiitol pentaacetate eluted with a retention time of 63.2 min, whereas xylitol pentaacetate eluted after 63.6 min. The peak at 62.6 min represents an unknown product formed by the derivatization reagents. No sugars other than apiose and xylose were detected.
To verify the identity of the reaction products by an independent method, the assay was repeated with unlabeled UDP-d-glucuronate and affinity-purified AXS1 protein. After acid-catalyzed hydrolysis of the reaction products, monosaccharides were separated and quantitated by gas-liquid chromatography (GLC) of alditol acetates. This experiment indicated the formation of products with retention times identical to those of authentic apiitol and xylitol pentaacetate (Figure 5, panels b and c). Boiled enzyme preparations did not exhibit any detectable activity (Figure 5d). Incubation of purified AXS1 with UDP-d-[14C] galactose, UDP-d-[14C]-glucose, or UDP-d-[14C]-xylose did not yield any detectable products (data not shown).
To verify that the reaction products from the AXS1 assays with radiolabeled substrate represented nucleotide sugars rather than monosaccharides or sugar phosphates, the reaction products were purified using anion-exchange chromatography (Bonin and Reiter, 2000; Burget et al., 2003). Ionic strengths required to elute the three sugar types were determined by loading and eluting d-xylose, d-apiose, α-d-xylose-1-phosphate, and a mixture of unlabeled and radiolabeled UDP-d-Xylose. d-xylose and d-apiose eluted at 1 mm potassium phosphate (pH 6.0), whereas α-d-xylose-1-phosphate eluted at 50 mm and UDP-d-xylose eluted at 100 mm of this buffer. Once these conditions were established, the reaction products from the assays with AXS1 were loaded onto an anion exchange column and eluted with two column volumes each of 10, 60, 120, and 200 mm potassium phosphate (pH 6.0). More than 85% of the radioactivity eluted with 120 mm of this buffer, indicating that the reaction products represented nucleotide sugars.
pH optimum, enzyme stability, and effect of co-factors
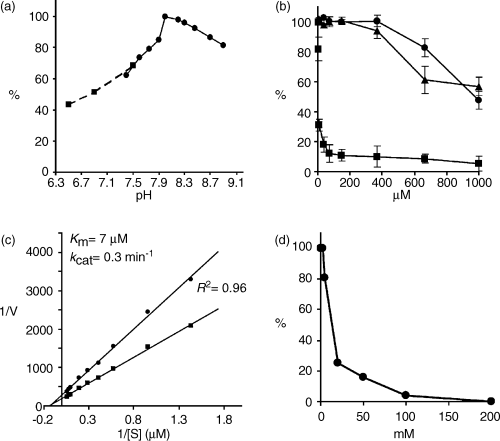
To examine the influence of pH on UDP-d-apiose/UDP-d-xylose synthase activity, enzyme preparations were assayed in 100 mm potassium phosphate buffer (pH 6.5–7.5) and 100 mm Tris–HCl (pH 7.5–9.0) using UDP-d-[14C] glucuronate as a substrate. The pH optimum of AXS1 was determined to be 8.0 (Figure 6a).
Characterization of recombinant AXS1.
(a) pH profile obtained by assaying AXS1 in 100 mm potassium phosphate buffer (pH 6.5–7.5; ▪) and 100 mm Tris–HCl buffer (pH 7.5–9.0; ●).
(b) Effect of UDP-d-galacturonate (▪), UDP (●), and UDP-d-xylose (▴) on AXS1 activity.
(c) Lineweaver-Burk plot showing the effect of UDP-d-glucuronate concentration on the reaction rate for UDP-d-apiose (●) and UDP-d-xylose (▪) synthesis.
(d) Effect of (NH4)2SO4 on AXS1 activity.
Storage of purified AXS1 at 0 or 25°C overnight resulted in 40 and 50% loss of activity, respectively, whereas storage at −20°C in the presence of 50% glycerol caused a twofold decrease in the activity over 1 week. The enzyme was also sensitive to freezing in the presence of 50% glycerol at −80°C (74% loss of activity), and was completely inactivated by freezing in the absence of cryoprotectant. For these reasons, only freshly purified AXS1 was used to characterize the enzyme further.
The effect of oxidized and reduced forms of pyridine nucleotide co-factors (NAD+, NADH, NADP+, and NADPH) on the ability of AXS1 to convert UDP-d-[14C]-glucuronate to UDP-d-[14C]-apiose and UDP-d-[14C]-xylose was also determined. Results of this analysis showed that purified recombinant AXS1 was active in the absence of added co-factors, although addition of 2 mm NAD+ stimulated the enzyme activity by 8.5 folds. This suggests that some of the affinity-purified enzyme contained tightly-bound NAD+, leading to a low but detectable enzymatic activity. Addition of 2 mm NADH, NADP+, or NADPH to the assay had no effect. However, pre-incubating purified AXS1 with 2 mm NADH at 25°C overnight resulted in total loss of enzyme activity, which could not be rescued by addition of exogenous NAD+ to the assay. This suggested that enzyme-associated NAD+ could be slowly replaced by exogenous NADH, which would prevent the enzyme from catalyzing the first step in the reaction sequence, i.e. the oxidation of C-4 of the glucuronate moiety.
Addition of up to 1 mm DTT has been reported to have a stabilizing effect on UDP-d-apiose/UDP-d-xylose synthase from parsley and duckweed (Baron et al., 1972; Mendicino and Abou-Issa, 1974; Wellmann and Grisebach, 1971). However, up to 2 mm DTT did not have any effect on the recombinant Arabidopsis enzyme. Addition of EDTA to 5 mm final concentration had no effect, indicating that divalent cations are not required for AXS1 activity.
Effect of nucleotides and nucleotide sugars on AXS1 activity
UDP-d-glucose, UDP-d-galactose, and UDP-d-xylose did not serve as substrates for AXS1, and addition of up to 2 mm UDP-d-glucose or UDP-d-galactose had no inhibitory effect on AXS1 activity. UDP and UDP-d-xylose inhibited AXS1 at concentrations higher than 370 μm (Figure 6b), which is equal to a 109-fold excess over the substrate UDP-d-glucuronate. However, addition of 3.7 and 7.4 μm UDP-d-galacturonate (equal to 1.1- and 2.2-fold substrate concentration) reduced AXS1 activity to 82 and 31%, respectively (Figure 6b), suggesting that the concentration of UDP-d-galacturonate might regulate the activity of AXS1 in vivo.
AXS1 is a dimer
To determine whether AXS1 is a monomeric or multimeric enzyme, the molecular weight of the recombinant AXS1 was analyzed by size-exclusion chromatography. A calibration curve with protein standards was used to determine the molecular weight of the recombinant AXS1. The elution volume for purified AXS1 and AXS1 in crude extracts corresponded to a molecular weight of 84 kDa, suggesting that the recombinant AXS1 exists as a dimer (predicted molecular weight of 91.6 kDa).
Kinetic parameters of recombinant AXS1
The kinetics of AXS1 were analyzed using 3 μg purified AXS1 in the presence of 2 mm NAD+ and various concentrations of UDP-d-[14C]-glucuronate. The data were analyzed both by a computerized non-linear regression fit and a Lineweaver-Burk plot (Figure 6c). The Km value was determined to be 7 μm for both plots (R2 = 0.96), indicating a high affinity for the substrate. This value is comparable to those of the enzymes from parsley and duckweed (2 and 5 μm, respectively; see Grisebach, 1980). The Vmax value for the conversion of UDP-d-glucuronate to UDP-d-apiose plus UDP-d-xylose was 3.1 nmol min−1 mg−1 protein, giving a kcat value of 0.3 min−1, calculated based on the molecular weight of the dimeric protein. The apiose/xylose ratio was determined to be 0.6, based on data obtained by both TLC and GLC. As this ratio has been reported to increase in a potassium phosphate buffer system (Grisebach, 1980; Matern and Grisebach, 1977), we performed the enzyme assay under these conditions, but did not observe any change in the relative amounts of apiose and xylose.
NH4+ has been reported to influence the activities of the UDP-d-apiose/UDP-d-xylose synthases from parsley and duckweed by inhibiting the formation of UDP-d-apiose and stimulating the biosynthesis of UDP-d-xylose (Baron et al., 1972; Wellmann and Grisebach, 1971). To test if NH4+ has the same effect on the recombinant Arabidopsis enzyme, AXS1 was assayed in the presence of 2 mm up to 200 mm (NH4)2SO4. We found that increasing concentrations of (NH4)2SO4 inhibited the activity of AXS1 (Figure 6d) without changing the apiose/xylose ratio. Similar effects were obtained by adding NH4Cl, KCl, and MgCl2 (data not shown).
Expression pattern of the AXS1 gene in A. thaliana
The expression pattern of AXS1 was analyzed by an AXS1::GUS construct transformed into Arabidopsis. All plant organs were analyzed for GUS staining. Results from this experiment showed that AXS1::GUS is expressed throughout the Arabidopsis plant (Figure 7a–d). The GUS staining data were in agreement with RT-PCR results using AXS1-specific oligonucleotides, which indicated that the AXS1 transcript could be amplified from total RNA extracted from all tissues analyzed (roots, rosette leaves, stems, flowers, and siliques; Figure 7e).
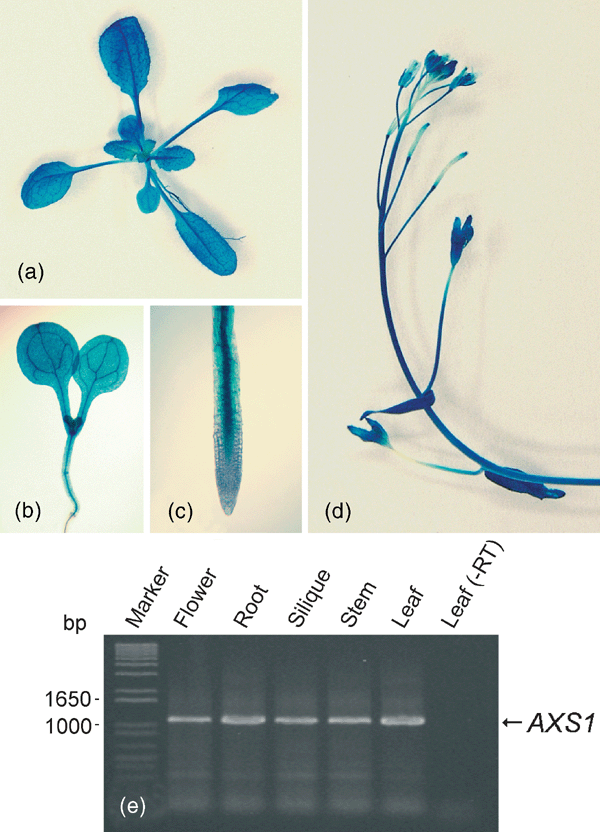
Expression analysis of AXS1::GUS in Arabidopsis.
GUS staining in (a) rosette leaves, (b) young seedling, (c) root tip, and (d) inflorescence. (e) RT-PCR analysis of AXS1 expression: RNA was isolated from various organs and used for RT-PCR with a total of 35 amplification cycles.
Discussion
In this study, we present the identification, heterologous expression, and characterization of AXS1, an Arabidopsis UDP-d-apiose/UDP-d-xylose synthase. This study describes the identification of the first gene encoding an enzyme involved in the biosynthesis of a branched-chain sugar.
Database analyses revealed sequence similarities of AXS1 to UDP-d-glucuronate decarboxylases from various sources and to the UDP-d-glucuronate dehydrogenase domain of ArnA, an enzyme involved in the biosynthesis of UDP-4-amino-4-deoxy-l-arabinose in E. coli, suggesting an evolutionary relationship (Figure 3). These similarities may be based on catalytic properties shared by the three enzymes, which utilize UDP-d-glucuronate as a substrate and NAD+ as a co-factor. All three enzymes share an initial catalytic step involving oxidation of C-4, giving a 4-keto intermediate that is decarboxylated to generate UDP-l-threo-4-pentosulose (Bar-Peled et al., 2001; Breazeale et al., 2002; Grisebach, 1980). Whereas both UDP-d-glucuronate decarboxylases and UDP-d-apiose/UDP-d-xylose synthases reduce the 4-keto-pentose intermediate stereospecifically to give UDP-d-xylose (Ankel and Feingold, 1965, 1966; Bar-Peled et al., 2001; Grisebach, 1980), only UDP-d-apiose/UDP-d-xylose synthases catalyze the complex re-arrangement events that lead to the formation of UDP-d-apiose.
The enzymatic properties of AXS1 are similar to those of UDP-d-apiose/UDP-d-xylose synthases from parsley and duckweed with some exceptions. Kinetic analysis showed that the Km of AXS1 is 7 μm, which is similar to values determined for the synthases from parsley (Km = 2 μm) and duckweed (Km = 5 μm; Grisebach, 1980). The kcat value for AXS1 is unusually low (0.3 min−1), but similar to the specific activity of the highly purified UDP-d-apiose/UDP-d-xylose synthase from parsley (kcat value of ca. 1 min−1). These low turnover numbers may explain why AXS1 is highly expressed (19 AXS1-derived cDNAs in dbEST), even though the demand for UDP-d-apiose in Arabidopsis is expected to be limited to the synthesis of RG-II.
The UDP-d-apiose/UDP-d-xylose from parsley has been reported to co-purify with a protein of 65 kDa, which appears to consist of two identical subunits (Matern and Grisebach, 1977). Although this 65-kDa protein was not required for enzymatic activity, its removal from the enzyme preparation caused a marked decrease in enzyme stability over time (90% loss of activity upon storage for 15 h at 4°C). We observed an approximately 40% loss of activity of AXS1 under the same conditions, which may be related to the absence of this stabilizing protein factor.
The formation of a mixture of UDP-d-apiose and UDP-d-xylose by a single enzyme is unusual and difficult to rationalize in regard to its mechanism and functional significance. In apiose-rich plants such as duckweed and other hydrophytes, UDP-d-apiose/UDP-d-xylose synthase may make a significant contribution to the pools of both UDP-d-apiose and UDP-d-xylose; however, in Arabidopsis and most other land plants, virtually all of the UDP-d-xylose is expected to be synthesized by monofunctional UDP-d-glucuronate decarboxylases. The bifunctionality of UDP-d-apiose/UDP-d-xylose synthases may be accidental but without negative consequences for the plants as UDP-d-xylose is needed in any case for the synthesis of cell wall polysaccharides. In this sense, UDP-d-apiose/UDP-d-xylose synthases can be considered functionally redundant with UDP-d-glucuronate decarboxylases in regard to the formation of UDP-d-xylose.
While AXS1 from Arabidopsis produced more UDP-d-xylose than UDP-d-apiose with an apiose/xylose ratio of 0.6 (Figure 5, panels a and c), the parsley and duckweed enzymes both synthesized more UDP-d-apiose than UDP-d-xylose with an apiose/xylose ratio of 1.2–1.4 in the same buffer system (Grisebach, 1980). This suggests that the synthesis of the two products is not mechanistically linked, and that apiose-rich plants have evolved enzymes that favor the production of UDP-d-apiose. The bifunctionality of UDP-d-apiose/UDP-d-xylose synthases raises the question whether UDP-d-xylose can be converted to UDP-d-apiose and vice versa. We found that UDP-d-xylose is only a weak inhibitor of AXS1 activity, and did not observe its conversion into any detectable products. This suggests that the formation of UDP-d-xylose and UDP-d-apiose from UDP-d-glucuronate are essentially irreversible processes, and that the two products are not interconverted into each other.
The expression pattern of AXS1 was analyzed by RT-PCR and a promoter-GUS fusion. Both methods showed expression of AXS1 throughout the plant, reflecting the function of AXS1 as a housekeeping gene. A search of the EST database indicated that AXS1 and its homolog AXS2 are expressed at similar levels, suggesting that AXS2 encodes a functional UDP-d-apiose/UDP-d-xylose synthase that is genetically redundant with AXS1. This hypothesis is supported by the expression of an AXS2::GUS construct in all major plant organs, and the absence of a visible phenotype in an axs1 knockout mutant (M. M. and W.-D. R., unpublished results). Elimination of UDP-d-apiose/UDP-d-xylose synthase activity is expected to cause the absence of the two major side chains of RG-II, presumably with severe consequences for cell wall structure and function. This hypothesis can be tested by analyzing the phenotype of an axs1/axs2 double mutant.
Experimental procedures
Plant material and growth conditions
Plants were grown in an environmental chamber at 23°C and 60–70% humidity under continuous fluorescent light (60–70 μmol m−2 sec−1). Arabidopsis plants of the ecotype Columbia were used for transformation and the isolation of DNA and RNA.
Plasmid constructs and cloning
A cDNA encoding the AXS1 protein was amplified by RT-PCR from total leaf RNA using the oligonucleotides 5′-ATTTGCCCATGGCGAATGGAGCTAATAGAGTGGATCTCGACG-3′ and 5′-CGTTAAGAATTCGCGGAAGCCACTGGTTTGGATGTTGCCTTCTTCAC-3′ (NcoI and EcoRI sites engineered into the primers are underlined). After cleavage with NcoI and EcoRI, the cDNA was cloned in frame with the polyhistidine tag into the E. coli expression vector pET28b (Novagen, Madison, WI, USA), giving the construct pET28b-AXS1 in E. coli host strain BL21 (DE3).
For gene-expression studies, 2 kb of DNA directly upstream of the AXS1 coding region were PCR-amplified from genomic DNA using the oligonucleotides 5′-ATGCTGAATTCAAAGCAACGAGAAATTAGCATCTTTTTTTCATC-3′ and 5′-GCCTAAAGATCTTCCATTTTTTTTCTCAGATCAGAAAAAATAATC-3′ (EcoRI and BglII sites are underlined) and cloned in frame with the gusA reporter gene in pCAMBIA1301 (CAMBIA, Canberra, Australia), giving pCAMBIA1301-AXS1. All PCR reactions were performed with Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA, USA), and the sequences of all constructs were verified using the ABI Prism Big Dye Terminator Cycle Sequence Reaction Kit (Perkin-Elmer Applied Biosystems, Foster City, CA, USA) and an ABI Prism 377 DNA sequencer. A. thaliana was transformed using Agrobacterium tumefaciens strain GV3101 (pMP90) essentially as described by Bechtold et al. (1993).
Expression of AXS1 in E. coli and purification of the AXS1 protein
Luria-Bertani medium containing 50 μg ml−1 kanamycin was inoculated with E. coli BL21 (DE3) transformed with pET28b-AXS1 and shaken at 260 r.p.m. and 37°C until an OD600 of 0.6–1.0 was attained. Gene expression was induced by addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mm and further incubation for 3 h. Cultures were cooled on ice for 5 min, and cells were harvested by centrifugation at 5000 g for 5 min at 4°C. Cell pellets were stored at −80°C. To prepare a crude protein extract from recombinant E. coli, frozen cells obtained from a 50-ml culture were re-suspended in 1 ml of 100 mm Tris–HCl (pH 8.0), followed by the addition of 1× Complete protease inhibitor (Boehringer Mannheim, Indianapolis, IN, USA) and 1 mg ml−1 lysozyme (Sigma, St Louis, MO, USA). After incubation at 4°C for 30 min, cells were lyzed by sonication. Measurements of protein concentration were performed with a bicinchoninic acid protein quantitation kit (Sigma). Purification of the native recombinant AXS1 protein from crude extracts was carried out with Ni-NTA agarose columns according to the manufacturer's protocol (Qiagen, Valencia, CA, USA).
SDS–PAGE and Western analysis
SDS–PAGE was performed in 10% polyacrylamide gels, and proteins were stained with 0.1% (w/v) Coomassie Brilliant Blue R-250 in methanol/acetic acid/water (9:2:9, v/v/v), and de-stained in the same solution without the dye. Western blotting was performed using a semi-dry electroblotter, nitrocellulose membranes from MSI Micron Separations (Westborough, MA, USA), and transfer buffer composed of 25 mm Tris–HCl (pH 8.3), 192 mm glycine, and 20% (v/v) methanol. For immunologic detection, membranes were incubated overnight at 4°C with a primary antibody against UDP-d-apiose/UDP-d-xylose synthase from parsley (Gardiner et al., 1980). This was followed by a 3-h incubation at room temperature with alkaline phosphatase-conjugated goat antirabbit-IgG (Sigma). Both incubations were carried out with 1:2000 dilutions of the respective antibodies in 50 mm Tris–HCl (pH 7.4), 500 mm NaCl, 0.5% (v/v) Tween-20, and 5% (v/v) horse serum. Alkaline phosphatase was detected colorimetrically using 0.03% (w/v) 4-nitro blue tetrazolium chloride and 0.02% (w/v) 5-bromo-4-chloro-3-indolyl-phosphate (p-toluidine salt) in 100 mm Tris (pH 9.5), 0.1 m NaCl, and 5 mm MgCl2.
Assay of AXS1 enzyme activity
Unless stated otherwise, purified recombinant AXS1 (3 μg protein) was incubated at 25°C overnight in 50 μl of 100 mm Tris–HCl (pH 8.0), 2 mm NAD+ (Sigma) in the presence of 25 nCi of UDP-d-[14C]-glucuronate (300 mCi mmol−1), UDP-d-[14C]-galactose (300 mCi mmol−1), UDP-d-[14C]-glucose (300 mCi mmol−1), or UDP-d-[14C]-xylose (238 mCi mmol−1; American Radiolabeled Chemicals, St Louis, MO, USA). To assay the effect of various nicotinamide dinucleotide co-factors, NAD+ was replaced by NADH, NADP+, or NADPH. The effects of all other additives were assayed in the presence of 2 mm NAD+. Substitution of enzyme-bound co-factor was tried by incubation with 2 mm NADH at 25°C for 10 h. Nucleotide sugars were hydrolyzed by the addition of trifluoroacetic acid to 1.6 m final concentration, and incubation at 95°C for 30 min. Samples were dried under vacuum and re-suspended in 15 μl of 80% (v/v) ethanol. Resultant monosaccharides were separated by TLC on silica TLC plates (Aldrich, Milwaukee, WI, USA) in a 6:2:1 (v/v/v) mixture of 1-propanol, saturated ammonia solution, and water. Radioactivity was visualized by phosphorimaging (Bio-Rad Laboratories, Hercules, CA, USA) and quantified using Molecular Analyst Software (Bio-Rad Laboratories, Hercules, CA, USA). Authentic sugar standards run in parallel were stained with aniline-hydrogen phthalate (Fry, 1988). In assays with unlabeled substrate, 20 μg AXS1 was incubated overnight at 25°C with 50 mm UDP-d-glucuronate and 3 mm NAD+ in 250 μl 100 mm Tris–HCl (pH 8.0). All assays were repeated at least once. Free apiose was obtained by hydrolyzing 1,2:3,5-di-O-isopropylidene-α-d-apiose (Sigma) at 80°C for 30 min in 2 m trifluoroacetic acid followed by drying under vacuum and dissolving the residue in water.
For kinetic evaluation, linearity with respect to protein concentration (2–5 μg) and time (2–45 min) was first established. The concentration of UDP-d-[14C]-glucuronate was then independently varied while keeping the concentrations of all other reactants constant (3 μg purified AXS1, 2 mm NAD+ in 50 μl of 100 mm Tris–HCl (pH 8.0)). The incubation time was 30 min. Computerized non-linear regression fits and double reciprocal plots were constructed using GraphPad Prism Version 3.03, and the equation of the best-fit was determined. To examine the influence of pH on AXS1 activity, purified enzyme preparations were assayed using 25 nCi UDP-d-[14C]-glucuronate and 2 mm NAD+ in 100 mm Tris–HCl or 100 mm potassium phosphate at 25°C for 30 min.
Gas-liquid chromatography
Nucleotide sugars were hydrolyzed with trifluoroacetic acid, and monosaccharides were converted to alditol acetates as described by Kindel and Cheng (1990). Separation and quantitation of alditol acetates was carried out by GLC on a 30-m SP2330 column (Supelco, Bellefonte, PA, USA) with an inner diameter of 0.25 mm. One microliter of sample was injected under splitless conditions at an oven temperature of 140°C. After 1 min at 140°C, the oven temperature was raised to 170°C at 25°C min−1, held at 170°C for 40 min, then raised to 240°C at 2°C min−1, and held at 240°C for 15 min. Helium was used as a carrier gas at a pressure of 125 kPa. The injector and detector temperatures were 240 and 250°C, respectively.
Anion-exchange chromatography
Four separate Superclean LC-SAX solid-phase columns (Supelco, Bellefonte, PA, USA) were used to determine the ionic strengths required to elute 200 μg of d-xylose, 200 μg of d-apiose, 200 μg of α-d-xylose-1-phosphate, and a mixture of 200 μg of unlabeled UDP-d-xylose and 100 nCi of UDP-d-[14C]-xylose, respectively. Nucleotide sugars were identified by liquid scintillation counting and the monosaccharides and sugar phosphates were identified by total carbohydrate assays of the eluted fractions (Fry, 1988).
Size-exclusion chromatography
Purified AXS1 (70 μg) and AXS1 in crude extracts (400 μg total protein) were fractionated at 4°C on a 40 cm × 1 cm Sepharose CL-6B (Sigma) column equilibrated with 50 mm Tris–HCl (pH 7.5), 100 mm KCl. Four hundred microliter fractions were collected. Protein concentrations in each fraction were measured by determining the absorbance at 280 nm. The molecular-weight determination of AXS1 was made by comparing the ratio Ve/V0 (Ve is the elution volume and V0 is the void volume) to the Ve/V0 of 160–800 μg of protein standards (β-amylase from sweet potato, 200 kDa; yeast alcohol dehydrogenase, 150 kDa; bovine serum albumin, 66 kDa; bovine carbonic anhydrase, 29 kDa; horse cytochrome c, 12.4 kDa; Sigma) dissolved in 80 μl equilibration buffer containing 5% (v/v) glycerol. Blue dextran (2000 kDa, 160 μg in 80 μl) was used to measure V0. SDS–PAGE was used to verify fractions containing AXS1.
Histochemical β-glucuronidase assays
Arabidopsis plants transformed with pCAMBIA1301-AXS1 were selected on 0.8% (w/v) agar plates containing half-strength MS medium (Sigma), 3% (w/v) sucrose, 50 μg ml−1 hygromycin B (Calbiochem, La Jolla, CA, USA), and 500 μg ml−1 vancomycin (Sigma). The T2 generation was analyzed for GUS staining using a protocol adapted from Jefferson et al. (1987). To determine the expression pattern of AXS1::GUS, more than 15 individual transformants were analyzed.
RT-PCR
To determine the presence of AXS1 mRNA in various organs, the AXS1 transcript was amplified by RT-PCR using total RNA from various plant organs. RNA was purified with the RNeasy plant mini kit from Qiagen. The reverse transcription reactions were performed in a volume of 70 μl with 2 μg of RNA, 150 U M-MLV reverse transcriptase, 0.1 mm oligo (dT)12–18, and reverse transcriptase core reagents (Gibco-BRL, Rockville, MD, USA), according to the manufacturer's protocol. As a control, reverse transcriptase was omitted from the reaction mixture. PCR on reverse transcription reactions was performed in a volume of 50 μl using Pfu Turbo DNA polymerase, PCR core reagents (Stratagene, La Jolla, CA, USA), and the oligonucleotides 5′-ATCTCGACGGGAAACCGATACAA-3′ and 5′-GTTTGGATGTTGCCTTCTTCACA-3′. Restriction enzyme digests with PvuII were used to distinguish the AXS1-specific PCR products (which are cleaved into two fragments) from potential AXS2-specific products, which do not contain a PvuII cleavage site. In all cases, complete cleavage of the PCR product was observed indicating the absence of AXS2-derived amplification products.
Acknowledgements
We would like to thank Elmon Schmelzer and Klaus Hahlbrock (Max Planck Institut für Züchtungsforschung, Cologne, Germany) for the antiserum against the parsley UDP-d-apiose/UDP-d-xylose synthase, Scott Poethig (University of Pennsylvania) for providing an axs1 insertion mutant, and the Center for the Application of Molecular Biology to International Agriculture (CAMBIA) for plant transformation vectors. Support of this work by the DOE Energy Biosciences program (Grant no. DE-FG02-95ER20203) is gratefully acknowledged. M.M. was supported by a grant from the Danish Agricultural and Veterinary Research Council (Grant no. SJVF 23000237). Dedicated to Florence Dal Degan.




