Rapid and proven production of transplastomic tobacco plants by restoration of pigmentation and photosynthesis
Summary
Tobacco chloroplast transformation is typically achieved using dominant, selectable antibiotic resistance genes such as aadA, nptII and aphA-6. An improvement would be the combination of such a marker with a visual screening system for the early and conclusive detection of plastid transformants. As such, we investigated the use of three photosynthesis-deficient plastid mutants, ΔpetA, Δycf3 and ΔrpoA, for the development of a phenotypic selection system. Mutant plants were used as an alternative to the wild-type as source tissue for transformation, re-introducing deleted plastid sequences and using the aphA-6 gene as a selection marker. The reconstitution of the deleted genes in transformed regenerants resulted in shoots with a visually distinct phenotype comparable to the wild-type. This transformation/selection system overcomes the common problems associated with plastid transformation, e.g. the recovery of spontaneous mutants or nuclear insertions. In addition to the benefits offered by phenotypic selection, phenotype reconstitution leads to restoration of photosynthesis, which we assume drives reconstituted plants rapidly towards homoplasmy. As such, repeated cycles of regeneration in the presence of an antibiotic selection agent are no longer required.
Introduction
Compared to conventional nuclear transformation, the advantages of expressing genes in plastids are numerous and have been extensively reviewed (Bock and Hagemann, 2000; Daniell et al., 2002; Maliga, 2002). To date, four dominant selectable markers have been described for plastid transformation in higher plants: the aadA gene (aminoglycoside adenyltransferase) detoxifying spectinomycin and streptomycin (Svab and Maliga, 1993), the nptII and aphA-6 genes (aminoglycoside phosphotransferases) detoxifying kanamycin (Carrer et al., 1993; Huang et al., 2002) and the badh gene (betaine aldehyde dehydrogenase) in combination with betaine aldehyde as a selection agent (Daniell et al., 2001). A major disadvantage of such selection systems is that they can produce ‘false positives’ (a term used throughout this paper to describe all selected regenerants, which are not true plastid transformants). Random point mutations within plastid-encoded genes can result in the insensitivity to some antibiotics. Such a phenomenon has been documented for the antibiotics spectinomycin, streptomycin and lincomycin in Solanum nigrum (Kavanagh et al., 1994). In tobacco, vectors containing segments of tobacco plastid DNA carrying specific point mutations in the 16S rRNA gene (giving resistance to spectinomycin and streptomycin) were initially used to demonstrate the principle of plastid transformation in tobacco (Svab et al., 1990). However, in this pioneering work, the transformation frequencies were extremely low; from a total of 62 spectinomycin-resistant cell lines, only three were shown to be plastid transformants, the rest were spontaneous mutants. The recovery of spectinomycin-resistant mutants also remains an issue where the aadA gene is used as a plastid marker. During the last decade, the aadA gene has been used for the production of plastid transformants in tobacco (Svab and Maliga, 1993) and other species like Arabidopsis (Sikdar et al., 1998), potato (Sidorov et al., 1999) and tomato (Ruf et al., 2001). All of these reports describe the recovery of false positives because of the occurrence of spontaneous spectinomycin-resistant mutants. Double selection using spectinomycin and streptomycin can be used to alleviate this problem (De Santis-Maciossek et al., 1999; Koop et al., 1996), but streptomycin can decrease cell growth and prolonged exposure is also reported to be mutagenic (Sager, 1962).
False positives can also occur when the plastid-transformation vector gets inadvertently delivered to the nucleus, where random integration of the coding sequence (CS) behind an active promoter leads to expression. This was observed using the nptII marker (Carrer et al., 1993), where only three plastid transformants were obtained from a total of 99 kanamycin resistant lines; all other events analysed were nuclear transformants.
A further disadvantage of dominant selection markers is that gene expression in one cell can lead to localized detoxification of the selection agent in surrounding cells. This could also explain why false positives were recovered from experiments where plastid transformants were selected on betaine aldehyde (Daniell et al., 2001). Additionally, such cross-protection can have the undesirable effect of prolonging plastids in a heteroplastomic state (Dix and Kavanagh, 1995). Most laboratories describe the need to make several cycles of in vitro regeneration from small leaf explants in order to purge all wild-type plastomes from transplastomic lines. Such steps can considerably extend the time period of in vitro culture and are therefore undesirable.
In this paper, we describe a novel antibiotic/phenotypic selection system that utilizes plastid mutants as source tissue for chloroplast transformation in order to circumvent the problems outlined above. Several plastid mutants have been described in the literature, each of which displays a clearly distinguishable phenotype. Deletion of the plastid encoded petA gene leads to a chlorophyll-deficient phenotype with pale-green leaves (Monde et al., 2000). The petA gene product is the key protein of the cytochrome b6/f complex and mediates the electron transport between photosystem II (PS II) and photosystem I (PS I). Deletion of the plastid-encoded ycf3 gene leads to a light-dependent conditional phenotype. Under normal illumination, young leaves start out pale-green and slowly bleach to an off-white colour, while under reduced illumination the leaves have a green colour (Ruf et al., 1997). The ycf3 gene encodes a PS I-related protein, and although its exact function is not known, it is proposed to be involved in the assembly and/or stability of PS I. Specific deletion of the plastid-encoded rpoA gene, as with the genes encoding the other three subunits (rpoB, rpoC1 and rpoC2) of the plastid-encoded RNA polymerase (PEP) results in a non-functional PEP leading to altered plastid transcription (Allison et al., 1996; De Santis-Maciossek et al., 1999; Serino and Maliga, 1998). Under such circumstances, only non-PEP such as the nuclear encoded polymerase (NEP) are operational, and transcripts for many plastid proteins are dramatically reduced, resulting in a photosynthesis-deficient tissue with an albino phenotype.
For the generation of petA-, ycf3- and rpoA-deficient mutants, we constructed vectors to make specific deletions in the tobacco plastid genome. Our constructs were similar to those previously published for mutant production, where the targeted deletion was achieved through the insertion of the aadA selection gene (Monde et al., 2000; Ruf et al., 1997; Serino and Maliga, 1998). Homoplastomic mutant lines were then re-transformed using the aphA-6 gene as a second selectable marker, simultaneously re-introducing the deleted gene and restoring the wild-type phenotype. The key feature of the system is that, unlike standard transformation/selection protocols, green regenerants arising from re-transformation experiments are guaranteed to be plastid transformants. Furthermore, the approaches presented here have the additional advantage that reconstitution restores photosynthesis, which rapidly drives the transformants towards homoplasmy. The method of reconstitution of wild-type pigmentation and photosynthetic capacity described in this paper will also be applicable for the introduction of genes of interest into the plastid genome.
Results
Generation of plastid mutants
Plant transformation
Plastid mutants were generated from wild-type tissue by particle bombardment of leaves or grids containing protoplast-derived microcolonies using vectors pICF558, pICF577 and pICF585 (Figure 1). Numerous green calli/shoots were selected on RMOP medium (Svab et al., 1990) containing spectinomycin for each targeted deletion (petA, ycf3 and rpoA). Preliminary analysis was made by PCR to prove correct integration of the aadA gene into the plastome (data not shown). From petA-deletion experiments, we obtained 41 plastid transformants from 15 bombardments; from ycf3 deletion experiments, 14 transformants from 7 bombardments; and from rpoA deletion experiments, 18 transformants from 17 bombardments. Characteristically for spectinomycin selection, we also observed regenerants that were resistant to the antibiotic but did not carry the aadA gene (35 lines from a total of 108 selected). In order to obtain homoplastomic mutant lines with characteristic phenotypes, primary transformants were subjected to three to five rounds of regeneration on RMOP medium containing spectinomycin. Segregation of wild-type and mutant plastomes gave pale-green (ΔpetA, Δycf3) or white (ΔrpoA) sectors within leaves. To accelerate the segregation process, only explants from leaf sectors showing a mutant phenotype were chosen for repeated cycles of regeneration on selection medium. Further in vitro maintenance of homoplastomic lines was performed on antibiotic-free RMOP medium.
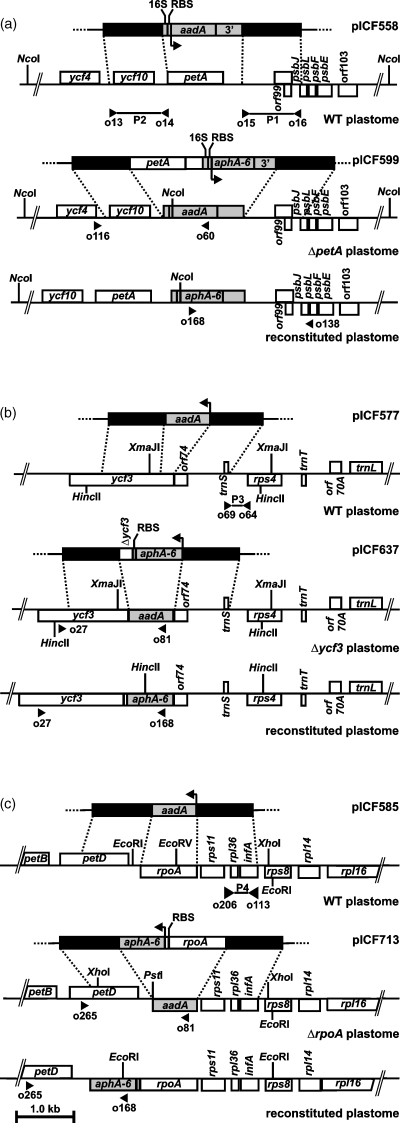
Plastid-transformation vectors.
(a) Deletion and reconstitution of the petA gene. Homologous recombination between pICF558 and tobacco wild-type (WT) plastome sequences results in a ΔpetA plastome containing an aadA expression cassette (16S rRNA promoter-aadA-3′UTR). Re-transformation of ΔpetA lines with pICF599 leads to the repair of the petA gene to give a reconstituted plastome containing the aphA-6 expression cassette (16S rRNA promoter-aphA-6-3′UTR) inserted downstream to the petA gene.
(b) Deletion and reconstitution of the ycf3 gene. Homologous recombination between pICF577 and WT sequences results in a Δycf3 plastome containing the aadA CS, under the control of endogenous ycf3 regulatory elements. Re-transformation with pICF637 leads to the repair of the ycf3 gene to give a reconstituted plastome where the aphA-6 gene is inserted upstream of the ycf3 gene as part of an artificial operon.
(c) Deletion and reconstitution of the rpoA gene. Homologous recombination between pICF585 and WT sequences results in a ΔrpoA plastome containing an aadA CS, under the control of endogenous rpoA regulatory elements. Re-transformation with pICF713 leads to the repair of the rpoA gene to give a reconstituted plastome where the aphA-6 gene is inserted downstream of the rpoA gene as part of an artificial operon. Flanking sequences used for homologous recombination are shown as black boxes, insertion sites and positions of the flanking homologies are indicated by dotted lines. The arrows indicate the transcription direction of the marker genes. Sites of primer annealing used for PCR analysis and probe synthesis (P1–P4) are indicated by filled triangles.
Southern analysis
Non-segregating lines from each of the three classes of plastid mutants were further analysed by Southern hybridization to confirm the predicted plastome deletion because of aadA insertion. Probes were chosen that hybridized to both, transformed and wild-type plastomes, in order to detect the two plastome types simultaneously (see Figure 1 for restriction sites and position of the probes). Total DNA from ΔpetA mutants and wild-type was digested with NcoI and hybridized with probe P1. The wild-type control gave a fragment of 13.082 kb, while all transformants gave the expected fragment of 6.212 kb (Figure 2a, lanes 1–4). For the analysis of Δycf3 mutants, total DNA was restricted with XmaJI and hybridized with probe P3. The wild-type control gave a fragment of 2.219 kb, while fragments of 2.792 kb were obtained from the transplastomes because of the insertion of the aadA coding region directly downstream of the ycf3 5′ regulatory elements (Figure 2b, lanes 1–5). In the third case, total DNA from ΔrpoA transformants was digested with XhoI and EcoRV and analysed by probing with P4. The wild-type control gave a band of 1.562 kb, while in all transformed lines, a fragment of 2.740 kb was observed because of the replacement of the rpoA coding region with the aadA coding region (Figure 2c, lanes 1–5).
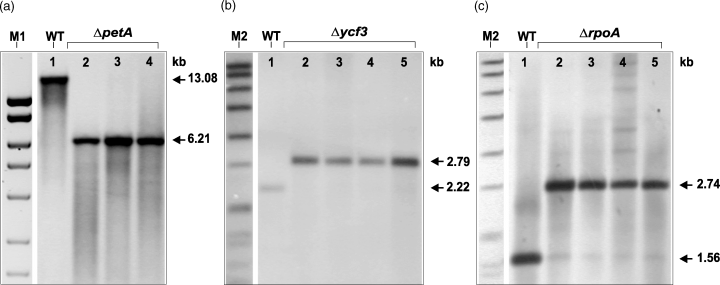
Southern analysis of plastid mutants.
For the respective restriction sites and probes (P1, P3 and P4) see Figure 1.
(a) Total DNA was NcoI digested and probed with P1 giving a fragment of 13.082 kb in the case of WT (lane 1) and 6.212 kb in the case of ΔpetA mutant lines (T9-3, T9-4, T9-5; lanes 2, 3 and 4, respectively).
(b) Total DNA was XmaJI digested and probed with P3 giving a fragment of 2.219 kb in the case of WT (lane 1) and 2.792 kb in case the of Δycf3 mutant lines (T44-10, T44-16, T44-18 and T44-20; lanes 2, 3, 4 and 5, respectively).
(c) Total DNA was digested with XhoI and EcoRV and probed with P4 giving a fragment of 1.562 kb in case the of WT (lane 1) and 2.740 kb in the case of ΔrpoA mutant lines (T63-7, T63-12, T63-23 and T64-10; lanes 2, 3, 4 and 5, respectively). M1, MassRuler DNA Ladder (10, 8, 6, 5, 4, 3 and 2.5 kb; Fermentas (MBI)); M2, DIG marker (8.576, 7.427, 6.106, 4.899, 3.639, 2.799, 1.953, 1.882, 1.515, 1.482, 1.164 and 0.992 kb; Roche).
The Southern analysis of ΔpetA and Δycf3 mutants showed no wild-type signals, and as such these lines can be considered homoplastomic. In contrast, faint bands of comparable size to the wild-type (1.562 kb) were observed in all of the four ΔrpoA mutant lines analysed. These, however, do not represent wild-type plastome copies as all attempts to amplify the rpoA gene by PCR failed (data not shown) and segregation of green sectors in these mutants was never observed.
Propagation of plastid mutants
Homoplastomic mutant lines (ΔpetA, Δycf3 and ΔrpoA) were propagated in vitro as shoot cultures and used for the production of mutant plants. After a growth period of 5–8 weeks, leaves from these plants were used for reconstitution experiments. In contrast to ΔrpoA mutant plants, ΔpetA and Δycf3 mutant lines were grown under low illumination in order to avoid problems associated with light-dependent photosystem degradation.
The production of tobacco seeds from homoplastomic mutant plants grown in vitro is difficult and their growth in the glasshouse is impossible as they are photosynthesis deficient. As such, heteroplastomic visually segregating plants from all mutants were transferred to the glasshouse and grown to maturity. Representative examples are shown for a ΔpetA plant (Figure 3a) and a ΔrpoA plant (Figure 3b), from which we obtained seedpods with a wild-type, segregating (Figure 3c,d) or mutant phenotype. Seeds from individual segregating and mutant seedpods were collected, surface-sterilized and germinated on antibiotic-free MS medium (Murashige and Skoog, 1962). Seedlings from the segregating seedpods were typically homoplastomic wild-type or mutant (Figure 3e,f), whereas seedpods with a non-segregating mutant phenotype yielded only homoplastomic mutant progeny.
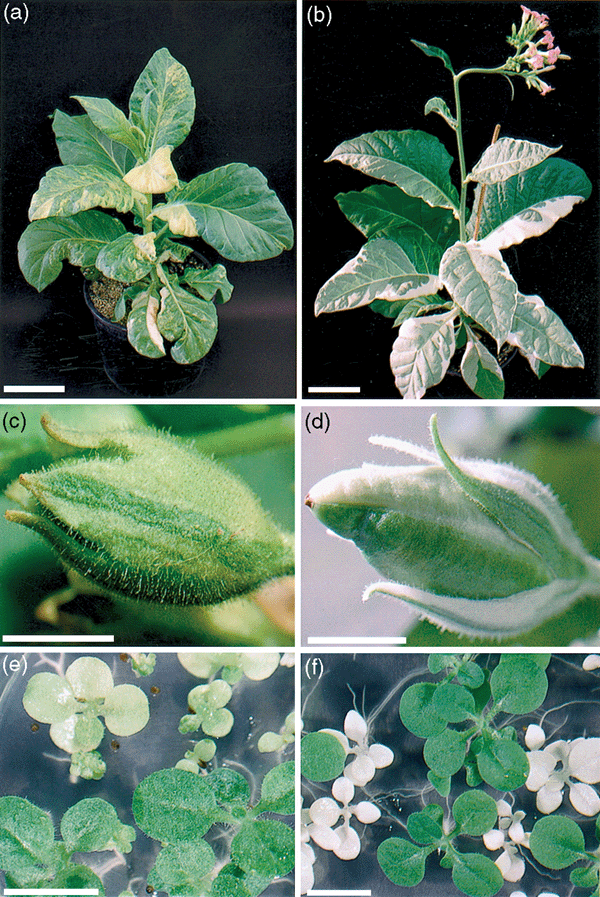
Production of seeds from ΔpetA and ΔrpoA mutants.
Visually segregating chimaeric plants grown in the glasshouse: (a) ΔpetA transformant and (b) ΔrpoA transformant containing mixed populations of WT and mutant plastids. Seedpods from self-pollinated heteroplastomic plants (c) ΔpetA and (d) ΔrpoA showing green (WT) and mutant sectors. T1 progeny, obtained from the seedpods shown in (c, d), germinated on antibiotic-free MS medium (e) ΔpetA and (f) ΔrpoA. The scale bars are equivalent to 1 cm except for (a, b), where it represents 10 cm.
Reconstitution of plastomes
Plant transformation
Leaves from homoplastomic mutants used for the second transformation showed characteristic phenotypes (Figure 4a). As described previously, leaves from ΔpetA and Δycf3 lines have a pale-green phenotype under low illumination and get bleached to a pale-yellow colour under standard illumination. Leaves from ΔrpoA lines show a non-conditional off-white phenotype. These mutant plants were used in transformation experiments for reconstitution of the petA, ycf3 and rpoA genes with vectors pICF599, pICF637 and pICF713, respectively (Figure 1). The deleted gene sequences were re-introduced by particle bombardment of mutant tissue using the aphA-6 gene in combination with kanamycin selection. As a consequence of the reconstitution, the primary marker gene (aadA) was removed and photosynthesis was restored to give a clearly distinguishable phenotype compared to the mutant tissue.
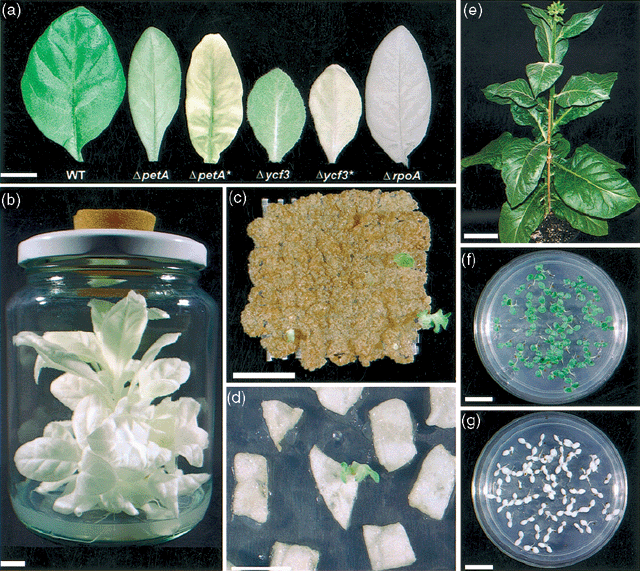
Reconstitution of plastomes.
(a) Phenotypic comparison of WT and ΔpetA, Δycf3 andΔrpoA leaves. The ΔpetA and Δycf3 mutants show a conditional response under low illumination and standard (*) illumination.
(b) ΔrpoA plant growing on VBW medium.
(c) Bombarded Δycf3 protoplast-derived colonies selected on RMOP medium containing 25 mg l−1 kanamycin. The green shoot and the colony to the right-hand side of the grid are two reconstitution events (T174, after 7 weeks of culture).
(d) Green reconstituted shoot from a bombarded ΔrpoA leaf explant selected on RMOP medium containing 15 mg l−1 kanamycin (T308, after 6 weeks of culture).
(e) Phenotypically normal rpoA-reconstituted plant flowering in the glasshouse (T308-5). T1 progeny from a self-pollinated rpoA reconstituted plant (T308-3) germinated on (f) MS medium containing 100 mg l−1 kanamycin and (g) MS medium containing 500 mg l−1 spectinomycin. The scale bars are equivalent to 1 cm except for (e) where it represents 10 cm.
Grid-embedded protoplast-derived colonies have previously been shown to be an efficient target tissue for the production of tobacco plastid transformants (Huang et al., 2002). Therefore, we developed protoplast regeneration systems for each of the three mutant types. Reproducible protoplast yields were obtained by doubling the enzyme concentration to that normally used. Yields of mutant protoplast preparations were up to 50% lower compared to the wild-type, and the reduced plating efficiencies were compensated by higher plating densities. Protoplasts isolated from ΔpetA and Δycf3 plants were embedded in grids, cultured under low illumination and transferred to standard illumination following bombardment. In contrast, protoplasts from ΔrpoA lines divided only when cultured in the dark. Following bombardment, the grids were transferred to low illumination for 2 weeks and then to standard culture conditions.
For the reconstitution of rpoA, we also investigated leaves as an alternative target tissue for bombardment. In vitro grown plants (Figure 4b) typically developed smaller leaves (compared to wild-type), and as such, several leaves were used collectively to generate a comparable target area. Bombarded grids containing protoplast-derived colonies were selected on RMOP medium containing 25 mg l−1 kanamycin, and regenerants were observed after 4–8 weeks of culture. From grids with ΔpetA and Δycf3 microcolonies, two types of regenerants were observed: those that displayed a pale-green rapidly bleaching phenotype, and others with a green non-bleaching phenotype (as shown in Figure 4c for ycf3 reconstitution). From the bombarded ΔrpoA leaves selected on RMOP medium containing 15 mg l−1 kanamycin, and from grids selected on 25 mg l−1 kanamycin, we recovered only green non-bleaching regenerants (Figure 4d).
Primary regenerants were removed from grids or leaf explants and transferred to individual dishes containing antibiotic-free RMOP medium. Only regenerants with a green non-bleaching phenotype were analysed by PCR to prove that all contained the aphA-6 marker and the re-introduced plastid sequences. Without exception, all 36 lines analysed gave the expected PCR products, indicating successful reconstitution (data not shown). The results obtained from the reconstitution experiments are summarized in Table 1, showing that all three different mutant plastomes were successfully reconstituted, although the overall transformation efficiencies were variable. The efficiency of the leaf bombardment experiments involving reconstitution of rpoA was high; on average, five transformants were obtained from every shot.
| Transformation experiment | Vector | Reconstitution | Target tissue/(number of actual bombardments) | Green regenerants/PCR positive lines |
|---|---|---|---|---|
| T202 | pICF599 | petA | microcolonies (14) | 1/1 |
| T174 | pICF637 | ycf3 | microcolonies (5) | 6/6 |
| T307 | pICF713 | rpoA | microcolonies (12) | 2/2 |
| T308 | pICF713 | rpoA | leaves (6) | 27/27 |
The first shoots obtained from PCR positive reconstituted lines were transferred directly to antibiotic-free B5 medium (Gamborg et al., 1968) for plant development without previous cycles of regeneration. In contrast to wild-type plants, ΔpetA, Δycf3 and ΔrpoA mutants show almost no growth on B5 medium, and this developmental difference was used to purge mutant plastomes and amplify reconstituted plastomes. On B5 medium, reconstituted shoots grew rapidly and developed into plants that were comparable to the wild-type. Only in very rare cases did we observe segregating leaf sectors because of the presence of residual mutant plastomes.
Southern analysis
For the confirmation of successful plastome reconstitution, Southern analysis was performed from glasshouse-grown plants together with control samples isolated from wild-type and mutant plants (Figure 5). Probes were chosen that hybridized to wild-type, mutant and reconstituted plastomes in order to be able to detect all plastome types simultaneously (see Figure 1 for restriction sites and position of the probes). Total DNA from the petA reconstituted line T202-1, the wild-type and a ΔpetA mutant line were digested with NcoI and hybridized with probe P2. The wild-type control gave a fragment of 13.082 kb (Figure 5a, lane 1), the mutant line gave a fragment of 7.025 kb (lane 2), whereas the reconstituted line T202-1 gave the expected fragment of 8.288 kb (lane 3). For the analysis of reconstituted ycf3 lines, total DNA from the wild-type, a Δycf3 line and reconstituted ycf3 lines was restricted with HincII and hybridized with probe P3. The wild-type control gave a signal of 3.257 kb (Figure 5b, lane 1), the mutant gave a fragment of 3.830 kb (lane 2), whereas fragments of 2.059 kb (lanes 3–6) were obtained in the reconstituted lines. In the third case, total DNA from the wild-type, a ΔrpoA mutant line and rpoA reconstituted lines were digested with PstI and EcoRI and analysed by probing with P4. The wild-type control gave a fragment of 2.384 kb (Figure 5c, lane 1), the mutant gave a fragment of 2.046 kb (lane 2), whereas the reconstituted lines gave fragments of 2.538 kb (lanes 3–5). Southern analysis confirmed the complete absence of mutant plastomes in the reconstituted plants. Thus, it can be concluded that all glasshouse-grown plants were homoplastomic with respect to reconstituted plastome content. Such a result is remarkable considering that none of the lines were subjected to repeated cycles of in vitro regeneration on antibiotic-containing medium.
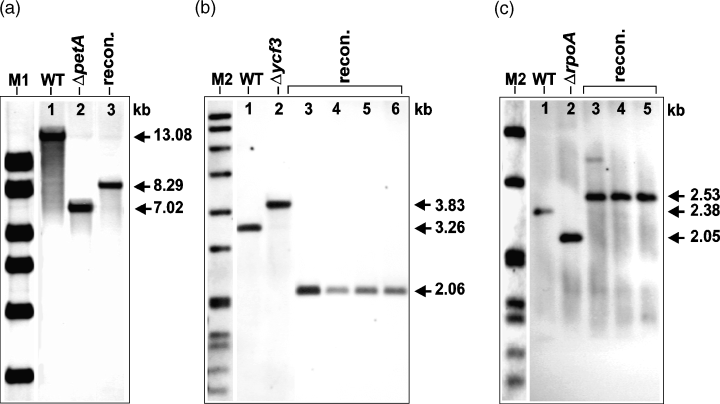
Southern analysis of reconstituted plastomes.
The respective restriction sites and probes (P2–P4) are shown in Figure 1. For confirmation of successful reconstitution, DNA extracts from WT and the respective mutants were used as additional controls.
(a) Total DNA was digested with NcoI and probed with P2 giving a fragment of 13.082 kb in the case of WT (lane 1), 7.025 kb in the case of a ΔpetA mutant control (T9-3, lane 2) and 8.288 kb in the case of the reconstituted line T202-1 (lane 3).
(b) Total DNA was digested with HincII and probed with P3 giving a fragment of 3.257 kb in the case of WT (lane 1), 3.830 kb in the case of a Δycf3 mutant control (T44-10, lane 2) and 2.059 kb in the case of reconstituted lines (T174-1, T174-2, T174-3 and T174-4; lanes 3, 4, 5 and 6, respectively).
(c) Total DNA was digested with PstI and EcoRI and probed with P4 giving a fragment of 2.384 kb in the case of WT (lane 1), 2.048 kb in the case of a ΔrpoA mutant control (T63-7, lane 2) and 2.533 kb in the case of the reconstituted lines (T308-3, T308-5 and T308-10; lanes 3, 4 and 5, respectively). M1 and M2 as detailed in Figure 2 (M2 in (c): 3.639, 2.799, 1.953, 1.882, 1.515, 1.482, 1.164 and 0.992 kb).
PCR monitoring of aadA and aphA-6 genes in reconstituted lines
The fate of the two marker genes (aadA and aphA-6) in three representative petA (T202-1), ycf3 (T174-3) and rpoA (308-3) reconstituted lines was monitored by PCR, using primer combinations to specifically amplify plastome insertions (Figure 6). To achieve this aim, one primer binding within the selection gene and a second one from outside of the flanks used for homologous recombination were chosen. Total DNA was isolated at four distinct time points from: (i) callus tissue of primary regenerants cultured on RMOP medium, (ii) leaves from plants grown on B5 medium, (iii) leaves from glasshouse-grown plants (GH) and (iv) the first transgenic progeny (T1, approximately 10 individuals for each analysis). For controls, total DNA was prepared from the leaves of a wild-type plant and each of the respective mutant lines. As expected, all wild-type (WT) control reactions were negative for both marker genes, and all mutant lines (Mut.) were only positive for plastome-integrated aadA. All primary reconstituted lines selected on RMOP medium containing kanamycin (RMOP) showed signals for both aadA and aphA-6 plastome insertions, indicating that these regenerants were heteroplastomic with respect to mutant and reconstituted plastome content. As described above, the growth of reconstituted plants on antibiotic-free B5 medium is a selective process that leads to the loss of mutant (aadA-containing) plastomes. Evidence for this phenomenon is also presented here, where the PCR-product from the aadA-specific amplification is clearly reduced in the petA reconstituted line and completely absent in the ycf3 and rpoA reconstituted plants grown in vitro on B5 medium (B5). When DNA from glasshouse-grown plants and T1 progeny of the respective reconstituted lines was analysed by PCR, all showed products for plastome-integrated aphA-6 and no products indicative of the presence of aadA.
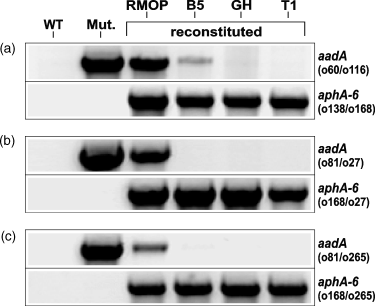
PCR monitoring of plastome integrated aadA and aphA-6 genes in reconstituted plants.
Analyses were performed using samples prepared from each of the reconstituted lines (a) petA (T202-1) (b) ycf3 (T174-3) and (c) rpoA (T308-3) at different time points. The presence of plastome integrated aadA (upper panels) is indicated by PCR products of (a) 1.717 kb, (b) 2.0 kb and (c) 1.886 kb, while the presence of plastome-integrated aphA-6 (lower panels) is indicated by products of (a) 2.171 kb, (b) 2.237 kb and (c) 1.878 kb. DNA samples were prepared from the following: the wild-type (WT), homoplastomic ΔpetA, Δycf3 and ΔrpoA mutants (Mut.), primary kanamycin-resistant reconstituted regenerants (RMOP), 4-week-old reconstituted plants grown on B5 medium (B5), glasshouse-grown plants (GH), and the first transgenic progeny (T1). The primer combinations are shown for each of the PCR reactions. The respective binding sites are given in Figure 1.
Additional PCR analyses were conducted with 10 other ycf3 and rpoA reconstituted lines in order to confirm that the rapid loss of aadA-containing plastomes during plant development is indeed a common phenomenon (Table 2). Here, samples were only analysed for the presence of aadA and not for aphA-6, which is guaranteed to be present in all reconstituted lines. The data obtained were similar to that shown in Figure 6 in that reconstituted lines rapidly loose the aadA marker following transfer of primary shoots from RMOP to B5 medium and are thus generally homoplastomic by the time glasshouse-grown plants and T1 progeny are analysed. In rare cases, mutant plastome copies were already undetectable in some lines (T174-11 and T307-1) prior to transfer of primary shoots to B5 medium. Taking all data together, mutant reconstitution and restoration of photosynthesis is a powerful selective tool for rapidly obtaining homoplastomic plants.
| Line | RMOP | B5 | GH | T1 |
|---|---|---|---|---|
| T174-2 | + | +/− | +/− | − |
| T174-4 | + | + | − | nd |
| T174-7 | + | − | − | nd |
| T174-10 | + | − | − | nd |
| T174-11 | − | − | − | nd |
| T307-1 | − | − | − | − |
| T308-5 | + | − | − | nd |
| T308-10 | + | − | − | − |
| T308-15 | + | − | − | nd |
| T308-20 | +/− | − | − | − |
- RMOP, primary reconstituted regenerants cultured on RMOP medium; B5, reconstituted plants grown on B5 medium; GH, glasshouse-grown plants; T1, first transgenic progeny.
- +, strong PCR signal; +/−, weak PCR signal; –, no PCR signal; nd, not determined.
Analysis of T1 progeny from reconstituted plants
Seeds from self-pollinated reconstituted plants (a representative example of an rpoA reconstituted plant is shown in Figure 4e) were surface-sterilized and germinated on MS medium containing spectinomycin or kanamycin, in order to confirm that all of the progeny were kanamycin resistant and spectinomycin sensitive. As an example, seeds from an rpoA reconstituted line (T308-3) germinated and remained green on 100 mg l−1 kanamycin (Figure 4f), whereas on 500 mg l−1, spectinomycin seedlings rapidly bleached (Figure 4g). No segregation was observed in either of the selection dishes with respect to bleaching of seedlings on kanamycin or greening of seedlings on spectinomycin. Further germination experiments gave comparable results to those described above for petA and ycf3 reconstituted lines (data not shown).
Discussion
In this work, we have shown that plastid mutants can be used as source tissue for the efficient and reproducible production of plastid transformants in higher plants. Mutants carrying targeted deletions in the chloroplast genes petA, ycf3 or rpoA display characteristic phenotypes as previously described (Monde et al. 2000; Ruf et al., 1997; Serino and Maliga, 1998). In contrast to standard selection protocols using antibiotic selection alone, the restoration of pigmentation in these mutants has the advantage that the reconstituted regenerants show a distinct visual phenotype that can be clearly differentiated from mutant tissue. Previous attempts to combine antibiotic selection with a non-destructive phenotypic marker have been described using the aadA gene in combination with the green fluorescent protein (GFP) from the jellyfish Aequorea victoria. In the published examples, the screening of plastid transformants was supported by examining spectinomycin-resistant tissue for GFP activity (Khan and Maliga, 1999; Sidorov et al., 1999). Both of these fluorescence selection systems are comparable to our reconstitution approach, where all phenotypically distinguishable regenerants were plastid transformants. The main advantage of our phenotypic selection system is that reconstituted regenerants can be detected simply by eye, whereas GFP-screening procedures require expensive fluorescence-detection equipment. The problems of using an antibiotic selection gene without an additional phenotypic marker are clearly illustrated from our transformation experiments for the generation of the ΔpetA, Δycf3 or ΔrpoA mutants. Here, only 73 of the 108 spectinomycin-resistant lines analysed by PCR were shown to be real plastid transformants; the others were false positives (spontaneous mutants or nuclear insertions).
In addition to the benefits given by phenotypic selection, reconstituted lines rapidly became homoplastomic without repeated cycles of regeneration on selection medium because of the restoration of photosynthesis. It is known from the literature that repeated regeneration on antibiotic-containing medium can be very time consuming, but it is necessary to obtain homoplastomic plants. Typically, the number of cycles required to purge all wild-type plastomes from transplastomic lines varies from one to two cycles (Staub and Maliga, 1995; Sugita et al., 1997) to four to six cycles (Eibl et al., 1999; Ruf et al., 1997). In our reconstituted lines, rapid segregation from mutant to reconstituted plastome content was observed once primary regenerants were removed from kanamycin selection and transferred to B5 medium.
The overall transformation efficiencies from all the reconstitution experiments using the aphA-6 gene as a marker were variable but comparable to results obtained from plastid transformation experiments using the aadA gene as a selection marker. Published transformation efficiencies using particle bombardment of wild-type tobacco leaves are in the range of 0.5–5 transformants from every bombardment (Daniell et al., 1998; Khan and Maliga, 1999; Sugita et al., 1997; Svab and Maliga, 1993). Our best results were obtained with ΔrpoA leaves where we were able to obtain on average five transformants from each bombardment. Even higher plastid-transformation efficiencies have been described using the badh gene, although these results are so far based on a single publication (Daniell et al., 2001).
In the earlier report describing the use of the aphA-6 gene as a plastid marker in tobacco (Huang et al., 2002), it was not possible to obtain transformants from bombarded wild-type leaves selected on 50 mg l−1 kanamycin. In contrast, here ΔrpoA leaves were shown to be a good target tissue for the efficient production of plastid transformants. We believe, this can be explained by the reduced kanamycin selection level (15 mg l−1) and the albino tissue, which enables the detection of green plastid transformants very easily.
Long-term maintenance of mutant shoot cultures in vitro is undesirable and can result in plants with abnormal phenotypes or reduced fertility because of somaclonal variation (Larkin and Scowcroft, 1981). As described previously (Allison et al., 1996), seeds from a ΔrpoB mutant were obtained following the grafting of a variegated periclinal chimaera to a wild-type tobacco plant. In this example, the periclinal shoot was homoplastomic for the rpoB deletion in the L2 layer, which forms the germ-line cells and gave homoplastomic mutant progeny. In our work, we used an alternative approach for the production of seeds from ΔpetA, Δycf3 and ΔrpoA mutant lines. Chimaeric heteroplastomic mutant lines with clearly segregating phenotypes were transferred to the glasshouse for seed production. As the segregation of mutant and wild-type plastomes in these plants is random, it gives rise to sectorial chimaeras, which may or may not contain mutant plastomes in the L2 layer. Some seedpods gave progeny, which were exclusively mutant in appearance, and plants raised from these seeds never showed segregating sectors indicative of residual wild-type plastomes.
The high transformation efficiency and the ease with which albino mutant tissue and green reconstituted tissue can be differentiated makes the reconstitution of rpoA the most attractive of the three different mutant reconstitution systems described here. Initially, we thought it was questionable whether the reconstitution of rpoA would be possible at all as homoplastomic lines are totally devoid of PEP. In contrast to ΔpetA and Δycf3 mutant lines, ΔrpoA mutants lack not only the rpoA gene product, but also as a consequence of a non-functional PEP, all PEP specific transcripts are absent (Allison et al., 1996; De Santis-Maciossek et al., 1999). As a complete shut down of all plastid housekeeping genes in ΔrpoA lines would result in a regulatory cul-de-sac, basal transcription of the whole plastome occurs by additional RNA polymerases such as the NEP, which are responsible for the maintenance of the basic plastid genetic machinery (Legen et al., 2002). We believe that the recovery of photosynthetically active plants from ΔrpoA mutants is dependent upon these alternative RNA polymerases, which trigger the production of functional PEP following reconstitution.
Our results also confirmed that marker genes can be effectively expressed from endogenous plastid regulatory elements rather than from chimaeric expression cassettes. Similar strategies have previously been described by Staub and Maliga (1995) and Huang et al. (2002). In the examples involving the deletion and reconstitution of ycf3 and rpoA genes, both marker genes (aadA and aphA-6) were introduced as coding sequences alone without additional regulatory elements. Operon-based insertions can avoid the duplication of plastid-derived expression elements, typically contained in chimaeric expression cassettes. Several publications describe undesirable recombination events, leading to the loss of plastome fragments enclosed within direct repeats (Eibl et al., 1999; Huang et al., 2002; Iamtham and Day, 2000; Svab and Maliga, 1993).
In this work, we have shown that the disruption of essential plastid genes and their reconstitution can be readily achieved in tobacco. As mutants of potato and tomato can also be maintained in vitro as shoot cultures and plastid transformation of these two species has recently been described (Ruf et al., 2001; Sidorov et al., 1999), our system based on the inactivation and reconstitution of chloroplast genes should be applicable here as well. As with standard plastid transformation systems, our approach can also be used to introduce genes of interest during the reconstitution step. Therefore, these mutants can be used instead of wild-type plants as target material for chloroplast transformation for the production of biotechnologically interesting proteins. A particularly desirable enhancement of our system would be the development of alternative selection protocols using reconstitution for the production of transplastomic plants that do not contain undesirable antibiotic marker genes. In conclusion, our work has shown that chloroplast mutants with a clearly distinguishable phenotype can be used for the rapid and proven production of plastid transformants in tobacco. Although two different selection markers have been used for co-transformation experiments previously (Carrer and Maliga, 1995), here we have shown the consecutive use of two independent antibiotic selection markers for plastome engineering, which should open new possibilities in the field of higher plant plastid transformation.
Experimental procedures
Vector construction
A schematic representation of the constructs used to delete and reconstitute petA, ycf3 and rpoA genes is shown in Figure 1.
Deletion and reconstitution of the petA gene using vectors pICF558 and pICF599
For the deletion of the petA gene, an aadA expression cassette was introduced replacing the petA CS and a 0.3-kb downstream region (deleted plastome nucleotides 64335–65597, GenBank Accession number Z00044). The two flanks for homologous recombination (63335–64334 and 65598–66597) were amplified by PCR and ligated together with the aadA cassette (pUC-16S-aadA; Koop et al., 1996). For the reconstitution of the petA gene, vector pICF599 was used (Huang et al., 2002).
Deletion and reconstitution of the ycf3 gene using vectors pICF577 and pICF637
For the deletion of the ycf3 gene, the aadA CS was introduced replacing the first exon and part of the first intron (deleted plastome nucleotides 46042–46266). The two flanks for homologous recombination (plastome nucleotides 45033–46041 and 46269–47205) were amplified by PCR and ligated together with the aadA CS (pUC16SaadA) into pUC19 to give vector pICF577. For the reconstitution of the ycf3 gene, vector pICF637 was used (Huang et al., 2002).
Deletion and reconstitution of the rpoA gene using vectors pICF585 and pICF713
For the deletion of the rpoA gene, the aadA CS was introduced replacing the CS of the rpoA gene (deleted plastome nucleotides 80455–81468). The two flanks for homologous recombination (79401–80454 and 81469–82470) were amplified by PCR and ligated together with the aadA CS into pUC19 to give vector pICF585. The left flank (79401–80454) of the rpoA reconstitution vector pICF713 was amplified by PCR. The right flank of this vector was amplified as one fragment together with the CS for the rpoA gene (80455–82470), incorporating a synthetic ribosomal binding site (atcactagttgtagggagggatcc) at the 5′ end of the amplified fragment. The two PCR fragments were ligated together with the aphA-6 CS (pSK.KmR; Bateman and Purton, 2000) into pUC19 to give vector pICF713.
Plant material
Wild-type Nicotiana tabacum L. cv. Petit Havana plants were grown in vitro on B5 medium (Gamborg et al., 1968) as described by Koop et al. (1996). Homoplastomic mutant lines were maintained in vitro on antibiotic-free RMOP medium (Svab et al., 1990). For the routine growth of mutant plants, shoots from these cultures were transferred to glass jars containing MS medium (Murashige and Skoog, 1962) for ΔpetA lines or VBW medium (Aviv and Galun, 1985) for Δycf3 and ΔrpoA lines. Plants from ΔpetA and Δycf3 lines were cultured under low illumination (5.0 ± 1.0 µmol m−2 sec−1, 16-h day, 27 ± 1°C), whereas ΔrpoA plants were grown under standard illumination (70 ± 10 µmol m−2 sec−1, 16-h day, 27 ± 1°C). Alternatively, mutant plants were grown from seed on MS medium and then transferred to glass jars containing MS or VBW medium.
Protoplast isolation and culture
Leaves from 3–4-week-old wild-type and 4–8-week-old mutant tobacco plants were used for protoplast isolation (Koop et al., 1996), and grid-embedding was performed as previously described by Dovzhenko et al. (1998) with the modifications given by Huang et al. (2002). For the isolation of protoplasts from mutant leaves, the enzyme concentration was doubled (0.5% w/v cellulase Onozuka R10 and 0.5% w/v macerozyme R10). The plating density of mutant protoplasts was increased to 7.0 × 104 cells per alginate grid. Following embedding, protoplasts from ΔpetA and Δycf3 plants were cultured in the dark for 1 day and then transferred to low illumination. ΔrpoA protoplasts were maintained in the dark until the first divisions were observed (approximately 7 days) and then transferred to low illumination.
Plant transformation and selection
For plastid transformation, we used leaves or microcolonies as target tissue in combination with particle bombardment (PDS-1000/He, Bio-Rad) as described by Huang et al. (2002). Following transformation, selection was made on RMOP medium containing 500 mg l−1 spectinomycin (aadA vectors) or RMOP with 15–25 mg l−1 kanamycin (aphA-6 vectors). In order to amplify the desired transplastomes in mutant lines, small leaf explants (2 mm × 2 mm) were subjected to several rounds of regeneration on selective medium to achieve homoplasmy.
DNA isolation, PCR and Southern analysis
DNA isolation, PCR and Southern analysis were made as detailed by Huang et al. (2002). For the synthesis of digoxigenin-labelled probes (P1–P4), tobacco wild-type DNA was used as template in combination with the relevant PCR primers (see Figure 1 and Table 3). For all PCR reactions, 100 ng of template DNA was used as standard.
| Name | Sequence (5′→3′) | Binding positions |
|---|---|---|
| o13 | gggaattccatatggtataaaactcatgtgtgtaagaaa | 63335–63359a |
| o14 | tcccccgggggtccaatcattgatcgcgaaa | 64313–64334a |
| o15 | ttccccgggttctaaatagaaagaaagtcaaatttg | 65598–65624a |
| o16 | acatgcatgcgaatcaataagattctcttagctc | 66574–66597a |
| o60 | cactacatttcgctcatcgcc | 943–963b |
| o64 | gaattaccaaaccatttgaccc | 47646–47667a |
| o69 | cattggaactgctatgtaggc | 47149–47169a |
| o81 | ctatcagaggtagttggcgtc | 439–459b |
| o113 | tcccccgggtaattactgaatcgcttccca | 82450–82470a |
| o168 | tcagtcgccatcggatgttt | 168–187c |
| o169 | accaatctttcttcaacacg | 628–647c |
| o206 | tgagtcagagatatatggat | 81971–81990a |
T1 analysis of reconstituted plants
Progeny analysis was performed with seeds collected from wild-type and self-pollinated petA, ycf3 and rpoA reconstituted lines grown in the glasshouse. Seeds were surface-sterilized and germinated on B5 or MS medium containing spectinomycin (500 mg l−1) or kanamycin (100 mg l−1).
Acknowledgements
We thank Carolin Adams for expert technical assistance. We are also grateful to Dr S. Mühlbauer for helpful comments and Dr C. Stettner for critical reading of the manuscript.




