In Vitro Production and Characterization of Partly Assembled Human CD3 Complexes
Abstract
Pairwise assembly of human CD3 chains takes place in the endoplasmic reticulum of T cells. Subsequently, the CD3 heterodimers form complexes with Tiα and Tiß chains forming hexameric TiαβCD3γεδε complexes. Finally, association with the ζ2 homodimer occurs in Golgi apparatus before the fully assembled T-cell receptor is transported to the cell surface. To study the structural properties of the human CD3 chains, we have developed new methods to produce and fold the extracellular domains of CD3γ, CD3δ and CD3ε. Proteins were expressed in Escherichia coli as denatured chains and de novo folded in vitro. CD3γ and CD3ε folded as soluble monomers, whereas CD3δ did not yield any soluble proteins. When folding the chains pairwise, soluble CD3γε and CD3δε heterodimers could be isolated, whereas CD3γδ heterodimers were not produced. Using antibodies as structural probes, we identified two different types of antigenic epitopes that were dependent on heterodimerization. Our data indicate that CD3ε undergoes a conformational change after dimerization with CD3γ or CD3δ. Furthermore, we demonstrated that the CD3γε heterodimer could be purified using immunoaffinity chromatography.
Introduction
The human T-cell receptor (TCR) consists of the disulphide-linked Tiαβ heterodimer in association with the CD3γε and the CD3δε heterodimers and the ζ2 homodimer [1]. TCR recognizes a foreign peptide antigen presented by major histocompatibility complex (MHC)-encoded molecules. Upon recognition of foreign peptide, signals are delivered across the membrane, leading to the activation of T cell [2]. The exact structure and stoichiometry of TCR is still a matter of debate [3, 4], but generally it is believed that CD3ε interacts with CD3γ or CD3δ, forming CD3γε and CD3δε heterodimers prior to the association with Tiα and -β. The assembly of Tiαβ and the CD3 molecules takes place in the endoplasmic reticulum (ER). Subsequently, the hexameric TiαβCD3γεδε complex is transported to the Golgi apparatus, where it associates with the disulphide-linked ζ2 homodimer [1, 5]. If the hexameric complex fails to assemble with the ζ chains, it is targeted to lysosomal degradation [6].
Based on sequence homology, the CD3 molecules belong to the immunoglobulin (Ig) superfamily. This family of proteins is characterized by domains built of antiparallel β-strands in a sandwich-like structure stabilized by an intradomain disulphide bond between two cysteine residues usually separated by 55–60 residues [7]. Assembly of the CD3 heterodimers is dependent on a variety of motifs in the different chains. Distinct sites in CD3γ and, in particular, a conserved CXXC motif present in all CD3 chains seem to play crucial roles for the assembly of the CD3 heterodimers [8, 9]. The CXXC motif has been shown to be the active site for the formation of interchain disulphide bonds [10]. The presence of interchain disulphide bonds has been reported for CD3γε heterodimers [11], but these dimers are apparently not expressed in normal T cells [12–14]. In addition, both CD3ε and CD3δ disulphide-linked homodimers have been reported in T cells [15, 16]. However, most probably they represent a misfolded fraction of the chains and seem not to be expressed at the T-cell surface [17].
TCR assembly has been studied mostly in transfection-based expression systems. The requirement of the chains for assembly and a number of important sites on the involved chains have been described, but a structural characterization of the interactions of the chains has received far less attention. To analyse the structural interactions of human CD3 chains in TCR assembly, we have expressed the extracellular domains of CD3γ, CD3δ and CD3ε as recombinant proteins in Escherichia coli. Using a novel de novo folding strategy to produce soluble chains, we found that when folding the extracellular domains together, CD3γε and CD3δε heterodimers formed spontaneously, whereas CD3δγ heterodimers did not form. A panel of different CD3 antibodies (Abs) was then used to analyse antigenic epitopes and structural properties of the folded protein. Comparing denatured proteins and soluble dimers by Western blotting and immunoprecipitations, we found that the CD3 Abs defined two distinct conformational epitopes on the CD3γε and CD3δε heterodimers. Thus, the extracellular domain of CD3ε exists in at least two different conformations, dependent on its association with CD3γ and CD3δ. Finally, we describe a method to purify CD3 heterodimers using immunoaffinity chromatography.
Materials and methods
Cells and antibodies E. coli BL21 DE3 was used for the production of the CD3 chains. The CD3 Abs used were UCHT-1 (Dako, Glostrup, Denmark), F101.01 [18], Leu-4 (clone SK7) (Becton Dickinson, Mountain view, CA, USA) and OKT-3 (ATCC# CRL-8001). Secondary Ab used in Western blotting analyses was horseradish peroxidase (HRP)-conjugated rabbit antimouse Ig Ab (Dako).
Cloning of CD3γ, -δ and -ε Expression cassettes were made by polymerase chain reaction (PCR) using Vent DNA polymerase (New England Biolabs, Beverly, MA, USA) and the plasmids pJ6T3γ-2 (human CD3γ– GenBank accession number X04145) [19], pPGBC9 (human CD3δ– GenBank accession number X01451) [20] and pDJ4 (human CD3ε– GenBank accession number X03884) [21] as templates. The primers were designed to incorporate a BamHI restriction enzyme site at the 5′-end and a stop codon and HindIII restriction enzyme site at the 3′-end. PCR products encoding the whole extracellular part of the CD3 genes excluding the leader sequence were cut with BamHI and HindIII and subsequently cloned into the BamHI–HindIII fragment of pT7H6 [22]. Finally, correct transfer of genes was confirmed by complete DNA sequencing.
Production and purification of recombinant CD3γ, -δ and -ε For protein expression, plasmids encoding the extracellular domain of the CD3 chains were separately transformed into E. coli BL21 DE3 by electroporation using a Bio-Rad Gene Pulser at settings 1.5 kV, 25 µF, 200 Ohm. A schematic view of the resulting proteins is shown in Fig. 1A. Mutant E. coli hosts allowing the production of proteins at high levels were isolated by isopropylthio-d-galactosid (IPTG) selection [23]. 50 ml of 2× YT with 10 mm MgSO4 and 100 µg/ml ampicillin was inoculated ovlernight at 37 °C with IPTG-selected mutants from a glycerol stock and poured into 1 l of 2× YT-containing 10 mm MgSO4 and 100 µg/ml ampicillin. At OD600 = 0.6–1.0, 1 ml of 420 mm IPTG was added, and the cells were subsequently harvested by centrifugation for 30 min at 4.500 × g, 4 °C, after overnight induction. Inclusion body purification was performed as described [24] with few modifications. In brief, the cell pellet was resuspended in 80 ml of lysis buffer (50 mm Tris–HCL, pH 8.0, 25% sucrose w/v, 1 mm ethylenediaminetetraacetic acid (EDTA)). Cells were lysed by a freeze-thaw cycle, followed by sonication and addition of 200 mg of lysosyme dissolved in 20 ml of lysis buffer. After being on ice for 30 min, DNaseI was added at a final concentration of 10 µg/ml and incubated for another 30 min. The lysate was centrifuged at 4.500 × g, 4 °C for 15 min. The supernatant was discarded and the pellet washed four times in 50 ml of 50 mm Tris–HCl, pH 8.0, 0.5 m NaCl. After a freeze-thaw cycle, the pellet was washed additionally four times in 0.5% Triton X-100 and 1 mm EDTA, followed by final washes in the Tris wash buffer until the pellet appeared white. The pellet was dissolved in 10 ml of 8 m urea, 50 mm Tris–HCl, pH 8.0, 0.5 m NaCl, giving approximately 1–2 mg/ml (urea stock solution), and frozen immediately at −20 °C.
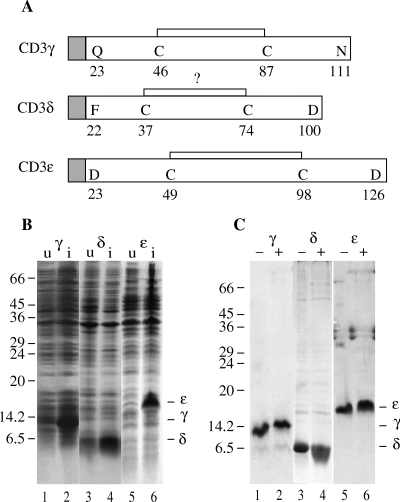
Expression and purification of the CD3 chains. (A) A schematic view of the expressed human CD3 chains with the proposed intrachain disulphide bonds. The grey squares represent the histidine tag sequence with the factor Xa cleavage site (in one letter code: MGSHHHHHHGSIEGR). (B) SDS-PAGE analysis of untreated (u) (lanes 1, 3 and 5) and isopropylthio-d-galactosid (IPTG)-induced (420 µm final concentration) (i) (lanes 2, 4 and 6) Escherichia coli BL21 DE3 transformed with CD3γ (lanes 1 and 2), CD3δ (lanes 3 and 4) and CD3ε (lanes 5 and 6). The protein bands were visualized by Coomassie staining. Molecular mass markers are indicated on the left (kDa), and the positions of the individual CD3 chains are indicated on the right of the gel. (C) SDS-PAGE analysis of the urea stock solution of CD3δ (lanes 3 and 4) and of CD3γ (lanes 1 and 2) and CD3ε (lanes 5 and 6) folded in the appropriate buffer. Uneven numbered lanes were run under nonreducing (–), and even numbered lanes were run under reducing (+) condition. The protein bands were visualized by Coomassie staining. Molecular mass markers are indicated on the left (kDa), and the positions of the individual CD3 chains are indicated on the right of the gel.
De novo folding by dilution of CD3 chains All CD3 chains were folded by dilution of approximately 1 mg of protein into 200 ml of buffer. CD3ε urea stock was diluted into 50 mm Tris–HCl, pH 8.0, at 4 °C and incubated overnight on a magnetic stirrer, whereas CD3γ was diluted into 20 mm NaAc, pH 5.0 and treated likewise. Precipitates were pelleted by centrifugation at 15.000 × g for 30 min. Supernatants were concentrated on an Amicon stirred cell using YM3 filter (Amicon, Millipore, MA, USA) or Centricon Plus-20, Biomax 5000 (Amicon) to 5–10 ml and used for experiments. Folding of the CD3 chains for heterodimer formation was performed in CD3ε folding buffer (50 mm Tris–HCl, pH 8.0). Here, approximately 0.5 mg of each protein was diluted into 200 ml of buffer and treated as described. The products were visualized on 15% SDS-PAGE.
Western blotting of folding mixtures Proteins were separated by SDS-PAGE on 15% nonreducing gels and transferred to nitrocellulose membranes (Hybond ECL – Amersham Pharmacia Biotech, Amersham, AB, Sweden). Electroblotting was performed at 0.8 mA/cm2 for 50 min, followed by blocking with 1% bovine serum albumin (Sigma, St. Louis, MO, USA) and 3% dry skim milk in phosphate-buffered saline (PBS) for 1 h. The membrane was incubated with primary Abs in blocking buffer for 2 h at room temperature, washed in PBS 0.05% Tween 20, followed by incubation for 1 h at room temperature with HRP-conjugated rabbit antimouse Ig. Immunoreactive proteins were subsequently visualized by ECL (Amersham Pharmacia Biotech).
Immunoprecipitation of heterodimer 100 µl of folding mixture was incubated for 1 h 30 min with 10 µg of F101.01 or UCHT-1 at 4 °C. Protein A sepharose (Amersham Pharmacia Biotech) was added and incubated for 2 h at 4 °C. The supernatant was transferred to another Eppendorf tube and the immunoprecipitate washed five times in wash buffer (20 mm Tris–HCl, pH 8.0, 137 mm NaCl, 1% NP-40). After the last wash, the beads and the supernatant were boiled for 5 min with Laemmli sample buffer (LSB) (50 mm Tris–HCl, pH 6.8, 2% SDS, 10% glycerol, 0.01% bromophenolblue) and run on 15% SDS-PAGE. The result was visualized with Coomassie staining.
Purification of heterodimers For the purification of the CD3γε heterodimer, an immunoaffinity column with approximately 1 mg of F101.01 monoclonal antibody (MoAb) coupled to 1 ml of CNBr-activated sepharose was produced according to the manufacturer's protocol (Amersham Pharmacia Biotech). The column was equilibrated with 20 mm of Tris–HCL, pH 8, 150 mm NaCl. 1 ml of CD3γε folding mix was sterile-filtered and run through the column. Bound proteins were eluted with 0.05 m diethylamine (DEA), 0.15 mm NaCl, pH 11, in 1 ml fractions into Eppendorf tubes containing 50 µl neutralization buffer, 2 m Tris, pH 8. Fractions were boiled with LSB and run on 15% SDS-PAGE. The purity of the recombinant protein was visualized by silver staining.
Results
Expression of CD3 chains
The extracellular domains of the CD3 chains were expressed in E. coli BL21 DE3. The expression resulted in a fusion-protein of the CD3 chains with a hexa histidine affinity-purification tag, cleavable at a factor Xa substrate recognition site at the N terminus (Fig. 1A). In the present study, the tag was not considered reactive in any way and was thus left on the proteins. All fusion-proteins were expressed in large quantities following IPTG induction (Fig. 1B). Lysing the bacterial cells with lysosyme and detergents and washing the insoluble protein with detergent-containing buffer produced highly purified proteins. Overnight expression of the recombinant fusion-protein after IPTG induction yielded approximately 10–20 mg/l of E. coli culture, as judged from SDS-PAGE analysis (data not shown).
De novo folding and characterization of the CD3 chains
The inclusion body proteins were solubilized without further purification in 8 m urea, 50 mm Tris, pH 8, 0.5 m NaCl. The CD3 chains were folded by diluting the urea stock solution in appropriate folding buffers. Theoretical isoelectric point pI values of the chains calculated by using the gcg package from Genetics Computer Group (Pharmacopeia, Princeton, NJ, USA) showed large differences between the different chains. Therefore, a screening of various folding buffers was carried out. It was found that CD3ε was soluble in 50 mm Tris, pH 8.0, whereas CD3γ was soluble in 20 mm NaAc, pH 5.0. We were unable to keep CD3δ in solution in any of the buffers examined. Approximately, 1 mg of CD3γ or CD3ε in the urea stock solutions was diluted into 200 ml of the appropriate folding buffer, and incubated overnight at 4 °C on a magnetic stirrer. Subsequently, the folding buffer was concentrated 20–40 times and subjected to 15% SDS-PAGE. Reducing the folded CD3γ and CD3ε with 2-ME resulted in a slower migration rate on SDS-PAGE, indicating the presence of an intrachain disulphide bond both in the CD3γ and the CD3ε chain (Fig. 1C– lanes 1, 2, 5 and 6). This suggested that the proteins had been oxidized after translation in the cells. In contrast, the urea-solubilized CD3δ chain showed no reduced mobility (Fig. 1C– lanes 3 and 4), questioning the existence of an intrachain disulphide bond in this chain [7].
Production of dimers
To examine the pairwise interaction of the CD3 components, approximately 500 µg of both CD3γ/CD3δ and CD3ε was folded together in 50 mm Tris, pH 8, at 1 : 1 molar ratio. Proteins were concentrated on an Amicon cell and subjected to 15% SDS-PAGE analysis. According to the sizes, the different bands were identified. A band with the size of a CD3γε heterodimer appeared between the bands corresponding to the CD3ε monomer and homodimer, indicating a heterologue assembly of CD3γε chains (Fig. 2A). CD3γ precipitated very quickly when folded alone in 50 mm Tris buffer, pH 8.0, but the presence of CD3ε partially rescued CD3γ from precipitation because of the assembly of the heterodimer. The heterodimer appearing on the SDS-PAGE was disulphide-linked. The soluble monomeric CD3γ chains observed in the folding mixture were most probably derived from nondisulphide-linked CD3γε dimers (Fig. 2A– lane 3). In comparison, CD3δ and CD3ε were less prone to form disulphide-linked heterodimers (Fig. 2B– lane 2). However, addition of glutathione (1.6 mm GSH and 0.2 mm GSSG) augmented the yield of disulphide-linked CD3δε heterodimers (data not shown). All attempts to produce CD3γδ heterodimers were unsuccessful. Reducing the CD3δε folding mixture showed no reduction in the mobility of CD3δ, again suggesting the lack of an intrachain disulphide bond in CD3δ (Fig. 2B– lane 3).
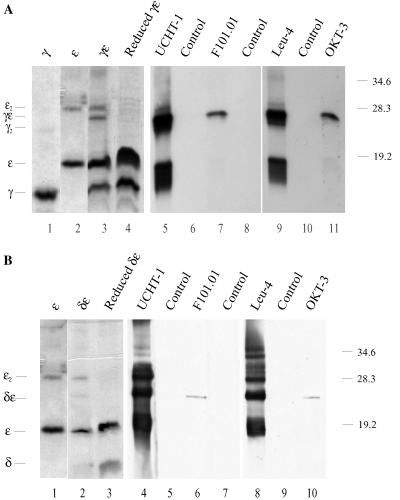
Identification of antigenic epitopes by Western blotting. (A) SDS-PAGE analysis of CD3γ and CD3ε folded either alone (lanes 1 and 2) or together (lanes 3–11). Lane 4 was run under reducing conditions; all other lanes were run under nonreducing conditions. Lanes 1–4 were stained with Coomassie. Lanes 5–11 were electroblotted to nitrocellulose membranes and incubated with the monoclonal antibodies (MoAb) UCHT-1, F101.01, Leu-4, OKT-3 or blocking buffer (control). The membranes were washed and incubated with horseradish peroxidase (HRP)-conjugated rabbit antimouse immunoglobulin (Ig) Ab, and immunoreactive proteins were visualized by ECL. (B) SDS-PAGE of CD3ε folded either alone (lane 1) or together with CD3δ (lanes 2–10). Lane 3 was run under reducing conditions; all other lanes were run under nonreducing conditions. Lanes 1–3 were stained with Coomassie. Lanes 4–10 were electroblotted to nitrocellulose membrane and incubated with the MoAbs UCHT-1, F101.01, Leu-4, OKT-3 or blocking buffer (control). The membranes were washed and incubated with HRP-conjugated rabbit antimouse Ig Ab, and immunoreactive proteins were visualized by ECL. Molecular mass markers are indicated on the right (kDa), and the positions of the CD3 mono-, homo- and heterodimers are indicated on the left of the gel.
Serological characterization of dimers
To further identify the different bands in the Coomassie-stained gel, Western blot analyses were performed on the concentrated folding mixtures. As determined by their molecular size, the CD3γε and CD3δε heterodimers could be recognized by different monoclonal Abs (Fig. 2A,B). Interestingly, the Abs could clearly be divided into two groups: F101.01 and OKT-3 Abs only recognized CD3γε and CD3δε heterodimers, but neither of the chains folded alone. Thus, a conformational nonlinear epitope only accessible upon heterodimerization was recognized by these MoAbs. In contrast, UCHT-1 and Leu-4 recognized an epitope on both the CD3ε mono-, hetero- and homodimerized CD3ε. The isolated CD3γ or CD3δ chains were not recognized by UCHT-1 and Leu-4. This indicated that a linear epitope on CD3ε was recognized by these Abs.
Immunoprecipitation of heterodimers
So far, the epitopes were characterized on denatured proteins by Western blotting. To determine whether antigenic epitopes were presented by the soluble heterodimers, immunoprecipitation experiments on the CD3γε folding mixture were performed. The CD3γε folding mixture was incubated with the MoAbs F101.01 and UCHT-1, and the resulting immune complexes were subsequently precipitated with protein A beads. The resulting precipitates and the remaining supernatants were separately run on SDS-PAGE (Fig. 3). Bands corresponding to the monomeric CD3γ and CD3ε chains and the disulphide-linked CD3γε heterodimer appeared in the precipitate (Fig. 3– lanes 2 and 4). As isolated CD3γ was insoluble in the folding mixture buffer and therefore not present as free monomers, the observed monomeric CD3γ and CD3ε bands most probably were derived from nondisulphide-linked CD3γε heterodimers. In agreement with this, the CD3γ and CD3ε bands in the precipitates were equally strong in contrast to the bands in the supernatants. The pattern of the precipitation with UCHT-1 was identical to that of F101.01. Thus, both Abs recognized native folded CD3γε heterodimers independent of the interchain disulphide bond. Neither of the MoAbs precipitated the isolated CD3γ or CD3ε chains (data not shown). Taken together, these experiments indicated that the linear CD3ε epitope recognized by UCHT-1 in the Western blot was masked in native CD3ε chains folded alone but became exposed following association of CD3ε with CD3γ.
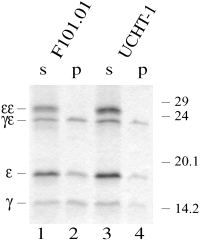
Identification of antigenic epitopes by immunoprecipitation. The CD3γε folding mix was incubated with F101.01 (lanes 1 and 2) or UCHT-1 (lanes 3 and 4). Immune complexes were precipitated by protein A beads. The resulting precipitates (p) and the remaining supernatant (s) were run on 15% SDS-PAGE, which were subsequently Coomassie stained. Molecular mass markers are indicated on the right (kDa), and the positions of the CD3 mono-, homo- and heterodimers are indicated on the left of the gel.
Purification of heterodimers
Large quantities of proteins are needed to perform structural analyses. In order to purify the heterodimers, we therefore developed a method for this purpose. We produced an immunoaffinity chromatography column with the MoAb F101.01. The folding mixture was loaded on the column, which was subsequently washed and eluted with DEA buffer, pH 11. Very pure heterodimers were eluted from this column, as judged by the SDS-PAGE analysis (Fig. 4). In agreement with the precipitation studies, monomers most probably representing nondisulphide-linked heterodimers were also present on the gel. The silver staining method used was not quantitative, explaining the different intensity of the CD3ε and CD3γ bands.
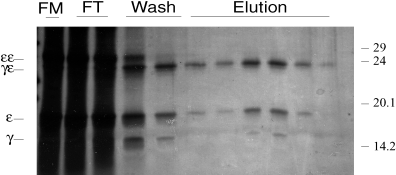
Immunoaffinity purification of CD3 heterodimers. The CD3γε folding mix was run through a F101.01 immunoaffinity chromatography column. The folding mix (FM), the flow-through (FT), fractions from the wash (Wash) and fractions from the elution (Elution) were run on 15% SDS-PAGE, and the gel was subsequently silver stained. Molecular mass markers are indicated on the right (kDa), and the positions of the CD3 mono-, homo- and heterodimers are indicated on the left of the gel.
Discussion
This study presents in vitro pairwise assembled human CD3 heterodimers. We have used a novel method to produce the extracellular domain of the three human CD3 chains in E. coli as denatured (inclusion body) proteins, followed by in vitro de novo folding by dilution.
By excluding reducing agents in the urea buffer, we maintained the disulphide bonds, which were made either by the cultured bacteria or during the process of cell rupture and purification of the inclusion bodies. The preformed disulphide bonds allowed us to fold the recombinant CD3 chains efficiently, without adding GSH/GSSG to the folding buffer. We confirmed the presence of intrinsic disulphide bonds, a characteristic feature of the Ig domains for CD3γ and CD3ε, but were unable to detect this in CD3δ. The lack of an intrinsic disulphide bond in CD3δ was surprising as it was present in CD3γ and CD3ε, and these genes originated from gene duplication [19, 25]. According to the sequence of CD3δ, only 37 residues separates the two cysteines in the proposed Ig domain. This is a shorter sequence as compared with 41 residues in CD3γ, 49 residues in CD3ε, and in particular to the 55–60 residues commonly found in Ig C-domains [7]. These differences could be explained if one or more of the β strands are missing in the β-sheets of CD3δ or if CD3δ does not fold as an Ig domain. However, final proof of this observation needs to be pursued in more detail, e.g. by titration of the free thiol groups.
The pairwise interaction between the extracellular domains of CD3ε and CD3γ or CD3δ was demonstrated by de novo folding of CD3 chains added pairwise to the folding buffer. Using MoAb against CD3ε, we showed a conformational change in CD3ε after the heterodimerization. The MoAb F101.01 has previously been regarded as a conformational TCR Ab [18]. Here we more precisely mapped the binding site of F101.01 to a shared conformational epitope on CD3γε and on CD3δε. During SDS-PAGE, the nondisulphide-linked chains were denatured, and they appeared as linearized monomers. However, when the dimers were stabilized by an interchain disulphide bond, a fraction of the dimers retained their structure. In this situation, the antigenic epitope was expressed, and F101.01 and OKT-3 were able to recognize the heterodimers, as shown by Western blotting (Fig. 2). In contrast to this, a recent study that succeeded in producing human CD3 heterodimers as CD3 IgG1 Fc fusion-proteins was unable to show OKT-3 reactivity towards the CD3γε heterodimer in Western blots [26]. Furthermore, we found that UCHT-1 and Leu-4 readily recognized mono-, homo- and heterodimeric forms of denatured CD3ε, as shown in a Western blot analysis (Fig. 2). After reduction of the intrinsic disulphide bond with 2-ME, UCHT-1 and Leu-4 still recognized CD3ε in Western blots indicating that the epitopes recognized by these MoAb were linear. As CD3γ and CD3δ alone were insoluble in the folding mixture buffer used, the distinct bands of the size of CD3γ in Fig. 2A (lane 3) and CD3δ in Fig. 2B (lane 2) were surprising. One explanation is that the heterodimers were heterogeneous and consisted of both disulphide-linked and nondisulphide-linked dimers.
To pursue the different binding characteristics of the MoAb on the native-like heterodimers, we further characterized the CD3γε folding mixture by immunoprecipitation. Identical precipitation patterns for the two MoAbs UCHT-1 and F101.01 were found. Surprisingly, the CD3ε homodimer was not precipitated by UCHT-1 in this native-like structure. Therefore, CD3ε seemed to undergo a conformational change before exposing both epitopes, and proper folding of CD3ε is likely to happen only after dimerization with CD3γ or CD3δ. This is in agreement with a previous study, which found that proper folding of murine CD3ε requires the presence of CD3γ[27]. Further studies from this group have recently led to the solution structure of a CD3γε single chain construct [28].
We thus propose that native CD3 heterodimers present two types of antigenic epitopes: (i) A linear type on CD3ε which is recognized by the MoAbs UCHT-1 and Leu-4 following association of CD3ε with CD3γ or CD3δ, and (ii) a nonlinear type composed of residues on both CD3ε and CD3γ or CD3δ which is recognized by F101.01 and OKT-3. This is supported by a previous study using transient transfection of COS cells with the CD3 chains, which showed that only COS cells doubly transfected with CD3ε and CD3γ or CD3δ could be stained with these Abs [29].
Assembly of all the TCR chains is a prerequisite for surface expression of the TCR complex [30]. Several domains in the CD3 chains important for assembly have been described [8, 9]. The molecular chaperone calnexin associates with TCR-α, TCR-β, CD3δ and CD3γ-containing monoglycosylated N-linked glycan chains, as well as with CD3ε which is unglycosylated [17, 31]. However, TCR can be expressed in the absence of calnexin, which probably limits the role of calnexin to stabilization of the TCR-α subunit during assembly [31]. Consistent with this, our results showed that the CD3γε and CD3δε heterodimers were formed spontaneously when they were folded together. This finding shows that the extracellular domain of the CD3 chains is sufficient to form the heterodimers, thus extending previous observations of Wegener et al. [32]. CD3γδ heterodimers have been observed when the transmembrane charges were removed [1]. In conflict with this, we were unable to obtain any CD3γδ heterodimers between the extracellular domains. However, we cannot exclude that the buffers examined in the folding experiments were unable to promote dimerization of the chains.
A conserved CXXC motif known from the protein disulphide isomerase (PDI) family [10] is present in all CD3 chains [9]. In the PDI family this motif is responsible for the formation of disulphide bonds [33]. Disulphide-linked CD3 heterodimers are usually not expressed in T cells, but it is possible that such bonds could be formed and removed again during the folding process in the ER to produce proper folded heterodimers. In our set-up, we did not mimic the reducing environment present in the ER, which could explain our ability to form disulphide-linked heterodimers spontaneously. Presence of GSH/GSSG augmented the yield of disulphide-linked CD3δε heterodimers suggesting that changes in the redox potential affects the dimerization and thus the fraction of disulphide-linked dimers.
In conclusion, we here describe an efficient protocol to produce and fold the extracellular domain of CD3 molecules. This allowed us to study the nature of pairwise assembly of the human CD3 chains. We demonstrate that the dimerization of CD3ε with CD3γ or CD3δ involves a conformational change of CD3ε. Studies with the aim of determining the three-dimensional structure of the CD3 chains should therefore focus on resolving the structure of the heterodimers. Furthermore, we describe a method capable of purifying the dimers by immunoaffinity chromatography. Large-scale production of the heterodimers could lead to the determination of the structure of the human CD3γε and CD3δε heterodimers by crystallographic or NMR studies, adding further insight into the structure and function of TCR.
Acknowledgments
This work was supported by The Danish Medical Research Foundation, The Novo Nordic Foundation, Gerda and Aage Haensch's Foundation, The Danish Medical Association Research Fund, The AP Møller Foundation for the Advancement of Medical Science, King Christian the Tenths Foundation and the Danish Cancer Society. We thank Drs Peter S. Andersen, Luca Bolliger and Anne-Marie K. Wegener for critically reading the manuscript. The expert technical help of Bodil Nielsen is gratefully acknowledged. J.K., J.P.H.L. and C.M. were recipients of PhD scholarships from the University of Copenhagen.




