Circulating, Interferon-Producing Plasmacytoid Dendritic Cells Decline During Human Ageing
Abstract
Increased frequency and severity of infections in the elderly have been taken as indicative of declining immune function. Dendritic cells (DCs), the most important antigen-presenting cells, play a central role in initiating and modulating immune responses. One type, DC2, arises from precursor plasmacytoid DCs (pDCs), a rare population of circulating blood cells, whose hallmark function is rapid and copious production of interferon-α (IFN-α) upon microbial challenge. We found significant decreases of the circulating pDCs during ageing in healthy adult humans, as defined both by flow cytometry and IFN-α generation. Mean pDC/mm3 in peripheral blood declined from 7.8 for the youngest age group (18–39 years) to 4.2 for the oldest (60–91 years; P = 0.017). IFN-α generation declined similarly, from 3537 to 1201 IU/ml, respectively (P = 0.006). There was also a slight decline over the age range in the amount of IFN generated per pDC (slope = −0.0087; P = 0.046). CD4+ T cells decreased by approximately 20% over the same age range (P = 0.001), while there was no change in the total lymphocyte or monocyte counts.
Introduction
Dramatic involution of the thymus with age has long been recognized [1, 2], while modest changes in T-cell subpopulations and signalling, and changes in other components of the adaptive and innate arms of the immune system have been reported more recently [3–7]. These may collectively contribute towards immunosenescence, manifested in elevated rates and severity of infections and reduced responses to immunization in the elderly [8].
Dendritic cells (DCs), which play crucial roles in initiating and regulating immune responses [9], remained, until recently, largely unstudied during the ageing process. Recent analyses suggest stability of the monocyte-derived (myeloid) dendritic cells (DC1) during the ageing process [10–12].
Another major DC lineage, generated from CD34+ progenitors, possibly via a lymphoid pathway [13, 14], is represented in the peripheral blood by precursor plasmacytoid mononuclear cells (MNCs), termed pDC [15–17]. pDCs are found in around 0.1–0.3% of peripheral blood mononuclear cells (PBMCs), and are detectable both by their rapid secretion of large amounts of interferon-α (IFN-α) in response to microbial challenge and by cell-surface markers [15–18]. Both pDC and their terminally differentiated DC2 forms are capable of interacting with and presenting microbial antigens to naive T cells in T-cell areas of secondary lymphoid tissue, leading to T-cell activation, cytokine production and memory T-cell formation [18]. pDC participates in the initiation of T-helper-1 (Th-1) immunity within lymphoid organs, and are found in various inflammatory processes. These include skin lesions of systemic lupus erythematosus (SLE) [19], reactive lymphadenopathy [18], nasal secretions after allergic challenge [20] and in cerebrospinal fluid (CSF) associated with multiple sclerosis and Lyme borreliosis [21]. Maturation of pDC to DC2 is accompanied by a shift in biasing away from Th-1 and towards a Th-2 response [18]. pDCs have also been identified in human thymus tissue [22, 23].
Deficits in pDC are associated with the development of opportunistic infections (OI) in acquired immunodeficiency syndrome and have also been found in other patient groups at high risk of OI [24, 25]. It was during the course of our clinical studies on pDC deficits that we noted an age-dependent fall in pDC number and function in our normal control populations (unpublished observations; Yang OO, Hauser MA, Ferbas J et al., submitted). As we were investigating the extent and significance of these changes in adults, a study appeared showing dramatic declines in a paediatric population [12].
Materials and methods
Circulating pDC numbers and IFN-α-generating capacities were determined for each of 56 adults (25 men, 31 women) over a 10 month period. There were 48 Caucasians, six Asians (including South Asians) and two Hispanics. For some analyses, they were grouped according to the traditional divisions of young, middle and old age: 16 were 18–29 years old (three men, 13 women); 26 were 30–59 years old (14 men, 12 women); and 14 were 60 years old and over (60–91, eight men, six women). All were generally healthy, physically and mentally active and had no recent immunizations or treatment with corticosteroids or other drugs known to be immunosuppressive. Subjects >60 were taking an average of 2.1 (range 0–8) medications when studied. While not adhering to the stringent SENEIUR protocol [26], the criteria employed generally agreed with the reconsideration of SENEIUR as recently published [27], to better ensure representational results for the healthy elderly. PBMCs were isolated from Ficoll/Hypaque gradients as previously described [28]. Percentages of circulating pDC and CD4+ T lymphocytes among PBMC were quantified from flow cytometric scatter plots generated by FACScalibur (BDIS), based upon collection of 80 000 events per run. pDCs were identified as peripherally circulating MNCs that were positive for CD4 and CD123 (interleukin-3R-α (IL-3R-α)) but negative for CD11c and the lineage markers CD3, CD14, CD16, CD19, CD20 and CD56 [28] for each subject, and the percentage of pDC/MNC was used to calculate the absolute number of circulating pDCs. Absolute circulating pDC numbers were calculated by multiplying the absolute count of ‘MNCs’ (i.e. those cells included in Ficoll/Hypaque populations: monocytes + basophils + lymphocytes) from automated complete blood counts by the percentage of pDC in the cell populations gated as MNC by light scatter in flow cytometry.
IFN generation was determined as previously described [15, 24, 28]. PBMCs were stimulated with ultraviolet-inactivated herpes simplex virus (HSV – kindly provided by P. Fitzgerald-Bocarsly, New Jersey College of Medicine, UMDNJ, Newark, NJ), and supernatant IFN was determined by the fluorescent signal produced by PIL indicator cells transfected with IFN response elements upstream from a structural luciferase gene [28]. Michael Tovey (Laboratory of Viral Oncology, Villejuif, France) generously provided the PIL cell line.
Statistical analysis was carried out using spss for Windows, version 10.0.7 (SPSS Inc., Chicago, IL, USA). For subjects tested more than once over the course of study, the arithmetic means of each variable were used in the overall analysis.
Results
Circulating PBMC subpopulations from healthy volunteers were analysed. Data are shown either within age categories (Table 1) or as continuous distributions (Fig. 1A,B). We found that total lymphocyte numbers and monocyte counts (data not shown) remained constant with age, while the CD4+ T-cell population declined moderately (Table 1 and Fig. 1C). The CD4+ decline in our study was attributable almost solely to declines in the males; CD4+ T-cell counts in females did not change during ageing.
| Group | Ages | IFN-α generation (IU/ml) | %pDC of MNC† | %CD4+T cells of MNC | %Lymphocytes of MNC |
|---|---|---|---|---|---|
| I | 18–29 | 3537 ± 2165 | 0.29 ± 0.15 (7.8) | 38.6 ± 7.3 | 77.9 ± 8.2 |
| II | 30–59 | 2663 ± 1210 | 0.25 ± 0.1 (5.6) | 38.0 ± 8.15 | 76.31 ± 10.3 |
| III | 60–91 | 1201 ± 806 | 0.17 ± 0.1 (4.2) | 29.9 ± 10.2 | 74.5 ± 9.4 |
| All subjects | 18–91 | 2547 ± 1649 | 0.24 ± 0.12 (6.1) | 36.16 ± 9.1 | 76.4 ± 9.4 |
- * Mean+1 standard deviation (SD). Cell counts shown are as the percentage of all mononuclear cells gated in flow cytometry on forward- and 90°-light scatter.
- † Numbers in parentheses indicate the number of pDC per mm3 of peripheral blood (see text for details – pDC as percentage of MNC by flow×circulating MNC/mm3).
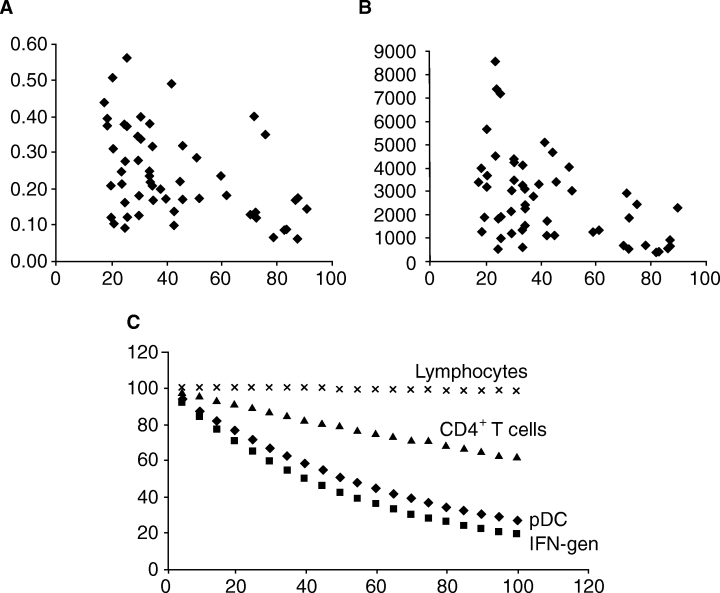
Relationship of circulating plasmacytoid dendritic cells (pDCs), CD4+ T cells and lymphocytes to the age of donor. Horizontal axes: donor age in years. (A) Vertical axis: percentage of peripheral blood mononuclear cells (PBMCs) that were pDC by flow cytometric criteria. (B) Vertical axis: interferon-α (IFN-α) generated (IU/ml) by PBMC in response to ultraviolet (UV)-inactivated herpes simplex virus (HSV). (C) Vertical axis: percentage of initial level of IFN-α generation (squares), and numbers of pDC (diamonds), CD4+ T cells (triangles) and lymphocytes (‘×’s) remaining, as predicted by best-fit curves (data only from ages 18–92 – see text for details).
In contrast to the above, the pDC population in circulating blood was found to decline significantly with age, and equally in men and women. This decline appeared to be continuous with time, with the best-fit curve (Fig. 1C) being an exponential decay, in which pDC numbers are inversely proportional to ‘e’ raised to the power of (0.0117 × age in years) (e−0.0117 × age) (r2 = 0.224). The generation of IFN also declined continuously with age, where the best-fit curve is represented by e−0.179 × age (r2 = 0.291). This would mean that pDC number and function are being lost at a rate of roughly 1–1.5% per year, starting from 18 years of age in our dataset. Rates of CD4+ T-cell loss with an exponential decay curve fit was roughly 0.5% per year (e−0.0048 × age).
These data were also analysed within age groups, using generally recognized age divisions into young, middle and old age – (i) those under 30; (ii) those between 30 and 59; and (iii) those over 60 (Table 2). There were significant decreases, as tested by one-way anova, of both pDC number (F2.51 = 7.95; P = 0.001) and IFN generation (F2.51 = 7.53; P = 0.001) in comparisons between the age groups, as well as lesser, but still significant declines for CD4+ cells (F2.53 = 5.13; P = 0.009).
| Comparison | IFN-α generation | %pDC | %CD4 ± T cells |
|---|---|---|---|
| Group I versus III | P = 0.006 | P = 0.017 | P = 0.001 |
| Group II versus III | P = 0.002 | P = 0.016 | P = 0.005 |
There was also an apparent but small decline in the IFN produced per pDC over the entire age range, based on an analysis comparing data for bulk IFN generation with %pDC in the MNC fraction from individual studies of each subject. Linear regression analysis revealed a slope of −0.0087, just barely achieving statistical significance (P = 0.046).
Discussion
The progressive losses of circulating pDC with age appear to be a relatively large effect in comparison with previously reported correlates of ageing and cellular immune function [3–7, 29]. The age-related losses of IFN-α-generating capacity of mixed MNC populations responding to HSV appear to be entirely attributable to losses of pDC. The reductions in IFN produced by MNC were linearly related to the proportion of pDC in the MNC fractions, and IFN produced per pDC changed little with ageing.
Given the age-related involution of the thymus, it is interesting to note that pDCs are present both in this organ [22, 23] and in T-cell areas of reactive peripheral lymphoid tissue [15, 16, 18]. pDC/DC2 are also regulators of Th-1/Th-2 balance, an equilibrium reported to be perturbed in the aged [30].
Our calculations of absolute numbers of circulating pDC are based on the assumption that pDC and other MNC subsets are recovered with equal efficiency in Ficoll–Hypaque separations. Should this not be the case, absolute pDC counts may differ from our estimates by some factor, but the relative effects observed would remain unchanged.
A very recent report, almost perfectly complementary to the present one, followed pDC and IFN production from birth to 18 years of age [12]. From 20 pDC/mm3 of circulating blood at birth, there was an initially rapid, almost 2.5-fold drop in the first 10 years of life, followed by an apparent plateau after about 10 years of age; by age 18, circulating pDCs were at 8/mm3. Our present study of adults began at 18 years of age, and shows mean circulating pDC at 7.8/mm3 in the 18- to 39-year-old age group (group I). The long decline we observed from age 18–92 of roughly 1–1.5% per year would not be apparent on the scale of the paediatric study. In contrast to dramatic changes in pDC, the study in children showed no changes in CD11c+ myeloid DC in the birth-to-18 age range [12], in line with earlier work in adults [10, 1].
Loss of pDC IFN-α generation by blood MNC, attributable chiefly not only to declining pDC numbers but also to a small reduction in IFN generated per pDC, as reported here, might play a role in the decreased capacity for immunization and increasing susceptibility to infections observed in the elderly.
Acknowledgments
Supported by an unrestricted grant from Pharmacia & Upjohn, by The Radin Foundation, and by institutional funds from Saint Vincents Catholic Medical Center/Manhattan. Dr Noel Warner of Becton-Dickinson Immunofluorescence Systems (BDIS) generously provided the flow cytometry reagents used in these studies. Ms Kokila Shah provided excellent technical assistance.




