Treatment of an Immortalized APC Cell Line with Both Cytokines and LPS Ensures Effective T-Cell Activation In Vitro
Abstract
Antigen-presenting cells (APCs) are crucial for the generation of a functional immune response to pathogens. Furthermore, there is abundant evidence for their importance in primary T-cell activation, B-cell maturation and maintenance of an ongoing immune response. In the present study, we have analysed phenotypic characteristics and functionality of a p53-deficient APC cell line (JawsII) derived from mouse bone marrow culture. We show that unstimulated JawsII cells express low surface levels of major histocompatibility complex (MHC) and costimulatory molecules, both of which can be upregulated upon treatment with cytokines in vitro. Cytokine stimulation also leads to an enhanced T-cell activation capacity but has only little effect on cytokine release by the JawsII cells themselves. On the contrary, stimulation of the JawsII cells with lipopolysaccharide (LPS) leads to the production and secretion of high amounts of interleukin-1 (IL-1), IL-6 and tumour necrosis factor-α (TNF-α) but no increase in the surface levels of MHC and costimulatory molecules, and has only little effect on the T-cell activation capacity. Our data suggest that the effects observed upon treatment with cytokines or LPSs are complementary, and that both stimuli are needed for mediating a strong and efficient JawsII cell-dependent T-cell activation.
Introduction
Priming of T cells relies on the activation of professional antigen-presenting cells (APCs) capable of taking up antigens. Professional APCs are located in the peripheral tissues (skin, mucosal areas, lung, etc.), and are characterized by the ability to express high levels of major histocompatibility complex (MHC) class I, MHC class II and costimulatory molecules (e.g. B7-1, B7-2 and CD40) upon activation. The repertoire of professional APCs comprises dendritic cells (DCs), macrophages (ΜΦs) and activated B lymphocytes. Murine DCs and MΦs can be isolated from bone marrow or lymphoid organs, and are usually distinguished based on their expression pattern of surface markers. Thus, DCs express the DEC205 (some, but not all), 33D1 and CD11c markers, while ΜΦs express F4/80, CD11b and CD68 [1–4]. However, cell subsets expressing markers of both MΦs and DCs have also been identified [5, 6]. Freshly isolated DCs express low levels of MHC class II and costimulatory molecules, and are known as immature DCs. However, maturation of the cells can be induced simply by transferring the cells from one well to another, and thus, after in vitro culture, many of the cells express intermediate or high levels of MHC class II and costimulatory molecules [7]. These cells might be called semimature. The final maturation of DCs in vivo takes place after exposure to antigens in the presence of pathogen-derived molecules such as lipopolysaccharide (LPS) or dsRNA. In addition, cytokines present during antigen-uptake might influence the maturation process (reviewed in [8]). Mature DCs express high levels of MHC and costimulatory molecules as well as a variety of other cell-surface molecules, and release large amounts of cytokines and chemokines. In vitro, a similar maturation process can be induced in numerous ways [7, 9–14], converting DCs into powerful APCs [15].
Another important feature of DCs is their capacity to determine the route of differentiation of naïve T cells after encountering antigen in the context of MHC on the surface of DCs. Correspondingly, cytokines released by APCs are believed to play a key role in the differentiation of T-helper (Th) cells into Th1 or Th2 cells. Thus, DCs have been reported to produce interleukin-12 (IL-12) or IL-6, which are strong modulators of Th cells, driving the differentiation of naïve T cells towards the Th1 or Th2 cell subsets, respectively, as well as pro-inflammatory cytokines (IL-1, IL-15, IL-18 and tumour necrosis factor-α (TNF-α)) important for the overall T-cell stimulatory effect [10, 16–21].
In the present study, we have investigated the phenotype and functionality of a long-term cultured, bone marrow-derived DC (BMDC) cell line – the JawsII cells. The JawsII cells were found to express both ΜΦ- and DC-associated surface markers, respond to cytokine stimulation by upregulating surface levels of MHC and B7 molecules and secrete pro-inflammatory cytokines upon LPS-mediated activation. Furthermore, by treating the JawsII cells with both cytokines and LPSs, we show that both stimuli are required for maximal T-cell stimulation during allogeneic mixed leucocyte reactions (MLRs) and antigen-mediated proliferation assays. Based on this characterization, we believe that JawsII cells will be useful for examining in vivo immunomodulating protocols and the effects of costimulation, and also examining for standardized screening assays, where cells with fixed phenotypes are required for comparable results.
Materials and methods
Cells and culture conditions The JawsII cells were isolated from bone marrow cultures of C57BL/6, p53-deficient mice (Dr V. MacKay, Zymogenetics Institute, Seattle – ATCC No. CRL-11904). The cells were grown in RPMI-1640 with glutamax (Gibco BRL, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (FBS) (Gibco – <1 EU/ml), 1% penicillin/streptomycin (Gibco), 50 µm 2-ME, 10 mm HEPES (pH 7.4) and 5 ng/ml of recombinant mouse granulocyte/macrophage-colony stimulating factor (GM-CSF) (Pharmingen, San Diego, CA, USA). For stimulation, the JawsII cells were treated with any of the cytokines (TNF-α, IL-4 or interferon-γ (IFN-γ)) (10 ng/ml of each – all from Pharmingen), LPS (10 µg/ml, Escherichia coli– Sigma, St. Louis, MO, USA) alone or a combination of both for 48 h, washed and added to purified T cells. The concentration of LPS used in this study was selected based on dose-titration assays analysing the cytokine profile of the JawsII cells after 48 h of stimulation (data not shown). We found that 10 µg/ml LPS was required for the maximum induction of cytokine production; however, it should be noted that concentrations as low as 10 ng/ml induced cytokine production above background.
The T-cell hybridoma lines 1AD2 and 2BB11 (I-Ab-restricted) are specific to the Der p1 p110–131 antigen peptide (RFGISNYCQIYPPNANKIREAL), and were kindly provided by Dr Jonathan Lamb. The GAD65-specific (SRLSKVAPVIKARMMEYGTT), I-Ag7-restricted MBP2.3 hybridoma was a gift from Dr Pia Reich. All hybridoma T cells were grown in RPMI-1640 with glutamax (Gibco), 5% FBS (Gibco), 1% penicillin/streptomycin (Gibco) and 50 µm 2-ME. Peptides were used at a concentration of 2 µg/ml, and Der p1 antigen (a gift from ALK Abello A/S, Hørsholm, Denmark) was used at the same molar concentration as Der p1 peptide (0.9 µm).
BMDCs were prepared as previously described [22]. Briefly, femur and tibia of C57BL/6 mice were flushed, single cells prepared and red blood cells lysed by treatment with 1× ACK buffer (150 mm NH4Cl, 10 mm KHCO3, 0.1 mm Na2EDTA, pH 7.2–7.4, filter sterilized (0.2 µm)) for 5 min on ice. T cells were removed by treatment with α-CD4 antibodies (RL174.2 hybridoma supernatant), α-CD8 antibodies (31M hybridoma supernatant), α-Thy antibodies (AT83 hybridoma supernatant) and rabbit complement (Harlan Sera Laboratory, Leicestershire, UK) for 75 min at 37 °C, in 5% CO2. The remaining cells (1 × 106 cells/well) were plated into a 24-well plate (Nunclon, Roskilde, Denmark). Every 2 days, nonadherent cells were removed and new media added. Cells grew in the presence of 20 ng/ml of GM-CSF (days 0–4) and 10 ng/ml of GM-CSF (days 5 and 6). On day 6, nonadherent cells were transferred to new plates in media containing 5 ng/ml of GM-CSF. Cells were harvested on day 8.
Immunizations Female C57BL/6 mice were immunized subcutaneously at the base of the tail with 50 µg of Der p1 p110–131 peptide, with or without 50 µg of OVA323–339 peptide (ISQAVHAAHAEINEAGR) in sterile 1× phosphate-buffered saline (PBS) and complete Freund's adjuvant (half the total volume), giving a final volume of 100 µl. After 5 days, the mice were killed by cervical dislocation, and inguinal and lumbar lymph nodes (LNs) were isolated. Single cells were obtained, and the red blood cells were lysed as described above. CD3+ cells were enriched using mouse T-cell enrichment columns from R&D Systems (Minneapolis, MN, USA) (MTCC-525), giving >95–98% CD4+/CD8+ single- and double-positive cells, as evaluated by flow cytometry analysis (FACS). All work on mice was performed according to the Danish Law of Animal Welfare and the ethical guidelines of Novo Nordisk A/S.
Antibodies Antibodies used were rat α-F4/80 (ATCC No. HB-198), rat α-33D1 (ATCC No. TIB-227), phycoerythrin (PE)-conjugated rat α-CD11b (M1/70), fluorescein isothiocyanate (FITC)-conjugated hamster α-CD11c (N418), rat α-CD14 (rmC5-3), rat α-CD16/CD32 (2.4G2), biotin-conjugated rat α-CD24 (heat stable antigen) (M1/69), rat α-CD34 (RAM34), FITC-conjugated rat α-CD40 (3/23), FITC-conjugated rat α-CD44 (IM7), FITC-conjugated rat α-CD80 (IG10), FITC-conjugated rat α-CD86 (GL1), mouse α-MHC class I H2-Kb (AF6-88.5), mouse α-MHC class II I-Ab (AF6-120.1), FITC-conjugated rat α-Mac-3 (M3/84), biotin-conjugated rat α-Gr-1 (Ly-6G) (RB6-8C5), FITC-conjugated mouse α-rat immunoglobulin (Ig) κ light chain (MRK-1), FITC-conjugated rat α-mouse IgG2a (R19-15) (all from Pharmingen), rat α-CD68 (MCA1957) (Serotec Ltd., Oxford, UK) and rat α-DEC205 (NLDC145) (BMA Biomedicals AG, Augst, Switzerland). For the detection of biotinylated antibodies, FITC–streptavidin conjugates (Pharmingen) were used. Isotype controls were rat FITC-conjugated IgG1, κ (R3–34), FITC-conjugated rat IgG2a, κ (R35–95), PE-conjugated rat IgG2b, κ (A95-1) and FITC-conjugated hamster IgG, group 1, λ (G235–2356) (all from Pharmingen).
FACS analysis Cells were isolated, washed once in PBS and incubated with primary antibodies for 1–2 h at 4 °C. Where required, the cells were washed twice and incubated with secondary antibodies for 30 min at 4 °C. Before analysis, the cells were fixed in 1% paraformaldehyde for at least 30 min. The cells were monitored by FACS analysis on a FACSCalibur™, calibrated using FACSComp™ version 4.0 software and CaliBRITE™ fluorescent beads (BD Biosciences, San Jose, CA, USA). Viable cells were gated based on forward- and side-scatter properties, and all data were analysed using CELLQuest™ version 3.1 software (Becton Dickinson).
Multiplex RT-PCR analysis Semi-quantitative multiplex (MPX) reverse transcriptase-polymerase chain reaction (RT-PCR) was performed as described in Jensen et al. [23]. Briefly, mRNA was isolated using the RNAzolB method by Biotecz Laboratories, Inc. One microgram of denatured RNA was used for cDNA synthesis by incubation with 40 U of RNAsin (Promega, Madison, WI, USA), 200 U of M-MLV reverse transcriptase (Gibco), 3 µg of random primers (Gibco), 10 mm dithiothreitol (DTT) (Gibco), 1 mm dNTPs (Amersham Pharmacia Biotech, Uppsala, Sweden) and 1× reverse transcriptase buffer (50 nm Tris–HCl, pH 8.3, 75 mm KCl, 3 mm MgCl2– Gibco), at a final volume of 25 µl. The reaction was left for 5–10 min at room temperature before incubation for 1 h at 37 °C. PCR reactions were done in 25 µl reactions using 0.5 µl of cDNA, 1× buffer (Mg2+-free – Invitrogen Life Technologies, Carlsbad, CA, USA), 1.5 mm MgCl2 (Life Technologies), 0.2 nm of each primer (Table 1), 1.25 U of Taq DNA polymerase (Life Technologies), dNTPs (0.04 mm each of dATP, dTTP and dGTP, plus 0.02 mm dCTP) (Ultrapure – Pharmacia Biotech) and 16.6 µmα32P-dCTP (Amersham, Uppsala, Sweden). MPX-RT-PCR was performed using the following primer sets: IL-1α/G6PDH, IL-1β/IL-6/G6PDH, IL-3/IL-10/G6PDH, IL-12 p35/IL-12 p40/G6PDH, IFNγ/G6PDH, TNF-α/IL-18/G6PDH and IL-15/transforming growth factor-β1 (TGF-β1)/G6PDH; the programme was always for 30 s at 96 °C, followed by 22 cycles of 30 s at 96 °C, 30 s at 55 °C and 30 s at 73 °C. The labelled PCR-products were identified using standard polyacrylamide gels (6%, 7 m urea) (Ultra Pure Sequagel® Sequencing System – National Diagnostics, Atlanta, GA, USA), and 0.4 µl/ml of Temed (N,N,N′,N′-tetramethylethylenediamine) (Bio-Rad, Hercules, CA, USA) and 8 µl/ml of 10% ammonium persulfphate (Sigma) were added. Gels were exposed to a phosphor-image storage screen overnight and scanned on a Typhoon imager (Molecular Dynamics, Amersham Biosciences, Uppsala, Sweden).
| Sequence 3′ | Sequence 5′ | bp | GenBank accession number | |
|---|---|---|---|---|
| Cytokines | ||||
| IL-1α | GCT TGA CGT TGC TGA TAC TG | CAA GAT GGC CAA AGT TCC TG | 241 | AF010237 |
| IL-1β | ACC GCT TTT CCA TCT TCT TC | CTC CAC CTC AAT GGA CAG AA | 222 | E04743 |
| IL-3 | ACA TTC CAC GGT TCC ACG GT | AGG AAT ATT ATC TTT CGG AG | 294 | K01850 |
| IL-6 | TTG GAT GGT CTT GGT CCT TA | CAA GAA AGA CAA AGC CAG AG | 172 | X54542 |
| IL-10 | TCC TTG ATT TCT GGG CCA TG | CAA TAA CTG CAC CCA CTT CC | 240 | M37897 |
| IL-12 p35 | GTT GAT GGC CTG GAA CTC TG | AGA CCA CAG ATG ACA TGG TG | 290 | M86672 |
| IL-12 p40 | CAG AGA CGC CAT TCC ACA TGT | AAG ACC CTG ACC ATC ACT GTC | 300 | M86671 |
| IL-15 | TTC TCC TCC AGC TCC TCA CA | GAC AGT GAC TTT CAT CCC AG | 200 | U14332 |
| IL-18 | AGC CTT CAC AGA GAG GGT CA | TGG AGA CCT GGA ATC AGA CA | 220 | U66244 |
| IFN-γ | CGA CTC CTT TTC CGC TTC CT | TGG ATA TCT GGA GGA ACT GG | 219 | K00083 |
| TGF-β1 | TTC CAC ATG TTG CTC CAC AC | AGG TCA CCC GCG TGC TAA TG | 188 | M13177 |
| TNF-α | TGA CTC CAA AGT AGA CCT GC | CTA CTC CCA GGT TCT CTT CA | 287 | X02611 |
| Standards | ||||
| G6PDH | TCC CAC CGT TCA TTC TCC A | AGA ACC ACC TCC TGC AGA TG | 268 | M26655 |
| TBP | ATG ATG ACT GCA GCA AAT CGC | ACC CTT CAC CAA TGA CTC CTA TG | 190 | D01034 |
- * Sequences, sizes and accession numbers (NCBI) of oligonucleotides used for the amplification of cytokine transcripts from RNA preparations of JawsII cells.
Enzyme-linked immunosorbent assay (ELISA) 5 × 105 JawsII cells were plated in 2 ml media in 6-well plates (Nunclon). After 1–2 days of culture, the cells were treated as indicated for 48 h, and supernatants were collected. The contents of IL-1β, IL-6, IL-10, IL-12p70, IL-18, IFN-γ and TNF-α were detected using the OptIEA ELISA kit from Pharmingen, following the manufacturer's protocol. Biologically active TGF-β was detected using the ELISA kit from Promega, following the manufacturer's protocol. Supernatants of T hybridoma cells activated with antigen peptides or whole Der p1 antigen in the presence of unstimulated or cytokine-stimulated JawsII cells were collected and diluted 10–40 times before the detection of IL-2 (BioTrak System, Amersham). In all cases, the washing procedure was carried out on a Labsystem Multiwash plate-washing machine, using 4–5 × 400 µl of wash solution (supplied with various kits), and cytokine was detected using a multiplate reader (Victor – EG & G Wallac, PerkinElmer Life Sciences, Boston, MA, USA) at 450 nm. Calculation of cytokine amount was done using the supplied recombinant cytokine standards.
Antigen-presentation assays Activation of T hybridoma cells: 5 × 103 JawsII cells were placed in a flat-bottomed 96-well plate (Nunclon) and cultured for 1 day, allowing the cells to attach to the surface. The cells were either left unstimulated or stimulated as described above. After 48 h, cells were washed thoroughly in 1× Hank's balanced salt solution (HBSS) (Gibco), and 5 × 104 T hybridoma cells were added together with 0.9 µm antigen peptides (Der p1 p110–131 or GAD65 p524-543) or Der p1 antigen. Supernatants were collected after 24 h, and the content of IL-2 was detected using the ELISA technique (see above).
Restimulation of in vivo-primed T cells: The JawsII cells were stimulated for 48 h as indicated, washed and treated with 25 µg/ml of mitomycin C (Sigma) for 25 min at 37 °C, while primary BMDCs were isolated, washed and treated with 6.25 µg/ml of mitomycin C for 20 min at 37 °C. Cells were washed extensively and adjusted to 105 cells/ml. 5 × 103 JawsII cells or BMDCs were plated in U-bottomed 96-well plates (Nunclon) in RPMI-1640, 10% FBS, 1% penicillin/streptomycin, 1× minimum essential medium (MEM) nonessential amino acids and 50 µm 2-ME. 0.75 × 105 purified CD3+ cells from the LNs of immunized mice were added per well in the presence or absence of 10 µg/ml of Der p1 peptide. After 66 h of culture, 0.5 µCi [3H]-thymidine ([methyl,1′,2′-3H]thymidine 5′-triphosphate – Amersham) was added per well, and T-cell proliferation was measured by incorporation of [3H]-thymidine after 72 h of cell culture into a microbeta counter (Wallac) and the supplied software (MicroBeta™ TriLux, Wallac).
MLR BMDCs were isolated as described above. The JawsII cells were stimulated for 48 h with cytokines and/or LPS as described above. CD3+ cells were enriched from BALB/c (Kd) spleens by negative selection (R&D systems). 105 cells were added to varying numbers of mitomycin C-treated BMDCs or JawsII cells (see above). After 4 days, 0.5 µCi [3H]-thymidine was added. Cells were harvested after another 16–18 h, and proliferation was measured as the amount of [3H]-thymidine incorporated (see above).
Statistics Statistical analyses of data were carried out using Student's t-test (two-tailed, unequal variance).
Results
JawsII cells show characteristics of a pre-immature DC subset
JawsII cells were expanded from the bone marrow of p53-deficient mice. As the bone marrow consists of many different cell types, the phenotype of the JawsII cells was investigated by FACS analysis of surface markers representing various bone marrow cell subsets. The cells expressed both ΜΦ- and DC-associated markers: CD11b, CD11c, CD14, CD24, CD34(low), CD44, CD68(low), DEC205, 33D1(low), Mac-3 and F4/80 but not markers of granulocytes (Gr-1) (Fig. 1), T cells (CD4, CD8α) and B cells (B220) (data not shown). For comparison, we analysed the expression pattern of DC and MΦ surface markers on in vitro-generated BMDCs. Ninety to ninety-five per cent of the cells expressed CD11c and was regarded as DCs (Fig. 1). In addition, all cells expressed high levels of CD11b and low levels of F4/80 and DEC205, reflecting the expression pattern of the JawsII cells. We could not detect CD3+ (T cells) or CD19+ (B cells) cells within our cell cultures (data not shown). Together, these data suggest that JawsII cells might represent a myeloid-derived precursor cell capable of terminally differentiating into either DCs or MΦs. Accordingly, we attempted to differentiate JawsII cells into ΜΦs by culturing the cells in the presence of 10–50 ng/ml of GM-CSF. This resulted in a reduced growth of the cells but no alteration in the pattern of surface markers, as evaluated by RT-PCR and FACS analysis (data not shown). Thus, the JawsII cells express a steady-state phenotype of a bone marrow progenitor cell type most likely corresponding to pre-immature DCs.
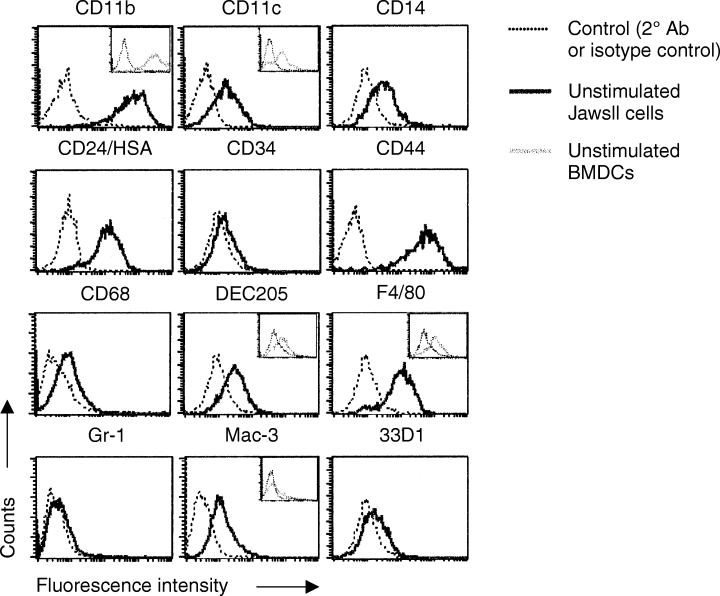
JawsII cells express markers of both macrophages (MΦs) and dendritic cells (DCs). JawsII cells were stained for surface antigens as described in Materials and methods. The expression pattern of CD11b, CD11c, CD14, CD24, CD34, CD44, CD68, DEC205, F4/80, 33D1, Gr-1 and Mac-3 (solid black lined histograms) and relevant controls (broken lined histograms) is shown. Viable JawsII cells were gated based on their forward/side-scatter properties. The insets show the surface expression of DC and MΦ markers on in vitro-generated bone marrow-derived DCs (BMDCs). The expression of CD11b, CD11c, DEC205, F4/80 and Mac-3 is represented as solid grey-lined histograms, while relevant controls are shown as broken lined histograms. BMDCs were prepared as described in Materials and methods. Data are representative of three to six independent assays.
Stimulation of the JawsII cells with cytokines enhances the surface levels of MHC and costimulatory molecules
The antigen-specific functionality of DCs is dependent on a pronounced upregulation in the surface levels of MHC and costimulatory molecules. We examined the expression pattern of these molecules on unstimulated JawsII cells as well as on cells stimulated with cytokines and/or LPS. Unstimulated JawsII cells expressed significantly high levels of both MHC class I and B7-1, and low, but detectable levels of B7-2 (Fig. 2). We were unable to detect MHC class II molecules on the surface of unstimulated JawsII cells (Fig. 2), although high amounts of MHC-II mRNA were clearly present (data not shown). Likewise, unstimulated JawsII cells did not express CD40 (Fig. 2). In order to induce a maturation-like process in the JawsII cells, we cultured the cells with different stimuli previously shown to induce DC maturation in vitro. Twenty-four hours of cell culture in the presence of cytokines (IL-4, IFN-γ and TNF-α) had no effect on the expression pattern of MHC and costimulatory molecules by the JawsII cells (data not shown). However, after 48 h of treatment, the levels of MHC-I, MHC-II, B7-1 and B7-2 expression increased significantly, while CD40 expression remained undetectable (Fig. 2– second row). This effect was solely owing to the effect of IFN-γ, as the stimulation of the JawsII cells with IFN-γ (not IL-4 and/or TNF-α) resulted in equal upregulations (data not shown). However, stimulation with IFN-γ alone resulted in less viable cells after 48 h, and thus we stimulated the cells with IL-4, IFN-γ and TNF-α for the remaining part of the study. We next analysed the effect of stimulating the JawsII cells with a high concentration of LPS (10 µg/ml). Surprisingly, LPS did not significantly influence the expression of any of the analysed surface molecules (Fig. 2), although an increase in the levels of MHC-II and B7-1 could be detected occasionally. Moreover, the levels of MHC and costimulatory molecules were not significantly different in the JawsII cells stimulated with cytokines in the presence or absence of LPS. CD40 expression was undetectable after stimulation with LPS in the presence or absence of cytokines. For comparison, we examined the levels of MHC and costimulatory molecules expressed by in vitro-generated unstimulated bone marrow-derived DCs. Based on the levels of MHC and costimulatory molecules expressed by these BMDCs, at least two subpopulations could be distinguished, presumably reflecting immature (MHClow, B7low, CD40neg) and semimature DCs (MHCint, B7int/high, CD40pos) (Fig. 2– lower panel). In agreement, the subset expressing high levels of B7-2 also expressed high levels of B7-1 as well as MHC class II (data not shown). Interestingly, cytokine-stimulated JawsII cells displayed similar levels of MHC-I expression as BMDCs. Moreover, the surface expression of MHC-II, B7-1, B7-2 and CD40 molecules on stimulated JawsII cells equalled the levels detected on the immature subset of BMDCs. Together, these data support the assumption that JawsII cells represent pre-immature DCs, and suggest that JawsII-like cells are present in the bone marrow of normal mice.
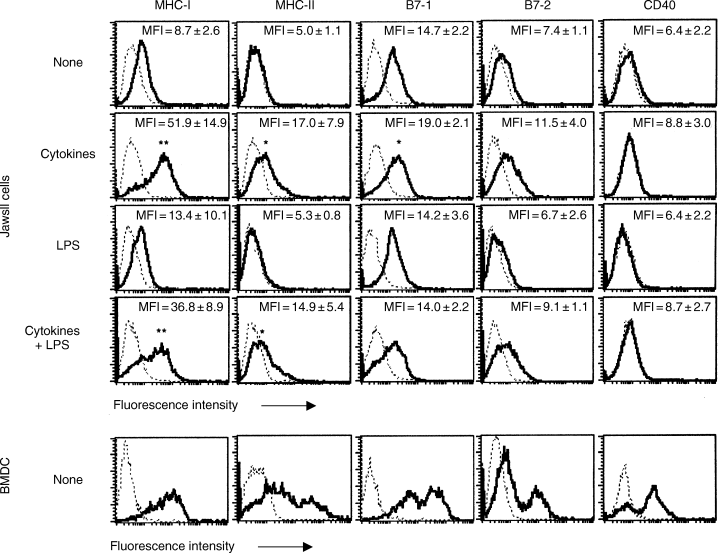
Expression pattern of activation markers: major histocompatibility complex (MHC) and costimulatory molecules. JawsII cells were treated with cytokines (interleukin-4 (IL-4), interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α)), lipopolysaccharide or a combination of these for 48 h, isolated and stained as described in Materials and methods. The mean fluorescence intensity (MFI) is given in each histogram, with the values representing the average of five independent experiments. Bone marrow-derived dendritic cells (lower panel) were isolated after 8 days of culture in vitro, as described in Materials and methods. Histograms represent viable cells gated based on their forward/side-scatter properties. Statistics were calculated based on the MFI values and are given by *P < 0.05 and **P < 0.005 relative to the expression level observed on unstimulated JawsII cells (none).
JawsII cells can produce several pro-inflammatory cytokines but not IL-12 in response to LPS
DCs are known to produce a variety of cytokines, and thus we tested the JawsII cells for the production and secretion of cytokines by RT-PCR analysis and cytokine-specific ELISA. Unstimulated JawsII cells produced IL-1α, IL-1β, IL-6, IL-15, IL-18, TGF-β1 and TNF-α (Table 2), while other APC-related cytokines such as IL-3, IL-10, IL-12 and IFN-γ were undetectable at all times (data not shown). Stimulation of the JawsII cells with cytokines had no or little effect on the content of IL-1β, IL-15, TGF-β and TNF-α mRNA, while the amount of IL-1α and IL-6 mRNA increased three- to fivefold. As expected, the rise in IL-6 mRNA was followed by an increase in the amount of IL-6 secreted. However, despite the fact that cytokine stimulation did not result in increased transcription of the IL-1β gene, cytokine-stimulated JawsII cells still secreted increased amounts of IL-1β (two- to threefold), indicating that either the cells contained intracellular storages of IL-1β that were readily released upon cytokine stimulation, or the IL-1β transcripts were more efficiently translated following stimulation.
| Cytokine profile of JawsII cells stimulated with | ||||
|---|---|---|---|---|
| None | IL-4, IFN-γ, TNF-α† | LPS‡ | IL-4, IFN-γ, TNF-α†+LPS‡ | |
| Transcript level § | ||||
| IL-1α | 0.28±0.08 | 0.87±0.32¶ | 1.99±0.60** | 4.60±0.81** |
| IL-1β | 0.06±0.03 | 0.06±0.03 | 0.31±0.09** | 0.51±0.13** |
| IL-6 | 0.002±0.0 | 0.01±0.01 | 0.7±0.05 | 0.32±0.16¶ |
| IL-15 | 0.02±0.00 | 0.03±0.00** | 0.02±0.01 | 0.05±0.01** |
| IL-18 | 0.36±0.10 | 0.16±0.03¶ | 0.17±0.08¶ | 0.07±0.01** |
| TGF-β1 | 0.67±0.09 | 0.90±0.26 | 0.72±0.11 | 0.67±0.18 |
| TNF-α | 0.14±0.07 | 0.24±0.11 | 0.35±0.08** | 0.82±0.25** |
| Protein secreted (pg/ml) | ||||
| IL-1β | 84±33 | 287±99¶ | 767±285¶ | 1420±416** |
| IL-6 | 118±35 | 707±390** | 5663±1949** | 17,607±7049** |
| IL-18 | 456±37 | 330±105 | 314±143 | 264±113¶ |
| TGF-ↆ | 212±110 | 213±136 | 168±87 | 189±230 |
| TNF-α | 52±15 | N/A | 228±82** | N/A |
- *Cytokine profile of JawsII cells. JawsII cells were stimulated with cytokines and/or LPS for 48h as described in Materials and methods. 0.5×10 6 JawsII cells were plated in 6-well plates for 1–2days and stimulated with cytokines and/or LPS. Cells and supernatants were collected after 48h. The amounts of cytokine-specific gene transcripts were measured by multiplexed RT-PCR analysis on total RNA isolated from JawsII cells stimulated as indicated. The levels of secreted cytokines were measured by enzyme-linked immunosorbent assay technique. Data represents average values (pg/ml) of four to six independent experiments±standard deviation, and the statistics were calculated using Student's t-test (two-sided and unequal variance). N/A: not applicable.
- † 10ng/ml of each.
- ‡ 10µg/ml.
- § Levels relative to internal standard (G6PDH) after 22–23 cycles of polymerase chain reaction.
- ¶ P<0.02.
- ** P<0.005 relative to unstimulated JawsII cells.
- †† Biologically active. Raw-values have been corrected for the amount present in foetal bovine serum (17ng/ml) (Promega).
Stimulation of the JawsII cells with LPS augmented the secretion of IL-1α, IL-1β, IL-6 and TNF-α mRNA, as well as the secretion of at least IL-1β, IL-6 and TNF-α (secretion of IL-1α was not analysed). Thus, LPS by itself induced pro-inflammatory cytokine production and secretion by the JawsII cells. Even more potent, however, was the combination of cytokines and LPS. Thus, the transcription of IL-1α, IL-1β, IL-6 and TNF-α genes as well as the secretion of bioactive cytokines was dramatically increased upon the stimulation of the JawsII cells with both cytokines and LPS (Table 2). We finally analysed whether stimulation with cytokines and/or LPS altered the pattern of TGF-β and IL-18 production and secretion. While the levels of TGF-β were largely unaffected by stimulation, the levels of IL-18 significantly decreased upon stimulation with cytokines, LPS or a combination of both. The functional significance of this result awaits further investigations.
JawsII cells activate T hybridoma cells in an antigen-dependent and MHC-restricted manner
We next investigated whether the JawsII cells could stimulate IL-2 production by MHC-compatible and -incompatible CD4+ T hybridoma cell lines, and whether this cytokine production was influenced by prior stimulation of the JawsII cells. The I-Ab-restricted, Der p1 p110–131-specific T-cell hybridomas (1AD2 and 2BB11) responded specifically to the JawsII cells in the presence of Der p1 peptides by releasing high levels of IL-2, while an I-Ag7-restricted, GAD65 p524–543-specific T-cell hybridoma (MBD2.3) did not (Table 3). Both unstimulated and cytokine-stimulated JawsII cells were capable of stimulating IL-2 production by T hybridoma cells in an antigen-dependent manner, although cytokine-stimulated JawsII cells induced the production of significantly larger amounts of IL-2 (P < 0.01).
| Amount of IL-2 released by responder T hybridoma cells | |||
|---|---|---|---|
| 1AD2, I-Ab | 2BB11, I-Ab | MBD2.3, I-Ag7 | |
| JawsII unstimulated | |||
| Der p1 p110–131 | 1450±300 | 1078±319 | 86.7±33.0 |
| Der p1 antigen | 1381±231 | ND | ND |
| GAD65 p524–543 | 44.4±16.4 | 32.2±1.9 | 35.0±7.1 |
| No peptide | 51.1±25.2 | 56.7±15.3 | 58.3±33.0 |
| JawsII+cytokines | |||
| Der p1 p110–131 | 5525±809† | 3136±657† | 156.7±4.7 |
| Der p1 Antigen | 3679±548† | ND | ND |
| GAD65 p524–543 | 61.1±34.0 | 44.4±7.7 | 40.0±4.7 |
| No peptide | 55.6±5.1 | 48.9±13.5 | 81.7±21.2 |
- * Antigen-dependent activation of T hybridoma cells. About 5×103 JawsII cells were plated in 96-well plates. JawsII cells were either unstimulated or stimulated with cytokines for 48h, washed repeatedly and antigen peptide (Der p1 p110–131) or whole antigen (Der p1) together with 5×104 T hybridoma cells was added. After 24h, supernatants were collected, and the quantity of secreted IL-2 was determined using the enzyme-linked immunosorbent assay technique. Statistics were calculated using Student's t-test (two-sided, nonpaired, unequal variance). ND: not done.
- † P<0.01 as compared with unstimulated JawsII cells.
A main feature of immature DCs is their capability to take up foreign antigens and process these into immunogenic peptides. Thus, to test whether cytokine stimulation of the JawsII cells resulted in maturation-like alterations, we cultured T-cell hybridomas with unstimulated or cytokine-stimulated JawsII cells in the presence of recombinant Der p1 protein. As expected, we found that unstimulated JawsII cells could process Der p1 antigen and present the major peptide antigen in the context of MHC class II to antigen-specific T-cell hybridomas (Table 3) (1AD2 T cells). Moreover, the level of activation was similar to that obtained when unstimulated JawsII cells were cultured with T-cell hybridomas in the presence of equal number of antigen peptides, indicating that JawsII cells were fully competent in processing and presenting recombinant antigens. Surprisingly, cytokine-stimulated JawsII cells also stimulated T-cell dependent IL-2 production when the antigen was provided as total recombinant Der p1 (Table 3), indicating that cytokine-stimulation did not influence the capacity of the JawsII cells to take up antigen, process it and present it in the context of MHC class II. Hence, although JawsII cells show a phenotype of pre-immature DCs, they seem to undergo activation (upregulation of MHC and costimulatory molecules) rather than maturation (decreased phagocytosis) in response to cytokines.
JawsII cells can activate naïve T cells in allogeneic MLR
Another main characteristic of DCs is their capability to activate naïve T cells. To test whether the JawsII cells could do so, we cultured the JawsII cells with allogeneic CD3+ T cells from Kd-restricted BALB/c mice for 5 days. Unstimulated JawsII cells primed low levels of proliferation of naïve T cells. Cytokine-stimulated JawsII cells induced significantly higher levels of T-cell proliferation, while LPS-stimulated JawsII cells did not (Fig. 3). Overall, these data reflect the diverse levels of MHC and costimulatory molecules expressed by the JawsII cells in response to these stimuli (see above). However, LPS appeared to have a synergistic effect on the activation of naïve T cells by cytokine-stimulated JawsII cells, as cells stimulated with both cytokines and LPS induced several fold more proliferation of allogeneic naïve T cells than did the cytokine-stimulated JawsII cells. These data not only clearly demonstrate a positive role for LPS-induced cytokine production in APC-mediated activation of naïve T cells, but also show that the pro-inflammatory cytokines themselves are unable to induce T-cell proliferation. For comparison, we stimulated allogeneic T cells with in vitro-generated BMDCs and measured the level of proliferation after 5 days. Unstimulated BMDCs induced proliferation similar to cytokine- and LPS-stimulated JawsII cells, reflecting the higher levels of MHC and costimulatory molecules expressed by unstimulated BMDCs. Thus, activated JawsII cells resemble unstimulated in vitro-generated BMDCs with respect to their T-cell stimulatory capacity.
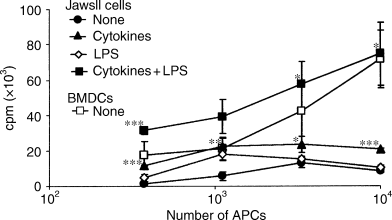
Cytokine and lipopolysaccharide (LPS)-stimulated JawsII cells are capable of activating allogeneic T-cell proliferation in a ratio-dependent manner. Allogeneic mixed leucocyte reaction was carried out as described in Materials and methods. 1 × 105 BALB/c T cells were added to variable numbers of JawsII cells (unstimulated (none), cytokine-stimulated, LPS-stimulated or cytokine and LPS-stimulated) or in vitro-generated bone marrow-derived dendritic cells (unstimulated (none)). Proliferation was measured after 120 h by [3H]-thymidine incorporation. Statistics: *P < 0.05, **P < 0.01 and ***P < 0.005, as compared with the T-cell activation mediated by unstimulated JawsII cells.
JawsII cells can restimulate in vivo-primed T cells in an antigen-specific manner
Finally, we used a standard immunization system, in which mice were immunized subcutaneously with or without the dust mite allergen peptide Der p1 p110–131 emulsified 1 : 1 (v/v) in complete Freund's adjuvant, to test whether the JawsII cells were capable of restimulating in vivo-primed T cells. T cells were isolated from lumbar and inguinal LNs and restimulated in vitro with varying numbers of either unstimulated or cytokine-stimulated JawsII cells in the presence of Der p1 p110–131 antigen peptide (Fig. 4A). T-cell proliferation was clearly dependent on the number of JawsII cells added and peaked at 15-fold excess of T cells. Using this T-cell-to-JawsII cell ratio, the JawsII cells either unstimulated or stimulated with cytokines and/or LPS were added to in vivo-primed T cells in the presence of peptide antigen. In accordance with our previous observations, the JawsII cells stimulated with both cytokines and LPS induced the highest level of T-cell proliferation (P < 0.001 when compared with unstimulated JawsII cells) (Fig. 4B). Also, cytokine-stimulated JawsII cells induced significant proliferation; however, as even unstimulated JawsII cells induced high levels of proliferation in the presence of peptide antigen, the effect of cytokine-stimulation was less pronounced (P < 0.01). As seen before, the JawsII cells stimulated with LPS alone had almost no activating effect, further emphasizing that it is the actual level of MHC and costimulatory molecules expressed by the JawsII cells and not the release of pro-inflammatory cytokines that is crucial for the activation of T cells. Finally, we included unstimulated BMDCs as the source of APCs in the assay to determine the relative functionality of the JawsII cells. The proliferation of T cells induced by cytokine/LPS-stimulated JawsII cells was once again comparable with that perceived using unstimulated BMDCs (Fig. 4B).
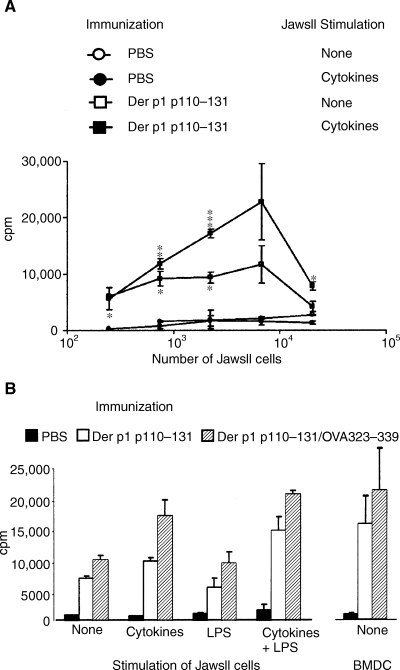
Combined stimulation of JawsII cells with cytokines and lipopolysaccharide (LPS) induces maximal T-cell proliferation during antigen-specific restimulation of in vivo-primed T cells. (A) 7.5 × 105 T cells isolated from inguinal and lumbar lymph nodes (LNs) of mice immunized with Der p1 p110–131 (squares, 50 µg) or phosphate-buffered saline (PBS) (circles) were added to variable numbers of JawsII cells, either unstimulated (open symbols) or cytokine-stimulated (closed symbols), in the presence of 10 µg/ml of Der p1 p110–131 peptide. Proliferation was measured after 72 h. Statistics: *P < 0.05, **P < 0.01 and ***P < 0.005, relative to the proliferation induced by unstimulated JawsII cells (none). (B) Mice were immunized with PBS, Der p1 p110–131 (50 µg), or Der p1 p110–131 and OVA323–339 peptide (50 µg of each). After 5 days, T cells from inguinal and lumbar LNs were isolated and added to JawsII cells or bone marrow-derived dendritic cells, giving an antigen-presenting cell (APC)-to-T-cell ratio of 1 : 15, in the presence of 10 µg/ml of Der p1 p110–131 peptide. Proliferation was measured after 72 h. Data are representative of three experiments, each comprising four to six mice per group. Statistics: *P < 0.05, **P < 0.01 and ***P < 0.005, relative to the proliferation induced by unstimulated JawsII cells.
Discussion
The JawsII cell line is becoming widely used for analysing the function of immature DCs [24–26]; however, no data have previously described the phenotype and general functionality of these cells. In this paper, we show that JawsII cells represent pre-immature DCs expressing a mixed MΦ/DC phenotype, as previously described for other bone marrow-derived cells and cell lines, as well as for freshly isolated spleen-derived cells [5, 22, 27–29]. In addition, we found that JawsII-like cells were present in primary BMDC cultures (Fig. 2); and it was recently published that a comparable population was residing in peripheral LNs [30].
Generally, immature DC lines have not survived long-term culturing without differentiating into mature DCs. However, the JawsII cells appeared to have retained an immature phenotype despite long-term culturing. Most likely, the JawsII cells have stayed alive owing to their p53-deficiency, because the lack of p53 expression might allow the cells to proliferate rather than differentiate during early cell culture procedures.
As immature DCs, the JawsII cells expressed low levels of MHC and costimulatory molecules (Fig. 2). In fact, the levels of surface MHC-II molecules expressed by unstimulated JawsII cells were practically undetectable (Fig. 2), although MHC-II mRNA was present in sound amounts (data not shown). The levels of both MHC class II protein and mRNA increased significantly (approximately three- to fourfold) upon stimulation with cytokines (Fig. 2 and data not shown). In parallel, the T-cell stimulatory capacity of unstimulated JawsII cells was basically high and increased only a few folds upon activation (3, 4). Thus, although JawsII cells basically expressed low levels of MHC class II molecules, this number still appeared to be high enough for T-cell hybridoma activation. In correlation, as little as 200 MHC class II molecules per cell have been shown to be enough for substantial T-cell activation [31]. Alternatively, a relatively high concentration of empty MHC class II molecules might be present at the JawsII cell surface, as previously suggested [32]. Using a specific antibody, Santambrogio et al. detected high levels of empty, otherwise undetectable, MHC class II molecules on the surface of immature DCs [33]. These empty MHC-II molecules bound extracellular soluble antigen peptides and presented these to antigen-specific T cells, while proteases secreted by the DCs themselves were shown to digest proteins within the extracellular space, producing immunogenic peptides also presented by DCs in the context of MHC-II [33]. Thus, in the presence of Der p1 peptides either added extracellularly or generated upon proteolytic digestion of Der p1 antigen, MHC-II molecules present on the surface of the JawsII cells would bind peptides and activate T cells. This mechanism could account for the significant amount of IL-2 produced by peptide-specific T-cell hybridomas after exposure to unstimulated JawsII cells and Der p1 peptide or antigen (Table 2), as well as for the high levels of proliferation of in vivo-primed T cells induced by unstimulated JawsII cells (Fig. 4). In contrast, mature DCs were not found to express empty MHC class II molecules on the cell surfaces [32], and hence the T-cell stimulatory capacity of stimulated JawsII cells should be directly related to the levels of MHC and costimulatory molecules expressed by the cells.
Mature DCs are known to have reduced phagocytic activity [15]. Thus, we anticipated that cytokine-stimulated JawsII cells would be less stimulatory than unstimulated JawsII cells in the presence of Der p1 antigen. However, cytokine-stimulated JawsII cells induced the secretion of significantly more IL-2 by T-cell hybridomas in the presence of Der p1 antigen than did the unstimulated JawsII cells (Table 3). Whereas cytokine-stimulated JawsII cells induced significant IL-2 production by T-cell hybridomas in the presence of antigen peptide, the cells induced substantially higher levels of IL-2 production when cultured in the presence of equal molar peptide antigen. These results suggested to us that cytokine-treated JawsII cells exhibited slightly reduced phagocytic activity, although the capacity to take up exogenous antigen was clearly intact. In agreement, we have previously observed that cytokine-stimulated JawsII cells took up less fluorescent microspheres than did the unstimulated JawsII cells (unpublished observation).
Another important observation of the current study was the finding that JawsII cells apparently lack all expression of CD40 (Fig. 2); also, the cells did not produce IL-12 under any of the investigated conditions (Table 2). In contrast, most DCs (mature > immature) express CD40, and have been found to express low amounts of IL-12 even when unstimulated, while large amounts of secreted IL-12 have been detected upon treatment of DCs in vitro with LPS, cytokines and bacterial DNA, uptake of antigen, or ligation of CD40 by CD40L or anti-CD40 antibodies (our unpublished data and [10, 12, 14, 16, 18]). Most likely, the lack of CD40 expression and IL-12 production by the JawsII cells is owing to the long-term culturing of the cells, as it is well known that primary cells might lose normal functions during long-term in vitro culture. Alternatively, TGF-β, which is constitutively expressed by the JawsII cells, might inhibit both the expression of CD40 and the production and secretion of IL-12 in an autocrine manner, as TGF-β has been found to inhibit IL-12 production by ΜΦs and microglial cells, as well as the expression of CD40 on ΜΦs after exposure to T cells [34, 35].
The lack of CD40 expression prompted us to investigate the priming capacity of the JawsII cells, as it has been proposed previously that CD40 is required for the priming of naïve T cells [36, 37]. We found that JawsII cells were clearly capable of activating allogeneic naïve T cells in the absence of CD40 in a dose-dependent manner (Fig. 3), thus supporting recent data from Lu et al. [38], who showed that CD40–/– APCs induced Th cell-mediated CD8+ T-cell proliferation as potently as did CD40+/+ APCs. Hence, when using the JawsII cells as APCs, CD40 is unnecessary for the activation of naïve T cells in vitro. As expected, the JawsII cells restimulated in vivo-primed T cells in an antigen-specific manner (Fig. 4), emphasizing the CD40-independence of antigen-dependent T-cell restimulation.
It has been suggested that cytokines produced by DCs in response to LPS acted as third-party activation signals during T-cell stimulation [39]. The same observation was recorded in the present study using the JawsII cells. The JawsII cells responded independently to cytokines and LPS by enhancing the expression of MHC/costimulatory molecules and pro-inflammatory cytokine production, respectively (Fig. 2 and Table 2); however, only cytokine-stimulated cells induced significant T-cell proliferation (3, 4). In the presence of both stimuli, T-cell activation was markedly increased, although the levels of both MHC and costimulatory molecules did not change dramatically. This indicates that pro-inflammatory cytokines produced in response to LPS positively affect antigen-dependent T-cell activation mediated by cytokine-stimulated JawsII cells. In contrast, BMDCs always responded to LPS by increased expression of MHC (classes I and II), B7 and CD40 molecules and by augmenting T-cell proliferation (data not shown). Whether this difference in LPS responsiveness is owing to an abnormal expression of the LPS receptor, Toll-like receptor 4, by the JawsII cells, or reflects intracellular deficits in the LPS signalling pathway has yet to be determined.
Finally, the JawsII cells were previously found to activate CD8+ T cells upon treatment with MHC class I-binding peptides or antigen-expressing adenovirus [24, 25]. Here, we show that, despite a lack in CD40 expression, the JawsII cells are able to activate naïve allogeneic CD4+ T cells as well as antigen-primed CD4+ T cells in an antigen-dependent manner. Thus, the JawsII cells have the same dual capability as primary BMDCs, which further supports a role for these cells in dissecting DC function. Furthermore, we have shown that stimulated JawsII cells resemble in vitro-generated BMDCs with regard to the expression of surface molecules (1, 2), cytokine production (except IL-12) (Table 2 and data not shown), T-cell priming (Fig. 3) and antigen-specific restimulation (3, 4). We believe that JawsII cells may be useful for studies of molecules, chemical drugs and environmental factors involved in T-cell activation in vitro, as recently confirmed [26]. Studies of T-cell priming, differentiation, induction of tolerance and target-cell-specific immunotherapy in vivo may be facilitated using such cells, as the interassay variance would most likely decline. Moreover, the importance of DCs during induction of immunity or tolerance towards well-defined antigens has been recently studied, emphasizing the therapeutic use of DCs in diseases such as cancers, allergies, infectious diseases and autoimmune disorders [40–45]. It is our belief that the JawsII cells will be a valuable tool for further investigations aimed at controlling or ameliorating these diseases.
Acknowledgments
We thank Dr V. Mackay for raising the JawsII cell line and making it available to us, Drs J. Lamb and P. Reich for kindly providing the T-cell hybridoma cell lines and Drs N. Brenden and A. Stubbs for a critical reading of the manuscript. Claus Haase was supported by a biotechnology grant from the University of Copenhagen (Grant No. 501-601-2/99). The Hagedorn Research Institute is a basic research component of Novo Nordisk A/S.




