Expression of CD137 (4-1BB) on Human Follicular Dendritic Cells
Abstract
Follicular dendritic cells (FDCs) are the antigen (Ag)-trapping accessory cells of the germinal centres (GCs), essential for the development of humoral immune responses and memory. FDCs reside in the microenvironment of secondary lymphoid tissue where Ag-activated B cells expand, and undergo isotype switching and affinity maturation prior to becoming memory B cells. In addition to delivering Ag, FDCs also provide potent nonspecific accessory signals to the B cells, which are important for the GC reaction. In this report, we show that human tonsilar FDCs express the costimulatory molecule CD137. Surface expression of CD137 on FDCs was confirmed by immunofluorescent labelling and fluorescence-activated cell sorter analysis. CD137 was also highly expressed by the human cell line HK, which displays many characteristics of in vivo FDCs. The interaction between B cells and FDCs is essential for the GC reactions, and our finding suggests that CD137 plays a role in FDC-regulated B-cell responses.
Introduction
The microenvironment in germinal centres (GCs) dictates many of the properties of T-cell-dependent B-cell immunity, such as somatic mutation, class switching and development of memory B cells [1, 2]. GCs in tonsils comprise antigen (Ag)-specific B cells, macrophages, dendritic cells, follicular dendritic cells (FDCs) and activated T cells, the last two being necessary for the GC formation [2–4]. FDCs are exclusively localized within B-cell follicles, where they deliver Ag to B cells. When antibodies are produced during a primary immune response, antibody–Ag (Ab–Ag) complexes are formed and retained on FDCs. High affinity centrocytes are positively selected in the light zone of GCs if they bind specifically to Ags trapped on the surface of FDCs. The main receptor for trapping immune complexes is CD21 long isoform, which is the target for many FDC-specific antibodies [2]. The Ags, which are trapped without being internalized or processed, can be presented by FDCs for long periods of time, a feature that is believed to be important for long-term maintenance of humoral immunity [5]. In addition to delivering Ags and converting the immune complexes into highly immunogenic forms, FDCs also provide the B cells with potent nonspecific accessory signals [6]. These costimulatory signals, together with the signal through the B-cell receptor, are necessary for the development of secondary Ab responses. However, the molecules involved in the Ag-independent costimulatory signalling are so far not completely elucidated.
Human CD137 (4-1BB) belongs to the tumour necrosis factor receptor (TNFR) superfamily and is expressed primarily by immune cells, such as activated CD4+ and CD8+ T cells [7], as well as by monocytes [8]. Cross-linking of CD137 triggers an intracellular signalling pathway, which leads to the activation of nuclear factor kappa B (NF-κB) and costimulation of T cells, inducing T-cell proliferation and cytokine secretion [9]. Its natural ligand, CD137L (4-1BBL), is a member of the tumour necrosis factor (TNF) superfamily. CD137L can be expressed by activated T cells and Ag-presenting cells (APCs), including B cells, macrophages and dendritic cells [10]. So far, the literature is sparse on the function of human CD137. In humans, signalling through CD137 using anti-CD137 Ab has been shown to regulate CD28 costimulation in CD4+ T cells to promote Th1 responses [11] and to induce the production of interleukin-8 and TNF-α in monocytes [8]. Recently, it was shown that CD137L can stimulate CD4+ and CD8+ T cells in the absence of CD28 and that CD137L signalling leads to increased expansion, cytokine production and development of cytolytic effector function by human T cells [12]. Bidirectional signalling has been shown for the CD137/CD137L system. While cross-linking CD137 leads to the activation of T cells, the reverse signalling following cross-linking of CD137L has been shown to inhibit proliferation, to upregulate CD95 expression on lymphocytes and to induce apoptosis in lymphocytes independently of CD95 [13].
In this study, we report that human FDCs in tonsils do express the costimulatory molecule CD137. Immunohistological staining and fluorescence-activated cell sorter (FACS) analysis confirm this expression of CD137 in tonsil. This discovery opens up new possible functions of CD137 and FDCs in the regulation of human immune responses.
Materials and methods
Histology Tonsils obtained from children undergoing tonsillectomy at Lund University hospital (Lund, Sweden) or at Malmö Academic Hospital (Malmö, Sweden) were frozen in liquid nitrogen. Cryostat sections (8 µm) were air-dried on microscope slides, fixed with ice-cold 4% paraformaldehyde and quenched for endogenous peroxidase activity with 0.3% H2O2. After blocking with 5% goat serum, the cells were incubated with primary Abs, rabbit anti-CD3, mouse anti-CD20 or mouse anti-DRC-1 (Dako A/S, Glostrup, Denmark). Staining with primary Abs was revealed by Alexa 488-conjugated goat antimouse immunoglobulin G (IgG) (Molecular Probes, Eugene, OR, USA) or rabbit antimouse Ig (Dako A/S), followed by Alexa 488-conjugated goat antirabbit IgG (Molecular Probes). Remaining antimouse reactivity on sections was blocked with mouse serum, and endogenous biotin was blocked with a biotin-blocking kit (Dako A/S). The cells were thereafter stained with a preformed complex of mouse anti-CD137 (Biosource, Camarillo, CA, USA) and biotin-conjugated Fab of goat antimouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Unbound Fab was blocked with 10% mouse serum before Ab complexes were added to the sections. CD137 staining was amplified using a tyramide signal amplification biotin system (NEN Life Science, Boston, MA, USA) and visualized by Alexa 568-conjugated streptavidin (Molecular Probes). Phosphate-buffered saline (PBS) with 5% goat serum was used to dilute the reagents, and PBS was used during washing. To assess the background staining in the tissue sections, the control slides were stained identically, with the primary Abs excluded. The slides were mounted using a prolong antifade kit (Molecular probes). For imaging, a laser-scanning confocal device (model MRC-1024; Bio-Rad, Hercules, CA, USA) equipped with a 15 mW krypton/argon laser and attached to an Eclipse E800 microscope (Nikon, Melville, NY, USA) was used.
Isolation and FACS analysis of tonsilar CD137+ cells Tonsils were cut into pieces and subjected to two enzymatic digestion cycles using 50 mg/ml of collagenase IV and 50 U/ml of DNase I (Sigma-Aldrich, St. Louis, MO, USA) in RPMI-1640 supplemented with 50 mg/ml of gentamicin (Invitrogen, Paisley, UK). For each cycle, tissue fragments were incubated at 37 °C for 30 min. Released cells were pooled, washed in RPMI supplemented with gentamicin and centrifuged on 50% isotonic Percoll (Pharmacia Biotec, Uppsala, Sweden) at 900 × g for 20 min. Cells in the interphase were washed in RPMI with gentamicin and incubated with mouse anti-CD137 (Biosource) for 30 min on ice. Cells were then washed and incubated with secondary Ab, goat antimouse IgG conjugated to magnetic microbeads (magnetic cell sorting (MACS); Miltenyi Biotec, Auburn, USA), for 15 min at 4 °C. CD137+ cells were then purified by MACS, using magnetic cell separation columns (Miltenyi Biotec). Rabbit antimouse Ig-PE was used to visualize CD137 expression. Cells were thereafter blocked with 10% mouse serum and incubated with mouse IgM anti-DRC-1 Ab (Dako A/S) for 30 min on ice. Secondary Ab rabbit antimouse IgM-biotin (PharMingen, San Diego, CA, USA) was added, and the cells were incubated for 30 min on ice. DRC-1 expression was visualized with Streptavidin-PECy5 (Dako A/S). PBS (w/o Ca/Mg; Invitrogen) containing 1% bovine serum albumin (BSA) (Sigma-Aldrich) was used in cell labelling and washing steps. The cells were stained with antibodies, diluted to the optimal staining concentration, for 30 min on ice, washed and resuspended in PBS/BSA for analysis. The FACS analysis was performed on a FACScan (Becton Dickinson, San Jose, CA, USA), using cellquest analysis software. Gates were set to exclude debris and nonviable cells.
Culture and immunostaining of HK cell line The HK cell line, kindly provided by Dr Yong Sung Choi (Cellular Immunology Laboratory, New Orleans, USA) was cultured in RPMI-1640 supplemented with 10% (v/v) fetal bovine sera (Gibco, Grand Island, NY, USA), 50 µg/ml gentamicin, 2 mm l-glutamine and 1% (v/v) nonessential amino acids (Invitrogen). To enable immunostaining, the adherent cells were grown directly on tissue culture-treated microscope slides (BD Falcon, Franklin Lakes, NJ, USA). The HK cells were air-dried, fixed in 4% ice-cold paraformaldehyde for 10 min and stained according to the protocol above. Staining with primary Ab mouse anti-DRC-1 (Dako A/S) was visualized with rabbit antimouse Ig (Dako A/S) and Alexa 488-conjugated goat antirabbit Ig (Molecular Probes). CD137 expression was visualized with goat anti-CD137-biotin (BAF838; R & D Systems, Minneapolis, MN, USA), tyramide signal amplification biotin system (NEN Life Science) and Alexa 568-conjugated streptavidin (Molecular Probes).
Results
To explore the tissue distribution of CD137, immunohistological staining of tonsil tissue sections was performed. The cryostat sections were double labelled with Abs to CD137 and DRC-1, CD20 or CD3 and imaged by confocal microscopy. Surprisingly, a majority of the CD137-expressing cells in human tonsil were localized in the GCs (Fig. 1), even though some expression also was detected in the T-cell zone. The dense network of CD137+ cells evident in the B-cell follicles was double stained with Ab to DRC-1, which binds the CD21 long isoform, specific for FDCs (Fig. 1B). Interestingly, the two Ags colocalized in the GCs (Fig. 1C), which indeed demonstrates that FDCs express high levels of CD137 Ag. To rule out B and T cells as producers of the high expression of CD137 observed in GCs, the sections were also double labelled with anti-CD20 (Fig. 1D–F) or anti-CD3 (Fig. 1G–I). CD20 expression by B cells in tonsil was detected in the entire GCs including the mantle zone, whereas CD137+ cells were highly confined to the interior of the follicles (Fig. 1F). Similarly, anti-CD3 Ab mainly labels the T-cell-dependent area of the tonsils, although some CD3 expression could be detected in GCs (Fig. 1H). The absence of colocalization of CD137 and CD20 or CD3 (Fig. 1F and 1I) clearly shows that FDCs are the main producers of CD137 in B-cell follicles. Furthermore, the tonsil sections were double stained with anti-CD137 and anti-CD11c monoclonal antibodies (MoAbs) to evaluate the CD137 expression on dendritic cells (data not shown). The CD11c+ cells were only sparely distributed in the follicles and could also be ruled out as contributors to the high CD137 expression in GCs. In addition to the MoAb antihuman CD137 (Dako A/S, clone BBK-2) used in the immunohistological stainings in Fig. 1, parallel labelling was performed with a polyclonal antihuman CD137 (R & D Systems) with identical results. This was done to rule out eventual unspecific reactivity of the Abs.
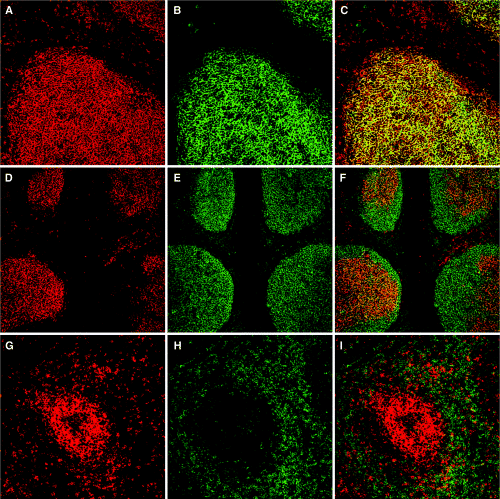
Immunohistological localization of CD137 expression in human tonsil. Cryostat sections were double stained with anti-CD137 monoclonal antibody (red) plus anti-DRC-1, anti-CD20 or anti-CD3 Abs (green), as described in Materials and methods and analysed in situ under the confocal microscope. (A–C) Anti-CD137 (A) and anti-DRC-1 (B) immunofluorescent double staining revealed high level of expression of CD137 in germinal centres (GCs) and colocalization with DRC-1+ follicular dendritic cells (yellow, C). (D–F) In contrast, labelling with anti-CD137 (D) and anti-CD20 (E) show CD137 and CD20 expression in tonsilar GCs but on different cell types (F). Anti-CD20 typically labels the entire B-cell follicle including the mantle zone, whereas anti-CD137 labels cells in the follicle interior. (G–I) Anti-CD137 (G) staining reveals expression of CD137+ cells in both the GC and the T-cell-dependent area of tonsils, whereas anti-CD3 (H) stains primarily the T-cell zone. Surprisingly, double staining (I) showed low colocalization of CD137- and CD3-expressing cells. Tonsil stainings are representative of three different donors.
To confirm the expression on FDCs, CD137+ cells in tonsils were isolated using MACS and analysed for their expression of FDC-specific marker DRC-1 (Fig. 2). Indeed, more than 50% of the cells expressing CD137 also expressed DRC-1. The forward and side scatter plots revealed that DRC-1+/CD137+ cells were larger and exhibited a more irregular cell shape than DRC-1–/CD137+ cells, showing that FDCs clearly constitute a separate population of CD137+ cells in tonsils.
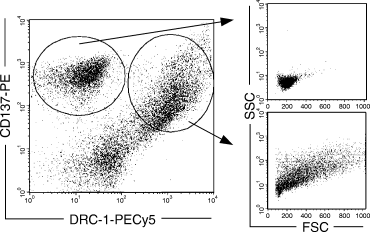
More than 50% of the CD137-expressing cells in human tonsil also express follicular dendritic cell (FDC)-specific marker DRC-1. CD137+ cells were positively isolated with magnetic cell sorting, double stained with anti-DRC-1 Ab and analysed by fluorescence-activated cell sorter. As indicated in the FSC/SSC (forward/side scatter) plots, CD137+/DRC-1– cells were homologous in size compared with the CD137+/DRC-1+ FDCs, which exhibited a more heterogeneous and enlarged cell morphology.
Next, the FDC cell line HK, which has been shown to display many characteristics of the in vivo counterpart such as binding, rescuing and stimulating GC B cells [14], was analysed for its ability to express CD137 Ag. Owing to the nature of this cell line, it was difficult to analyse the expression with flow cytometry. Instead, immunohistological staining with anti-CD137 and DRC-1 MoAb was performed, which revealed that both Ags are indeed expressed by the cell line, even though the expression of DRC-1 was weak (Fig. 3).
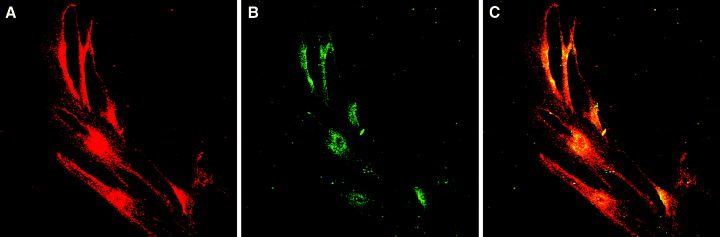
CD137 is expressed in the human follicular dendritic cell (FDC) cell line HK. The adherent cell line HK was grown directly on cell culture-treated microscope slides and labelled with anti-CD137 (red, A) and anti-DRC-1 (green, B) as described in Materials and methods. The immunofluorescent labelling was analysed with a confocal microscope. Yellow colour indicates the colocalization of CD137 and DRC-1 expression on HK (C).
Discussion
FDCs trap immune complexes with their abundant complement and Ig Fc receptors within the B-cell follicles, a feature which is crucial for the development of high-affinity, isotype-switched Ab responses [3]. The signals for survival, proliferation and differentation of GC B cells are, however, poorly defined partly because of the difficulties in the isolation and analysis of FDC–B-cell interaction. Our finding that FDCs express high levels of CD137 on the cell surface represents an additional way for the FDCs to interact with the CD137L+ GC B cells and is in agreement with recent data on the effect of CD137 on the activation of GC B cells [15]. Interestingly, the two main regulators of the humoral immune response, the T cells and the FDCs, have now been shown to produce CD137. T cells regulate the B-cell response by eliminating autoreactive B-cell clones, whereas FDCs present Ag and select for high-affinity clones [4]. In addition to providing survival signals, some FDCs have also been shown to express FasL and may also participate in the apoptosis of GC B cells [16]. In T cells, CD137 ligation provides costimulation and can inhibit activation-induced cell death [17]. The costimulatory CD137 has been shown to be especially potent for CD8+ T cells [18, 19], promoting enhanced cytokine production, proliferation and cytolytic function.
The TNF superfamily and their ligands appear to strongly influence the regulation of GC formation with affinity matured immune responses and FDC organization. Inhibition of lymphotoxin α/β or TNF signalling leads to the disappearance of multiple markers and elimination of trapped immune complexes on FDCs, rendering them nonfunctional [20]. In addition, mice knockout models such as LTα–/–, LTβ–/– and TNF–/– are devoid of splenic FDC networks and B-cell follicles [21]. Signals through the LTβR and TNFR-1, both expressed on FDCs, are thus required for the development of FDC clusters and GCs. It is still unknown whether a lack of FDCs in the knockout models leads to impaired development of primary B-cell follicles or whether a lack of follicles prevents the formation of FDC clusters. Mice deficient in either CD40 or CD40L are unable to form GCs and high affinity, class-switched antibodies [22, 23]. The same inability to mount T-cell-dependent humoral responses and develop GCs is seen in human patients with hyper IgM syndrome, which is caused by a mutation in CD40L [24].
Mice models deficient in either CD137 or CD137L have been generated recently to evaluate the role of the receptor/ligand pair in mediating immune responses in vivo[25, 26]. CD137–/– mice displayed normal ability to mount Ag-specific responses but demonstrated enhanced T-cell proliferation in response to mitogens and increased turnover of myeloid progenitors [26]. CD137L–/– mice developed normally and had normal humoral responses against vesicular stomatitis virus [25]. However, the CD8 cytotoxic T lymphocyte (CTL) responses were decreased when the mice were infected with influenza virus. Similarly, infection of CD137L–/– mice with lymphocytic choriomeningitis virus reduced the number of CTLs [27]. These reports indicate that CD137 costimulation in mice is not required for immune system organogenesis but rather important for the generation of antiviral CD8 T-cell responses.
The finding of reverse signalling through CD137L suggests that CD137L have broader functions than providing a costimulatory signal to CD137 on the regulation of immune responses. In a recent study, it was indeed shown that CD137L has a major impact on the regulation of B-cell survival [28]. CD137L transgenic (Tg) mice, where CD137L cDNA was expressed under the control of major histocompatibility complex class II I-Eα promotor, exhibited progressive depletion of peripheral B lymphocytes, low levels of circulating IgG and defective humoral responses to Ag challenge. In this model, where CD137L was exclusively produced by APCs, the number of T cells remained unchanged, whereas B cells were depleted. Interestingly, no B-cell follicles could be detected in the Tg mice model. In contrast to the B-cell follicles, enlarged, but less defined, T-cell areas were found. The mechanism leading to this phenotype is still unknown, but studies on the effect of FDC-expressed CD137 on GC formation in Tg mice would be interesting. Cross-linking of CD137L by CD137 has been shown to induce apoptosis in lymphocytes [8, 13]. The increased interaction between CD137+ FDCs and CD137L+ B cells may result in enhanced depletion of B cells, and thus lack of formation of follicles.
Functional studies concerning the CD137-CD137L signalling are important for better understanding of the interaction between FDC and B cells. The cell line HK was also found to express CD137 and can thus be used as a substitute for FDCs in future functional studies. Our finding that FDCs express CD137 contributes to better understanding of FDC biology and may have implications for the regulation of GC reactions.
Acknowledgments
This work was supported by a grant from the Vårdal Foundation.




