A genome-wide strategy for the identification of essential genes in Staphylococcus aureus
Summary
To address the need for new approaches to antibiotic drug development, we have identified a large number of essential genes for the bacterial pathogen, Staphylococcus aureus, using a rapid shotgun antisense RNA method. Staphylococcus aureus chromosomal DNA fragments were cloned into a xylose-inducible expression plasmid and transformed into S. aureus. Homology comparisons between 658 S. aureus genes identified in this particular antisense screen and the Mycoplasma genitalium genome, which contains 517 genes in total, yielded 168 conserved genes, many of which appear to be essential in M. genitalium and other bacteria. Examples are presented in which expression of an antisense RNA specifically reduces its cognate mRNA. A cell-based, drug-screening assay is also described, wherein expression of an antisense RNA confers specific sensitivity to compounds targeting that gene product. This approach enables facile assay development for high throughput screening for any essential gene, independent of its biochemical function, thereby greatly facilitating the search for new antibiotics.
Introduction
The spread of antibiotic resistance in bacteria has intensified the need for novel approaches to antimicrobial drug discovery. Antibiotics in current use target about 15 out of the approximately 265–350 essential genes (Hutchison et al., 1999) in bacterial genomes. The need to identify such untapped potential antibiotic targets in bacterial pathogens has fostered innovative approaches for essential gene identification. These include gene disruption or deletion (Joyce and Grindley, 1984; Link et al., 1997; Xia et al., 1999), promoter replacement (Jana et al., 2000; Zhang et al., 2000), saturation transposon mutagenesis in Mycoplasma genomes to deduce essential genes (Hutchison et al., 1999), transposon delivery of conditionally expressed promoters (Chow and Berg, 1988), comparative genomics combined with conditional expression (Arigoni et al., 1998; Freiberg et al., 2001), and approaches that use in vitro transposition in combination with genetic footprinting or with polymerase chain re-action (PCR) and Southern analysis (Akerley et al., 1998; Reich et al., 1999).
Staphylococcus aureus, the most frequent causative agent of nosocomial infections, has become a major public health threat as a result of the increased incidence of drug resistance in this organism. Since the emergence of methicillin-resistant S. aureus (MRSA) in the 1970s, the only effective antibiotics against such strains are vancomycin and linezolid. In the last few years, new strains of MRSA also resistant to vancomycin (glycopeptide intermediate resistance in S. aureus, GISA) have been isolated, making it very difficult to treat some S. aureus infections.
As a first step towards the development of new anti-biotics to combat such pathogens, we have developed a rapid shotgun antisense procedure for the comprehensive identification of S. aureus genes essential for growth. Inhibition of gene expression by antisense RNA has been observed in natural bacterial systems (Altuvia and Wagner, 2000; Wagner and Simons, 1994) and has been used for silencing gene expression (Engdahl et al., 1997; Ji et al., 1999). Key features of an effective antisense RNA are its stability and accessibility to participate in RNA–RNA duplexes (Zeiler et al., 1998), which are features that are not readily predictable. One major advantage of the genome-wide antisense fragmentation approach described here is that it selects the maximum growth inhibitory activity of antisense RNAs from large random populations. In this approach, essential genes are identified after conditionally expressing random genomic fragments, and then screening for fragments whose expression blocks growth. The genes targeted by anti-sense RNA are identified by DNA sequencing and BLAST analysis against the annotated genome sequence of the S. aureus MRSA strain, N315 (Kuroda et al., 2001). This approach has led to the identification of a comprehensive set of S. aureus essential genes that will enable the discovery of new antimicrobial compounds. In addition, we describe a cell-based assay for discovering new anti-biotics, which uses the expression of an antisense RNA, complementary to the mRNA of an essential gene, to reduce the level of the targeted mRNA and, thereby, target protein levels.
Results
pT5X xylose-inducible promoter: RNAs stability and abundance
The stability and abundance of an antisense RNA can play a critical role in determining its ability to block the expression of a cognate mRNA (Case et al., 1989). To determine the stability of RNA expressed from the pT5X promoter, cells containing the plasmid pEPSA5 (Fig. 1) were treated with 2% xylose for 10 min followed by rifampicin (200 μg ml−1) treatment. Total cellular RNA was isolated at various times, and the half-life of vector RNA transcribed from the pT5X promoter was determined to be >5 min by Northern analysis (data not shown), suggesting that, for a bacterial RNA, this molecule is relatively stable. In addition, the vector RNA transcribed from the pT5X promoter appears to be so abundant to be visible on ethidium bromide-stained agarose gels (data not shown). Given these observations, high steady-state levels of RNA from this promoter in S. aureus should be achievable.
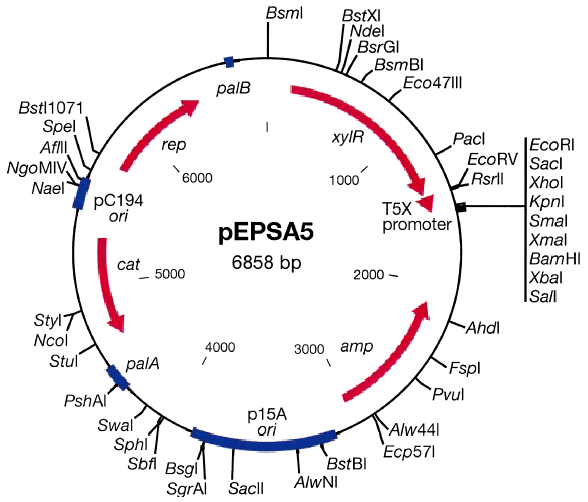
The pEPSA5 S. aureus/E. coli shuttle vector.
Shotgun antisense identification of essential genes
An example of the process for shotgun antisense identification of essential genes in S. aureus is described in Fig. 2. Genomic fragments from S. aureus strain RN450 between Å200 and 800 bp were ligated into pEPSA5, downstream of the xylose-inducible promoter pT5X. The genomic library was amplified by passage through Escherichia coli to provide sufficient amounts of DNA, and DNA from the pooled library was then transformed into S. aureus strain RN4220. The inserts of the resulting 3117 xylose-sensitive clones were sequenced, and the identity of the gene source and fragment orientation were determined by BLAST analysis against the annotated genome sequence of the S. aureus MRSA strain, N315 (Kuroda et al., 2001). From this particular screen, 2169 clones were found to contain genomic inserts in an antisense orientation relative to pT5X, representing 658 unique genes. The remaining clones contained inserts representing several classes, including inserts in the sense orientation relative to the promoter, mixed convergent and divergent inserts and intergenic inserts.
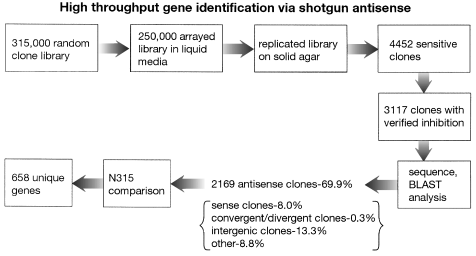
Flow chart for shotgun antisense gene discovery depicting numbers of clones screened.
Antisense induction resulted in various growth phenotypes ranging from a complete inability to grow to marginal reduction in growth rate (Fig. 3). In some cases, multiple clones targeting the same essential gene showed different levels of inhibition (data not shown). Only clones showing strong growth inhibition upon antisense induction were characterized further.
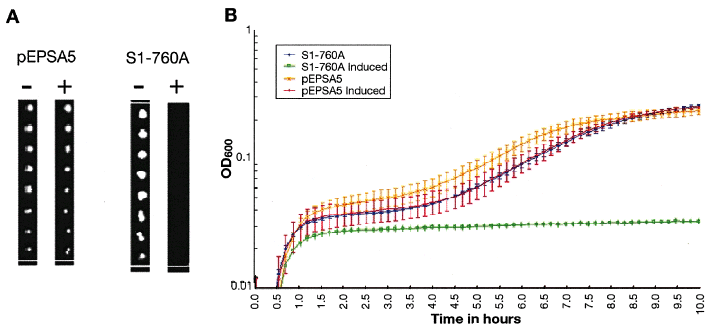
Assessment of growth inhibition of clones on solid agar (A) and in liquid media (B). A. Eight 10-fold dilutions were performed on RN4220 carrying pEPSA5 or S1–760A (produces antisense to rplQ) and replica-plated onto LBG + 2% xylose (inducing medium, +) and LBG (non-inducing medium, –) demonstrating specific growth inhibition of S1–760A. B. Exponential cultures of RN4220 carrying S1–760A were diluted into inducing and non-inducing media as duplicate cultures and the OD600 monitored over 10 h to track growth or its inhibition. Error bars depict the deviation of the duplicate samples.
Saturation analysis
The average size of the inhibitory inserts was 221 basepairs (bp), with 43 bp being the smallest and 854 bp being the largest fragment. Overall, 50% of the recovered fragments were 200–500 bp and 40% were 100–200 bp. Using the average length of the clones found in this screen, 221 bp, and applying the formula n = ln(1-Φf)/ ln(1–f), in which n is the number of clones necessary to obtain a single specific clone, Φ is the probability of obtaining at least one of any possible gene sequence, and f is the fraction of the genome contained in an average-sized cloned insert (Zissel et al., 1992), we calculate that by screening 208 226 inserts, we ensured that we sampled the entire genome with greater than 99.99% confidence.
Nearly 50% of the genes inhibited by antisense in this screen were targeted two or more times (Fig. 4). On average, 3.2 genomic fragments expressed antisense to a given gene. In one extreme example, 31 different antisense fragments to the rpoB gene were recovered. These fragments cover discrete regions of the gene, with a notable absence of fragments mapping to the N-terminal portion of the coding region (Fig. 5). We observed this for other genes identified in this screen, suggesting that there may be preferred sites for antisense inhibitory activity, as well as sites that are not favourable substrates for antisense inhibition. Alternatively, such regions may not be amenable to cloning, possibly because transcription of the DNA in that region is deleterious to E. coli.
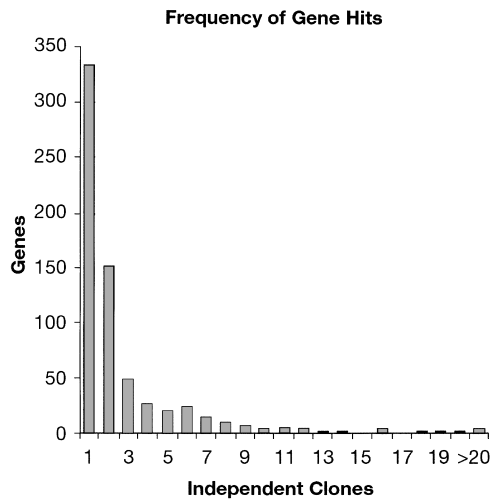
The frequency with which single genes were targeted by shotgun antisense.

Map of the rpoB gene, demonstrating the location and relative size of 31 different cloned antisense fragments.
Demonstration of specific targeting of the antisense RNA
To gain confirming evidence that the primary effect of antisense induction is the specific perturbance of the target mRNA, Real Time PCR (RT-PCR) was used to monitor the fate of target mRNAs and a non-targeted control mRNA early in response to induction of antisense (Fig. 6). The rplQ gene encodes the ribosomal protein L17 of the 50S subunit, and lig codes for DNA ligase, an activity shown to be essential in Bacillus subtilis (Petit and Ehrlich, 2000). RT-PCR revealed that rplQ mRNA decreases by 85% within 15 min of rplQ antisense RNA induction. No effect is observed on the unrelated mRNA of the lig gene. Conversely, lig mRNA decreases nearly 60% after lig antisense RNA induction, with no significant change in the rplQ mRNA.
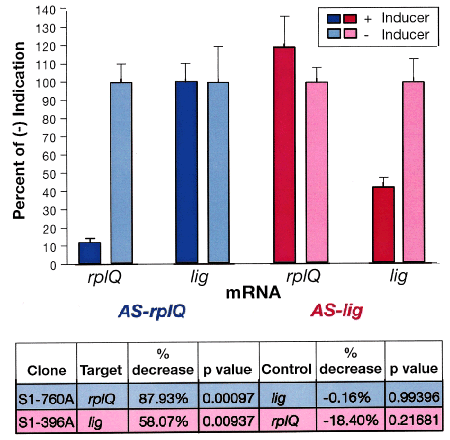
Targeted mRNAs decrease in concentration only with induction of their specific antisense. As measured by RT-PCR, rplQ mRNA decreases in response to induction of rplQ antisense (blue) whereas an unrelated lig mRNA shows no significant change. In the reciprocal case, lig mRNA decreases in response to induction of lig antisense (red) whereas the rplQ mRNA shows no significant change.
Comparison of S. aureus antisense inhibited genes to the M. genitalium gene set
The 658 S. aureus genes identified in this particular screen were compared with the gene set of M. genitalium to examine the efficiency of essential gene identification by antisense. Hutchison and colleagues (Hutchison et al., 1999), mapped most of the non-essential genes in M. genitalium by nearly saturating the genome using transposon mutagenesis. Those genes for which no insertions were recovered were inferred to be essential, thus providing a potential minimal set of 256–350 essential genes in the 517 genes comprising the genome of these bacteria. Comparison of the antisense RNA-inhibited S. aureus genes to the full M. genitalium gene set resulted in 168 Staphylococcus genes with clear Mycoplasma homologues (Table 1). Out of these 168 genes, 146 were not disrupted in the exhaustive transposon mutagenesis screen of the Mycoplasma genome, suggesting that these genes are essential in both organisms. Not surprisingly, M. genitalium and S. aureus share essential genes in most major functional categories; cell envelope pro-cesses, metabolism, DNA replication, RNA synthesis, protein synthesis and a set of unknown genes. Mycoplasma genitalium lacks a functional cell wall, and genes involved in cell wall synthesis are not present in this organism. In contrast, many of these genes were represented in the S. aureus antisense hits (data not shown).
| N315 gene | Strain N315 | # of independent | M. genitalium | Presumed essentiality | |||
|---|---|---|---|---|---|---|---|
| Class | Subclass | name | gene description | clones | gene | in M. genitalium | |
| I Cell envelope and cellular processes | |||||||
| I-1 Cell wall | |||||||
| murA | UDP-N-acetylglucosamine 1-carboxyvinyl transferase 1 | 7 | MG466 | Essential | |||
| I-2 Transport/binding proteins and lipoproteins | |||||||
| oppF | oligopeptide transport ATP-binding protein | 1 | MG079 | Essential | |||
| SA0272 | similar to transmembrane protein Tmp7 | 1 | MG424 | Essential | |||
| fruA | fructose specific permease | 6 | MG062 | Not essential | |||
| SA0675 | similar to ABC transporter ATP-binding protein | 1 | MG467 | Not essential | |||
| SA0774 | ABC transporter ATP-binding protein homologue | 1 | MG079 | Essential | |||
| SA1747 | similar to ABC transporter, ATP-binding protein | 1 | MG180 | Essential | |||
| opuCA | glycine betaine/carnitine/choline ABC transporter | 2 | MG468.1 | Essential | |||
| opp-1D | oligopeptide transporter putative ATPase domain | 1 | MG079 | Essential | |||
| SA2434 | fructose phosphotransferase system enzyme fruA homolog | 1 | MG062 | Not essential | |||
| vraD | hypothetical protein, similar to ABC transporter | 1 | MG180 | Essential | |||
| I-3 Sensors (signal transduction) | |||||||
| vraS | two-component sensor histidine kinase | 1 | MG397 | Essential | |||
| I-4 Membrane bioenergetics (electron | |||||||
| transport chain and ATP synthase) | |||||||
| atpF | ATP synthase B chain | 1 | MG403 | Essential | |||
| I-6 Protein secretion | |||||||
| secA | preprotein translocase subunit | 6 | MG072 | Essential | |||
| SA1078 | signal recognition particle | 2 | MG297 | Essential | |||
| ffh | signal recognition particle homolog | 6 | MG048 | Essential | |||
| secY | preprotein translocase SecY subunit | 11 | MG170 | Essential | |||
| SA2442 | preprotein translocase SecA homolog | 3 | MG072 | Essential | |||
| I-7 Cell division | |||||||
| ftsH | cell-division protein | 1 | MG457 | Essential | |||
| ftsZ | cell division protein | 9 | MG224 | Essential | |||
| gidA | glucose inhibited division protein A | 3 | MG379 | Essential | |||
| I-8 Sporulation | |||||||
| obg | Spo0B-associated GTP-binding protein | 13 | MG384 | Essential | |||
| II Intermediary metabolism | |||||||
| II-1 Metabolism of carbohydrates and related molecules | |||||||
| II-1–1 Specific pathways | |||||||
| fruB | fructose 1-phosphate kinase | 5 | MG063 | Essential | |||
| glpK | glycerol kinase | 2 | MG038 | Essential | |||
| SA1523 | acetyl-CoA carboxylase transferase beta subunit | 3 | MG325 | Essential | |||
| mtlD | mannitol-1-phosphate 5-dehydrogenase | 2 | MG362 | Essential | |||
| lacA | galactose-6-phosphate isomerase LacA subunit | 1 | MG396 | Essential | |||
| SA2279 | hypothetical protein, similar to phosphomannomutase | 1 | MG053 | Essential | |||
| gntK | gluconokinase | 7 | MG038 | Essential | |||
| II-1–2 Main glycolytic pathways | |||||||
| gap | glyceraldehyde-3-phosphate dehydrogenase | 3 | MG301 | Essential | |||
| eno | enolase | 4 | MG407 | Essential | |||
| pgi | glucose-6-phosphate isomerase A | 4 | MG111 | Essential | |||
| phdB | pyruvate dehydrogenase E1 component beta subunit | 1 | MG273 | Essential | |||
| pdhC | dihydrolipoamide S-acetyltransferase component of pyruvate dehydrogenase E2 | 4 | MG272 | Essential | |||
| pdhD | dihydrolipoamide dehydrogenase component of pyruvate dehydrogenase E3 | 5 | MG271 | Essential | |||
| tkt | transketolase | 3 | MG066 | Essential | |||
| SA1349 | dihydrolipoamide dehydrogenase | 8 | MG271 | Essential | |||
| pykA | pyruvate kinase | 2 | MG216 | Essential | |||
| pfk | 6-phosphofructokinase | 4 | MG215 | Essential | |||
| fbaA | fructose-bisphosphate aldolase | 3 | MG023 | Essential | |||
| II-2 Metabolism of amino acids and related molecules | |||||||
| bmfBB | branched-chain alpha-keto acid dehydrogenase E2 | 9 | MG272 | Essential | |||
| bfmBAB | branched-chain alpha-keto acid dehydrogenase E1 | 7 | MG273 | Essential | |||
| bfmBAA | branched-chain alpha-keto acid dehydrogenase E1 | 16 | MG274 | Essential | |||
| metK | S-adenosylmethionine synthetase | 2 | MG047 | Essential | |||
| glyA | serine hydroxymethyl transferase | 1 | MG394 | Not essential | |||
| II-3 Metabolism of nucleotides and nucleic acids | |||||||
| pta | phosphotransacetylase | 2 | MG299 | Not essential | |||
| pyrC | dihydroorotase | 1 | MG163 | Essential | |||
| upp | uracil phosphoribosyl transferase | 2 | MG030 | Essential | |||
| tdk | thymidine kinase | 1 | MG034 | Essential | |||
| SA1939 | deoxyribose-phosphate aldolase | 3 | MG050 | Essential | |||
| adk | adenylate kinase | 6 | MG171 | Essential | |||
| II-4 Metabolism of lipids | |||||||
| cdsA | phosphatidate cytidylyltransferase | 2 | MG437 | Essential | |||
| II-5 Metabolism of coenzymes and prosthetic groups | |||||||
| dfrA | dihydrofolate reductase | 1 | MG228 | Essential | |||
| hemC | porphobilinogen deaminase | 2 | MG238 | Essential | |||
| III Information pathways | |||||||
| III-1 DNA replication | |||||||
| dnaA | chromosomal replication initiator protein | 12 | MG469 | Essential | |||
| dnaC | replicative DNA helicase | 5 | MG094 | Essential | |||
| dnaX | DNA polymerase III gamma and tau subunits | 7 | MG420 | Essential | |||
| polC | DNA polymerase III, alpha chain PolC-type | 8 | MG031 | Essential | |||
| dnaE | DNA polymerase III, alpha chain | 1 | MG261 | Not essential | |||
| lig | DNA ligase | 3 | MG254 | Essential | |||
| pcrA | ATP-depentend DNA helicase | 1 | MG244 | Not essential | |||
| III-2 DNA replication/modification and repair | |||||||
| uvrA | exinuclease ABC subunit A | 2 | MG421 | Not essential | |||
| mutS2 | MutS-like protein | 1 | MG159 | Essential | |||
| III-4 DNA packaging and segregation | |||||||
| gyrB | DNA gyrase subunit B | 5 | MG003 | Essential | |||
| gyrA | DNA gyrase subunit A | 12 | MG004 | Essential | |||
| smc | chromosome segregation SMC protein | 3 | MG298 | Essential | |||
| parE | topoisomerase IV subunit B | 11 | MG203 | Essential | |||
| parC | topoisomerase IV subunit A | 6 | MG204 | Essential | |||
| III-5 RNA synthesis | |||||||
| III-5–2 Regulation | |||||||
| hprK | HPr kinase/phosphatase | 1 | MG085 | Not essential | |||
| III-5–3 Elongation | |||||||
| rpoC | RNA polymerase beta-prime chain | 27 | MG340 | Essential | |||
| rpoA | DNA-directed RNA polymerase alpha chain | 9 | MG177 | Essential | |||
| III-6 RNA modification | |||||||
| trmD | tRNA | 2 | MG445 | Essential | |||
| SA1885 | hypothetical protein, similar to ATP-dependent RNA helicase | 2 | MG425 | Essential | |||
| III-7 Protein synthesis | |||||||
| III-7–1 Ribosomal proteins | |||||||
| rplA | 50S ribosomal protein L1 | 1 | MG082 | Essential | |||
| rplB | 50S ribosomal protein L2 | 20 | MG154 | Essential | |||
| rplC | 50S ribosomal protein L3 | 5 | MG151 | Essential | |||
| rplD | 50S ribosomal protein L4 | 9 | MG152 | Essential | |||
| rplE | 50S ribosomal protein L5 | 8 | MG163 | Essential | |||
| rplF | 50S ribosomal protein L6 | 2 | MG166 | Essential | |||
| rplI | 50S ribosomal protein L9 | 2 | MG093 | Not essential | |||
| rplJ | 50S ribosomal protein L10 | 7 | MG361 | Essential | |||
| rplK | 50S ribosomal protein L11 | 8 | MG081 | Essential | |||
| rplL | 50S ribosomal protein L7/L12 | 7 | MG362 | Essential | |||
| rplM | 50S ribosomal protein L13 | 7 | MG418 | Essential | |||
| rplN | 50S ribosomal protein L14 | 12 | MG161 | Essential | |||
| rplO | 50S ribosomal protein L15 | 8 | MG169 | Essential | |||
| rplP | 50S ribosomal protein L16 | 5 | MG158 | Essential | |||
| rplQ | 50S ribosomal protein L17 | 7 | MG178 | Essential | |||
| rplR | 50S ribosomal protein L18 | 4 | MG167 | Essential | |||
| rplS | 50S ribosomal protein L19 | 5 | MG444 | Essentia | |||
| rplT | 50S ribosomal protein L20 | 1 | MG198 | Essential | |||
| rplU | 50S ribosomal protein L21 | 3 | MG232 | Essential | |||
| rplV | 50S ribosomal protein L22 | 8 | MG156 | Essential | |||
| rplW | 50S ribosomal protein L23 | 5 | MG153 | Essentiall | |||
| rplX | 50S ribosomal protein L24 | 3 | MG162 | Essential | |||
| rpmH | 50S ribosomal protein L34 | 5 | MG466 | Essential | |||
| rpmJ | 50S ribosomal protein L36 | 2 | MG174 | Essential | |||
| rpsB | 30S ribosomal protein S2 | 7 | MG070 | Essential | |||
| rpsC | 30S ribosomal protein S3 | 9 | MG157 | Essential | |||
| rpsD | 30S ribosomal protein S4 | 6 | MG311 | Essential | |||
| rpsE | 30S ribosomal protein S5 | 10 | MG168 | Essential | |||
| rpsG | 30S ribosomal protein S7 | 6 | MG088 | Essential | |||
| rpsH | 30S ribosomal protein S8 | 2 | MG165 | Essential | |||
| rpsI | 30S ribosomal protein S9 | 8 | MG417 | Essential | |||
| rpsJ | 30S ribosomal protein S10 | 4 | MG150 | Essential | |||
| rpsK | 30S ribosomal protein S11 | 7 | MG176 | Essential | |||
| rpsL | 30S ribosomal protein S12 | 5 | MG087 | Essential | |||
| rpsM | 30S ribosomal protein S13 | 5 | MG175 | Essential | |||
| rpsN | 30S ribosomal protein S14 | 3 | MG164 | Essential | |||
| rpsP | 30S ribosomal protein S16 | 1 | |||||
| rpsQ | 30S ribosomal protein S17 | 2 | MG160 | Essential | |||
| rpsR | 30S ribosomal protein S18 | 6 | MG092 | Essential | |||
| rpsS | 30S ribosomal protein S19 | 9 | MG155 | Essential | |||
| III-7–2 Aminoacyl-tRNA synthetases | |||||||
| metS | methionyl-tRNA synthetase | 7 | MG021 | Essential | |||
| lysS | lysyl-tRNA synthetase | 20 | MG136 | Essential | |||
| gltX | glutamyl-tRNA synthetase | 7 | MG462 | Essential | |||
| argS | arginyl-tRNA synthetase | 2 | MG378 | Essential | |||
| trpS | tryptophanyl-tRNA synthetase | 2 | MG126 | Essential | |||
| pheS | Phe-tRNA synthetase alpha chain | 3 | MG194 | Essential | |||
| pheT | Phe-tRNA synthetase beta chain | 16 | MG195 | Essential | |||
| ileS | Ile-tRNA synthetase | 19 | MG345 | Not essential | |||
| alaS | alanyl-tRNA synthetase | 14 | MG292 | Essential | |||
| aspS | aspartyl-tRNA synthetase | 8 | MG036 | Essential | |||
| hisS | histidyl-tRNA synthetase | 6 | MG035 | Essential | |||
| valS | valine-tRNA ligase | 4 | MG334 | Essential | |||
| thrS | threonyl-tRNA synthetase 1 | 3 | MG375 | Essential | |||
| tyrS | tyrosyl-tRNA synthetase | 2 | MG455 | Not essential | |||
| SA1563 | phenylalanyl-tRNA synthetase (beta subunit) homolog | 1 | MG449 | Essential | |||
| leuS | leucyl-rRNA synthetase | 18 | MG266 | Essential | |||
| SA1715 | glutamyl-tRNAGln amidotransferase subunit B | 12 | MG100 | Essential | |||
| SA1716 | glutamyl-tRNAGln amidotransferase subunit A | 16 | MG099 | Essential | |||
| III-7–3 Initiation | |||||||
| infB | translation initiation factor IF-2 | 4 | MG142 | Essential | |||
| infC | translation initiation factor IF-3 infC | 2 | MG196 | Essential | |||
| infA | translation initiation factor IF-1 | 4 | MG173 | Essential | |||
| III-7–4 Elongation | |||||||
| fus | translational elongation factor G | 19 | MG089 | Essential | |||
| tufA | translational elongation factor TU | 18 | MG451 | Essential | |||
| ssrP | ssrA-binding protein | 1 | MG059 | Not essential | |||
| SA0959 | GTP-binding elongation factor | 1 | MG138 | Essential | |||
| SA1100 | homolog elongation factor TS | 3 | MG433 | Essential | |||
| III-7–5 Termination | |||||||
| prfA | peptide chain release factor 1 | 1 | MG258 | Essential | |||
| III-8 Protein modification | |||||||
| ptsH | phophocarrier protein HPR | 1 | MG041 | Essential | |||
| ptsI | phosphoenolpyruvate-protein phosphatase | 4 | MG429 | Essential | |||
| SA1063 | protein kinase | 1 | MG109 | Essential | |||
| SA1854 | similar to O-sialoglycoprotein | 4 | MG046 | Essential | |||
| IV Other functions | |||||||
| IV-1 Adaption to atypical conditions | |||||||
| clpC | endopeptidase | 3 | MG355 | Not essential | |||
| clpB | ClpB chaperone homologue | 1 | MG355 | Not essential | |||
| clpL | ATP-dependent Clp proteinase chain ClpL | 2 | MG355 | Not essential | |||
| V Similar to unknown proteins | |||||||
| SA0351 | hypothetical protein, similar to GTP-binding protein | 1 | MG024 | Not essential | |||
| SA0437 | conserved hypothetical protein | 1 | MG134 | Essential | |||
| SA0447 | conserved hypothetical protein | 1 | MG056 | Essential | |||
| SA0449 | conserved hypothetical protein | 3 | MG009 | Not essential | |||
| SA0464 | conserved hypothetical protein | 2 | MG004 | Essential | |||
| SA0467 | conserved hypothetical protein | 3 | MG084 | Essential | |||
| SA0722 | conserved hypothetical protein | 3 | MG103 | Not essential | |||
| SA0940 | conserved hypothetical protein | 9 | MG139 | Essential | |||
| SA1031 | conserved hypothetical protein | 2 | MG198 | Essential | |||
| SA1086 | conserved hypothetical protein | 1 | MG442 | Not essential | |||
| SA1118 | conserved hypotehtical protein | 5 | MG139 | Essential | |||
| SA1187 | conserved hypothetical protein | 1 | MG247 | Essential | |||
| SA1252 | conserved hypothetical protein | 1 | MG448 | Essential | |||
| SA1307 | hypothetical protein, similar to GTP binding protein | 6 | MG329 | Essential | |||
| SA1445 | conserved hypothetical protein | 3 | MG002 | Not essential | |||
| SA1449 | (5-methylaminomethyl-2- | 5 | MG295 | Not essential | |||
| thiouridylate)-methyltransferase | |||||||
| SA0772 | conserved hypothetical protein | 3 | MG355 | Not essential | |||
| SA1957 | conserved hypothetical protein | 1 | MG265 | Essential | |||
| SA1966 | conserved hypothetical protein | 1 | MG105 | Essential | |||
| VI No similarity | |||||||
| SA0732 | hypothetical protein | 1 | MG104 | Essential | |||
| SA5049 | hypothetical protein | 1 | MG008 | Essential |
Antisense RNA expression leads to selective cell sensitization
We hypothesize that expression of an inhibitory antisense RNA results in a decrease in concentration of an essential protein, and that this may hypersensitize the cell to drugs that specifically inhibit that protein. The S. aureus clone expressing an antisense RNA to the gene, fab (fabF, yjaY), encoding β-ketoacyl-acyl carrier protein synthase, was examined for sensitivity to cerulenin (Schujman et al., 2001), a specific inhibitor of this enzyme. When the antisense RNA to the fab gene was induced with xylose, cells exhibited increased sensitivity to the inhibitor cerulenin (almost 12-fold, Fig. 7). In contrast, the same cells were not significantly sensitized to a variety of antibiotics that inhibit different protein targets, for example, targets involved in the synthesis of DNA, RNA, proteins, cell wall synthesis or even to triclosan (Slater-Radosti et al., 2001), an inhibitor of another fatty acid synthesis enzyme, enoyl-ACP reductase. All strongly inhibitory antisense clones to known antibiotic targets that we have tested show hypersensitivity to their respective antibiotics (data not shown).
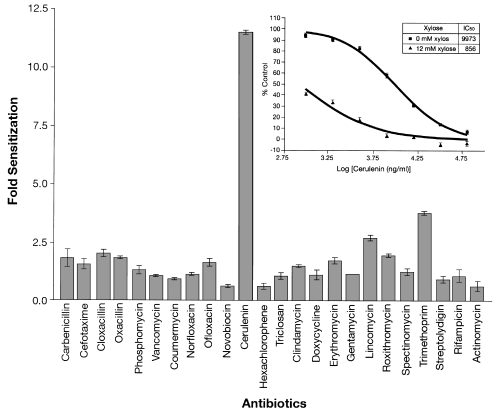
Induction of antisense to fab mRNA only sensitizes cells to the specific inhibitor, cerulenin. The average fold increases in sensitivity to various antibiotics exhibited by a S. aureus strain harbouring plasmid S1-1941, which expresses antisense RNA to the fab mRNA encoding β-ketoacyl-acyl carrier protein synthase. The highest concentrations (ng ml–1) of the antibiotics tested were: carbenicillin, 500; cefotaxime, 2000; cloxacillin, 250; oxacillin, 130; phosphomycin, 2000; vancomycin, 2000; coumermycin, 50; norfloxacin, 2000; ofloxacin, 1000; novobiocin, 600; cerulenin, 64 000; hexachlorophene, 1600; triclosan, 1000; clindamycin, 200; doxycycline, 100; erythromycin, 2000; gentamycin, 3000; lincomycin, 1000; roxithromycin, 1000; spectinomycin, 64 000; trimethoprim, 8000; streptolydigin, 64 000; rifampicin, 50; actinomycin D, 2000. The insert shows the effect of cerulenin on cell growth as a percentage of non-drug-treated controls under antisense induced and non-induced conditions.
Discussion
Here, we present a novel shotgun antisense approach used to identify genes essential for growth of S. aureus. An ideal strategy would comprise two main features, the rapid identification of all essential genes without prior knowledge of gene location, size, or function, and the comprehensive creation of conditional lethals as tools to assist in drug discovery.
While this paper was in review, Ji and colleagues (Ji et al., 2001) described a similar approach to ours in which expression of antisense RNA caused either a lethal or growth-inhibitory effect in 150 S. aureus genes, which were identified by their closest B. subtilis homologue. Ji and colleagues (Ji et al., 2001) also demonstrated that expression of antisense RNA directed against two known essential genes prevented infection in an animal model.
Our extensive analysis of the S. aureus genome resulted in a more comprehensive recovery of essential genes. This essential gene list includes representatives from all major essential functional groups such as cell wall biosynthesis, protein synthesis including the entire tRNA synthetase complement, fatty acid biosynthesis, DNA replication and RNA transcription.
We looked for homologues to the 658 unique S. aureus genes (N315 has 2595 genes; Kuroda et al., 2001) in which antisense expression resulted in growth inhibition, in the smaller genome of M. genitalium (517 genes; Fraser et al., 1995; Hutchison et al., 1999). Out of the 168 S. aureus genes sensitive to antisense inhibition and conserved in M. genitalium, 146 (86.4%) are homologues to Mycoplasma genes that have been implicated as essential open reading frames (ORFs) (Hutchison et al., 1999). Among the 22 S. aureus genes that were homologues to Mycoplasma knockouts (non-essential ORFs) are genes that are known to be essential in other bacteria. For example, Gram-positive organisms have two essential genes, polC and dnaE, homologous to the E. coli dnaE gene encoding the α-subunit of DNA polymerase III holoenzyme. Purified PolC contains both polymerase and 3′-5′-exonuclease activity, whereas DnaE has only polymerase activity (Sanjanwala and Ganesan, 1991; Bruck and O´Donnell, 2000; Klemperer et al., 2000). In our screen, we isolated antisense inhibitors to both genes. Both polC and dnaE are also essential in Gram-positive bacteria; (Flett et al., 1999; Tarantino et al., 1999; Dervyn et al., 2001; Inove et al., 2001), however, a transposon mutation was recovered only in the dnaE gene but not in the polC gene of M. genitalium (Hutchison et al., 1999).
Other probable essential genes identified in the Mycoplasma knockouts as non-essential were ileS, tyrS and ribosomal protein L9 (Hutchison et al., 1999). If one curates the putative non-essential gene list by removing these known essentials, which may represent incomplete gene disruptions or other false positives, nearly 90% of the S. aureus genes we isolated with identified homologues in M. genitalium were in the Mycoplasma putative essential list.
Many of the genes recovered by this screen did not have clear homologues in M. genitalium but are known to be essential. The lack of a cell wall in Mycoplasma (Razin et al., 1985) points to clear differences in the complexity of the membranes of both organisms. Consequently, some mur genes, penicillin binding proteins, D-ala-D-ala ligase, and other genes involved in the maintenance of the S. aureus cell wall, do not appear in M. genitalium.
Antisense induction results in a significant decrease in the target mRNA within 15 min of induction whereas a control mRNA exhibited no change (Fig. 6). Several mechanisms of antisense inhibition have been postulated, for example, mRNA decay via double-stranded RNase attack of the RNA duplex, ribosome occlusion resulting in inhibition of translation or premature translation termination. The mechanism of the premature degradation of specific mRNAs induced by antisense is an area of active experimentation (manuscript in preparation).
Antisense inhibition of a polycistronic mRNA may result in the rapid degradation of the entire mRNA, causing loss of expression of all of the proteins encoded on that mRNA, similar to a gene knockout, which may cause polar effects (Link et al., 1997). Thus, expression of an antisense RNA to an apparent non-essential gene might result in the degradation of an entire mRNA, which also may encode an essential gene.
A given antisense RNA also may inhibit a gene family by way of complementarity to a common motif. One example detected by the antisense screen was murA, which encodes the first concerted enzymatic step of cell wall biosynthesis and is represented by two loci, murA and murZ (murA2), in S. aureus and other low GC content Gram-positive bacteria (Du et al., 2000; Kuroda et al., 2001). As both loci must be ablated to cause an essential growth phenotype (Du et al., 2000), growth sensitivity caused by an antisense clone directed to one or the other genes suggests that both transcripts are affected. The S. aureus murA (seven antisense clones) and murZ (one antisense clone) genes have 59% identity at the DNA level, which has been shown in studies of naturally occurring antisense regulation to be sufficient for regulation of more than one locus (Altuvia et al., 2000). Experiments are ongoing to investigate whether there is, indeed, simultaneous antisense regulation of murA and murZ expression.
Shotgun antisense screening of a genome has proven to be readily scalable with robotics and applicable to other bacterial genomes. At present, we are able to screen over 1.5 million clones per month for growth-defective phenotypes. In comparison, the generation of conditional mutations, such as temperature sensitive mutants, requires mapping and sequencing. Furthermore, there is always the concern of mutations at multiple chromosomal locations (Schmid et al., 1989). When applied globally to Salmonella typhimurium, a number of gene products were refractory to temperature-sensitive mutations (Schmid et al., 1989). These considerations make the shotgun antisense approach more rapid and comprehensive.
Another major advantage of this technology is the ability to rapidly identify essential genes in an organism without prior knowledge of gene location, size or function. This removes biases associated with preselecting loci for analysis, a time-consuming and uncertain process. Freiberg and colleagues (Freiberg et al., 2001) found that only six out of 27 genes identified bioinformatically as highly conserved in a range of bacterial pathogens proved to be essential by laboratory experimentation. Arigoni and colleagues (Arigoni et al., 1998) also used a bioinformatic approach to select candidates for gene knockouts with similar results; only six out of 26 highly conserved genes chosen were essential.
The shotgun antisense technology is a very rapid and broadly portable way to identify essential genes in almost any microorganism. In principle, all that is required is an inducible promoter and a plasmid vector. In practice, expression systems and host strains need to be optimized to use this method successfully. Once optimized, we have used the shotgun antisense technology in other Gram-positive and Gram-negative bacterial pathogens with similar success in identifying essential genes.
The results of our genomic screens are being used to assemble a list of ‘universally conserved’ essential genes in bacteria. Such genes may serve as our optimal target set for the discovery of broad-spectrum antibiotics. Additionally, we are finding genes that are essential only in one or a subgroup of bacteria. These genes may serve as narrow-spectrum targets. An example of the need for a narrow-spectrum antibiotic is the treatment of persistent Pseudomonas aeruginosa infections in cystic fibrosis patients.
Over the last 50 years, nearly every major pharmaceutical company has screened their large chemical libraries against bacterial cells in attempt to find new antibiotics, and the limited success of this approach is now apparent. Less potent, but highly specific inhibitors are not readily identified in such screens. Similarly, simply cloning, expressing and purifying essential proteins for use in biochemical screens has also been unsuccessful; in vitro inhibitors are easy to identify but, typically, have failed to exhibit whole cell activity.
It is clear that a novel-screening paradigm is needed to find new antibiotics. By expressing the antisense to essential genes inside the cell, we have shown cells become hypersensitive to inhibitors of that target, with cells becoming 10–100 fold more sensitive to specific inhibitors. We are now using these hypersensitive strains in drug screens to find new classes of inhibitors for novel bacterial targets.
The shotgun antisense approach described here is a facile method to rapidly and comprehensively determine the genes necessary for the growth of microorganisms. This information will aid in the determination of true minimal genome sets and creates conditional lethal strains to be used in the identification of antimicrobial compounds against important pathogens.
Experimental procedures
Bacterial strains and plasmids
Staphylococcus aureus strains used include RN450 and RN4220 (Novick, 1990) and E. coli strains, DH5α and XL1Blue, which were obtained from Gibco-BRL and Stratagene respectively. Plasmids pRN5543 (Novick, 1991), pLEX5BA (Diederich et al., 1994) and pWH942 (Schnappinger et al., 1995) were described previously.
Construction of pEPSA5 containing the pT5X xylose-inducible promoter
The pEPSA5 S. aureus/E. coli shuttle vector (Fig. 1) contains elements that confer autonomous replication and CmR in S. aureus (obtained from the pC194-derived plasmid pRN5548; (Novick 1991). Also included are elements of the multiple cloning site, rrnB T1T2 terminators and the ampicillin resist-ance gene of the plasmid pLEX5BA (Krause et al., 1997), excluding the ColE1 origin of replication, which was exchanged for a NotI cassette containing the lower copy number p15a origin (Diederich et al., 1994). Upstream of the multiple cloning site and terminators is a Gram-positive optimized bacteriophage T5 PN25 promoter (LeGrice, 1990) in context with the operator sequence for the Staphylococcus xylosis XylR repressor protein (Schnappinger et al., 1995), the gene of which is also included as indicated in the map of pEPSA5.
Media and growth conditions
LB, B2 and SOC media and M9 salts were prepared as described (Sambrook et al., 1989). Where indicated, LB was supplemented with 0.2% glucose (LBG). The concentration of antibiotics as selective agents was 100 μg ml−1 for carbenicillin and 15 μg ml−1 for chloramphenicol, unless otherwise indicated.
Electroporation
All electroporations were conducted using a Bio-Rad GenePulser™. Escherichia coli electroporation was carried out according to the manufacturer’s instructions. Staphylococcus aureus electroporations were as described (Schenk and Laddaga, 1992).
Library construction and screening
Genomic DNA was isolated from RN450 using a kit obtained from GENTRA Systems. A final concentration of 50 μg ml−1 of lysostaphin was added during the lysis step, otherwise the isolation was carried out according to the manufacturer’s directions. Genomic DNA was fractionated by DNase I digestion and then blunt-ended with T4-DNA polymerase as described (Sambrook et al., 1989). The resulting genomic fragments were gel-isolated to enrich for fragments in the range of 200–800 basepairs (bp) using a Qiaquick Gel Extraction Kit (Qiagen) according to the manufacturer’s directions. Resulting genomic fragments were then ligated into the pEPSA5 vector, digested by SmaI and dephosphorylated with calf intestinal alkaline phosphatase (CIP). The resulting ligation was electroporated into E. coli strain DH5α to obtain greater than 1 × 106 individual colonies, which were then combined and subjected to plasmid purification with the use of a Plasmid Maxi Kit (Qiagen). This plasmid library was electroporated into RN4220 to generate approximately 315 000 transformants that were recovered on LBG + 15 μg ml–1 chloramphenicol agar plates. A total of 250 000 S. aureus transformants were arrayed into 384-well plates using the GeneMachine Gel-2-Well™ robot. Following overnight growth at 37°C, 384-well culture plates were replica-plated with a Genomic Solutions Flexys robot onto inducing (LBG + 2% xylose) and non-inducing (LBG) agar medium. Replica plates were incubated overnight at 37°C, and 4452 clones that did not form colonies in the presence of xylose were chosen for re-testing of sensitivity.
Validating clone sensitivity
The sensitivity of the 4452 clones to xylose induction was ranked according to the number of orders of magnitude of growth inhibition. Overnight cultures were diluted 1:100 and grown for 3 h in 96-well microtitre plates (Costar) with shaking at 37°C. Subsequently, cultures were serially diluted 10-fold eight times in 1× M9 salts in 384-well microtitre plates (Costar). Cultures were then robotically replica-plated (Genomic Solution Flexys) onto inducing and non-inducing (LBG + 2% xylose and LBG) media for overnight incubation. Comparative analysis of the number of dilutions that grew in the absence of induction to those that grew in the presence of induction yielded the log inhibition score. A total of 3117 clones had at least three logs of growth inhibition in the presence of xylose induction and was thus considered growth inhibitory.
Clones of interest were also evaluated for growth inhibition in liquid media. Overnight cultures were diluted 1:100 in LBG + 60 μg ml−1 of chloramphenicol. At an approximate OD600 = 0.2 cultures were diluted 1:500 into inducing and non-inducing media in duplicate wells of a 384-well microtitre plate. The OD600 was then followed over time in a SpectraMax plus plate reader.
Bioinformatic analysis
The original genomic location of the sensitive clone inserts was determined by BLASTN analysis of the insert sequences against the recently published S. aureus N315 genome (Kuroda et al., 2001). The identity and orientation of the genes covered were determined by comparison of BLAST hit co-ordinates to the published annotation. The protein sequences of the genes revealed as essential by this shotgun antisense screen were compared with BLAST analysis to the Mycoplasma genitalium proteome (Fraser et al., 1995). Gene products that shared more than 25% identity over more than 70% of the length of an S. aureus polypeptide were con-sidered to be potential homologues. Homologues reported here are the ones with the highest degree of similarity.
RT-PCR analysis
RN4220 carrying either an rplQ antisense clone (S1–760 A) or a lig antisense clone (S1–396 A) were grown to mid-log phase and diluted 1:10 into prewarmed Luria–Bertani (LB) broth. Then, 1 ml was transferred to each of six wells of a 96-deep well plate. Cells were grown to 0.1 OD600 by incubation on an orbital plate shaker at 37°C. Xylose was then added to three of the wells to a final concentration of 2%. After 15 min, the cells were pelleted, and RNA was isolated from each culture using a modified version of the RNeasy 96 RNA purification system (QIAGEN). TaqMan nucleotide primer/probe sets were designed specifically to measure a small segment (typically 100–200 bp) of either the rplQ or lig target mRNAs by Real Time PCR (RT-PCR) (ABI Prism 7700) using a standard curve method (Applied Biosystems). Data were normalized to a 16S rRNA loading control on a well- by-well basis, and +/− induction data was analysed by an unpaired, two-tailed t-test.
Cell-based assay for target-specific inhibitors
An overnight culture of RN4220 carrying plasmid S1- 1941 expressing antisense to the fab gene encoding β- ketoacyl-acyl carrier protein synthase was inoculated into fresh LB plus 34 μg ml−1 of chloramphenicol, and incubated with shaking at 37°C. Exponential cultures were diluted to a final OD600 of 0.002 into two flasks of the same medium containing 0 and 12 mM xylose. After 210 min at 37°C with shaking, the resulting cultures were diluted to an OD600 of 0.00022 into 1.1× LB medium containing either 0 or 12 mM xylose, and 45 μl of each cell suspension was dispensed into 384-well microtitre plates (Matrix Tech Corp.) containing 5 μl of antibiotic compounds or solvent per well.
Each plate contained duplicate sets of antibiotics with six twofold dilutions starting from the highest antibiotic concentration listed (a 7-point series). Antisense-induced and non-induced cells were tested against each set within a microtitre plate to enable direct comparisons of the relative potencies of antibiotics. Plates were sealed, shaken and incubated at 37°C. Cell growth was monitored (OD595) using an UltraMark microtitre plate reader (Bio-Rad Laboratories) for 15 h. IC50 values (concentration of a compound that inhibits growth by 50%) were generated using GRAPHPAD/PRIZM 3.0 to analyse the 7-point dose–response curves. The fold increase in sensitivity was calculated by dividing the IC50 value in the absence of antisense RNA induction, by the IC50 value in the presence of antisense RNA for a given antibiotic.
Acknowledgements
We wish to thank Peter Pattee, Steven Projan, Wolfgang Hillen, Walter Messer and Richard Novick for strains and plasmids. We are especially appreciative of the many manuscript improvements suggested by Jeff Winkelman, Moselio Schaechter and Doug Smith. A portion of these studies was supported by National Science Foundation Grant No. MCB-9507209 to J.W.Z. R.A.F. was a Predoctoral Fellow supported by the NIH National Institute of General Medical Sciences MARC F31 GM14967-0451 for part of these studies.