Chs1 of Candida albicans is an essential chitin synthase required for synthesis of the septum and for cell integrity
Abstract
CaCHS1 of the fungal pathogen Candida albicans encodes an essential chitin synthase that is required for septum formation, viability, cell shape and integrity. The CaCHS1 gene was inactivated by first disrupting one allele using the ura-blaster protocol, then placing the remaining allele under the control of the maltose-inducible, glucose-repressible MRP1 promoter. Under repressing conditions, yeast cell growth continued temporarily, but daughter buds failed to detach from parents, resulting in septumless chains of cells with constrictions defining contiguous compartments. After several generations, a proportion of the distal compartments lysed. The conditional Δchs1 mutant also failed to form primary septa in hyphae; after several generations, growth stopped, and hyphae developed swollen balloon-like features or lysed at one of a number of sites including the hyphal apex and other locations that would not normally be associated with septum formation. CHS1 therefore synthesizes the septum of both yeast and hyphae and also maintains the integrity of the lateral cell wall. The conditional mutant was avirulent under repressing conditions in an experimental model of systemic infection. Because this gene is essential in vitro and in vivo and is not present in humans, it represents an attractive target for the development of antifungal compounds.
Introduction
The enzyme chitin synthase catalyses the synthesis of chitin, a β1,4-homopolymer of N-acetyl glucosamine. Chitin and β1,3-glucan represent the key structural polysaccharides in fungal cell walls that counter cell turgor, determine cell shape and confer structural rigidity. Mutations in glucan synthase genes that reduce glucan levels in cell walls stimulate a salvage pathway in which chitin synthesis is elevated to restore the strength of the wall matrix (Popolo et al., 1997; Popolo and Vai, 1998; Smits et al., 1999). Chitin synthesis is therefore a critical aspect of fungal growth and is essential for fungal cell viability (Bulawa, 1993; Popolo et al., 1997).
Candida albicans is the major opportunistic fungal pathogen of humans. It exhibits the phenomenon of yeast–hypha dimorphism, which plays a role in tissue invasion and dissemination during infection (Lo et al., 1997; Brown and Gow, 1999). Hyphal cell walls have a higher chitin content than yeast cell walls (Chattaway et al., 1968; Sullivan et al., 1983; Munro and Gow, 1995; Munro et al., 1998), and the specific activity of chitin synthase is twofold higher in hyphae than in yeast cells (Braun and Calderone, 1978; Munro et al., 1998). Therefore, chitin synthesis is also important in the context of fungal morphogenesis.
Chitin synthases are found in all fungal pathogens but not in humans. Therefore, these enzymes are potential specific targets for antifungal drugs (Munro and Gow, 1995). However, multiple CHS (chitin synthase) genes are normally present in fungi, and existing chitin synthase inhibitors such as polyoxin and nikkomycin are most active against dispensable isoenzymes (Bulawa, 1993; Munro and Gow, 1995). It is therefore important to identify key members of the CHS gene family required for fungal growth to be able to optimize screens for inhibitors against chitin synthase isoenzymes.
Chitin synthesis has been studied most intensively in Saccharomyces cerevisiae. This yeast has three chitin synthase enzymes, each with a distinct function (reviewed by Bulawa, 1993). ScChs1p is involved in the repair of the cell wall after chitinase-mediated separation of the mature bud. This enzyme represents the major chitin synthase activity in cell extracts (Bulawa et al., 1986; Cabib et al., 1989; 1992). ScChs2p synthesizes chitin of the primary septum (Silverman et al., 1988; Bulawa and Osmond, 1990; Shaw et al., 1991). The viability of chs2 mutants has been shown to be strain and growth medium dependent (Silverman et al., 1988; Bulawa and Osmond, 1990). ScChs3p synthesizes most of the chitin in the lateral cell wall and forms the chitin ring at the presumptive bud site (Shaw et al., 1991; Valdivieso et al., 1991; Bulawa, 1992).
Candida albicans also has three chitin synthase genes: CaCHS1 (Au-Young and Robbins, 1990), CaCHS2 (Chen-Wu et al., 1992) and CaCHS3 (Sudoh et al., 1993). Both CaCHS2 and CaCHS3 are expressed preferentially during hyphal growth, whereas CaCHS1 appears to be expressed in both yeast and hyphae at a lower level (Chen-Wu et al., 1992; Munro et al., 1998). Candida albicans Chs1p displays the highest amino acid sequence identity with S. cerevisiae Chs2p (Chen-Wu et al., 1992) and may represent the orthologous enzyme.
Mutagenesis in C. albicans is complicated by the organism's diploid and constitutively asexual nature. The technique called ‘ura-blasting’ circumvents the need for multiple selectable markers for reverse genetics and allows sequential disruption of multiple gene copies by targeted gene replacement in which the URA3 gene is recycled as a single selectable marker (Fonzi and Irwin, 1993). This technique has been used successfully to disrupt the CaCHS2 (Gow et al., 1994) and CaCHS3 (Bulawa et al., 1995; Mio et al., 1996) genes. Neither CaChs2p nor CaChs3p are essential, as null mutants lacking these enzymes displayed normal growth rates and morphologies and produced normal hyphae (Gow et al., 1994; Bulawa et al., 1995; Mio et al., 1996). However, the Δchs3/Δchs3 null mutant had a low chitin content and was attenuated in virulence (Bulawa et al., 1995; Mio et al., 1996). CaChs3p synthesizes the lateral cell wall chitin and is orthologous to ScChs3p. Null Δchs2/Δchs2 mutants showed very low levels of chitin synthase activity in in vitro microsomal assays, had a marginally lower chitin content in hyphal walls and displayed normal virulence levels (Gow et al., 1994; Munro et al., 1998). CaChs2p therefore represents the main in vitro enzyme activity. CaChs2p is more similar to ScChs1p, although the Cachs2 null mutant does not have an identical phenotype to the Scchs1 mutant (Gow et al., 1994).
The role of the CaCHS1 gene in the biology of C. albicans was investigated in this study. By creating a conditional null strain, we show that CaCHS1 is required for both septum formation and lateral cell wall integrity in yeast and hyphal cells, and demonstrate that CaChs1p is the first essential chitin synthase to be identified in a fungus.
Results
Revision of the CaCHS1 sequence
In the course of performing genomic Southern analyses, we found that the restriction map generated from our data did not match the 5′ region of the restriction map generated from the published CaCHS1 sequence (Au-Young and Robbins, 1990; GenBank accession no. X52420). To address this discrepancy, we recloned the CaCHS1 locus from CAI4 by chromosome walking (see Experimental procedures, pKW035). Sequencing of the newly isolated clone yielded an open reading frame (ORF) of 3078 bp encoding a predicted protein of 1026 amino acids, some 753 bp longer than the original published CaCHS1 sequence. Comparison of the two sequences reveals two major differences. First, X52420 is missing a cytosine residue at nucleotide 189; correction of this frameshift extends the CaCHS1 ORF upstream of the previously published start. Secondly, the first 47 nucleotides of X52420 do not match our newly derived sequence. Identity between the two sequences begins at nucleotide 48. Because this corresponds to a Sau3A site and because the plasmid pJA-IV, the source of X52420, was isolated from a Sau3A library, we believe that pJA-IV contains two non-adjacent chromosomal fragments. The new 3078 bp ORF was also confirmed in the sequence of a 42.5 kb C. albicans cosmid-derived contig Ca35A5 (strain 1161), containing the CaCHS1 gene (accession no. AL033396; Tait et al., 1997).
Disruption of CaCHS1
The first copy of the CaCHS1 gene was disrupted by transforming CAI4 with XhoI–AspI-cut plasmid pKW015. This plasmid contained CaCHS1, with 1806 bp of the 3078 bp ORF replaced with the ura-blaster cassette. Attempts to disrupt the second CHS1 allele by the same procedure failed. To test whether this failure resulted from natural heterozygosity at this locus, we devised a strategy specifically to target the remaining wild-type CaCHS1 allele. Polymerase chain reaction (PCR) primers corresponding to DNA deleted from the Δchs1::hisG allele were used to amplify the remaining wild-type copy of CaCHS1 from genomic DNA of the heterozygote KWC167b. The resulting 600 bp product was cloned into a CaURA3-containing plasmid to create pKW025. This plasmid therefore contained a fragment of CaCHS1 that was only homologous to the remaining non-disrupted allele. Plasmid pKW025 was digested with ClaI to target integration into that site in the remaining wild-type CaCHS1 allele and transformed into KWC167b. Again, no Δchs1/Δchs1 null mutants were isolated. Two disrupted chs1 alleles were observed, but these strains had become triploid at the CaCHS1 locus, and a wild-type CaCHS1 allele still remained (not shown).
Because the viability of S. cerevisiaeΔchs2 mutants was strain dependent, we attempted to generate Δchs1/Δchs1 null mutants in independent genetic backgrounds. CaCHS1 disruption was attempted in the isogenic strains SGY243 (Ura−) and NGY10 (Ura−, Δchs2::hisG/Δchs2::hisG/Δchs2::hisG;Gow et al., 1994). Southern analysis suggested that these strains were triploid at the CaCHS1 locus, as has been described previously for CaCHS2 in the SGY243 strain (Gow et al., 1994). Attempts to knock out the final wild-type copy of CaCHS1 in these strains were also unsuccessful. A variety of conditions was tested in an attempt to recover potentially slow-growing, debilitated homozygous Δchs1/Δchs1 null mutants. No condition was found in which Δchs1/Δchs1 null mutants were recoverable. These data strongly suggest that CaCHS1 is an essential gene.
Construction of a conditional CaCHS1 mutant
We created a conditional Δchs1 null mutant by placing the remaining wild-type allele under the control of the regulatable promoter MRP1. This bidirectional promoter was isolated from a C. albicans promoter-trap library and is located upstream of an ORF with significant homology to maltase genes (US patent 5824545). The MRP1 promoter is induced by maltose and repressed by glucose. Plasmid pKW044 was constructed, which contained CaURA3 adjacent to the MRP1 promoter fused at the ATG to a 3′-truncated CaCHS1 gene. Integration into the remaining wild-type allele resulted in an inactive 3′-truncated CaCHS1 and a full-length CaCHS1 ORF under the control of the MRP1 promoter. Ura+ transformants were selected on maltose, and their ability to grow on glucose was then tested. Southern analysis confirmed that pKW044 had integrated correctly to create conditional Δchs1/MRP1p-CHS1 mutants (data not shown). No wild-type allele was present in these strains. One of these transformants, KWC340, was selected for further analysis.
Northern analysis of CaCHS1 expression under the control of the MRP1 promoter
Regulation of CaCHS1 expression by the MRP1 promoter was investigated by Northern analysis. SC5314 (CHS1/CHS1) and KWC340 (Δchs1/MRP1p-CHS1) cells were grown overnight in SMal, washed and resuspended in SD or SMal, incubated at 30°C and samples removed at hourly intervals up to 6 h. Total RNA was extracted from these cells and probed with a CaCHS1-specific probe N. No CaCHS1 mRNA was detectable after 1 h or at later time points in KWC340 cells resuspended in glucose-containing SD medium. Indeed, CaCHS1 mRNA could not be detected after 30 min (data not shown). In contrast, when KWC340 was grown in maltose medium SMal, the CaCHS1 mRNA was detected at all time points at levels similar to those seen in strain SC5314 (Fig. 1).
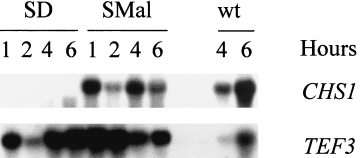
Northern analysis of CaCHS1 expression under the control of the Candida MRP1 promoter. Strain KWC340 was grown in SMal or SD, and cells were harvested at intervals. RNA was extracted and hybridized with a CaCHS1-specific probe N. The CaCHS1 probe was then stripped from the Northern filter, and the filter was hybridized with a TEF3-specific probe as a measure of RNA loading. The lanes marked ‘wt’ are RNA from isogenic strain SC5314 grown on SMal for 4 h and 6 h.
Viability of the Δchs1::hisG/MRP1p-CHS1 mutant
Growth and cell viability of strain KWC340 was measured spectrophotometrically. Samples were taken at intervals from cells grown on SMal and SD medium, and a diluted aliquot of cells was also plated on SMal plates. Cells in both media continued to grow exponentially for 6–8 h after dilution into SD and SMal. The number of viable cells grown in SMal continued to increase exponentially in proportion to the measured OD600. In contrast, the viability of cells grown in SD was always lower than the viability of SMal-grown cells, and the number of viable cells declined after 6–8 h in SD (Fig. 2). An initial increase in the OD600 of SD-grown cells was observed, accompanied by a decrease in cell viability, as assessed by colony counts and an increasing proportion of propidium iodide-staining cells (not shown). These results indicate that cells growing under repressing conditions in SD became non-viable.
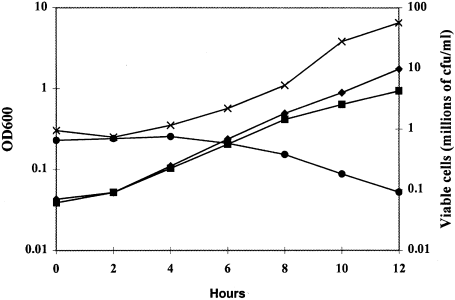
Growth rate and viability of strain KWC340. Cells were grown overnight in SMal, washed and transferred to SMal or SD at an inoculum of OD600 = 0.05. Cells were incubated at 30°C and sampled at 2 h intervals. The OD600 of cultures in SMal (◆) and SD (▪) and colony-forming units ml−1 of samples of these cultures plated onto SMal (x for SMal culture) or (● for SD culture) are shown.
Function of CaCHS1 during growth in the yeast form
To examine the role of CaCHS1 during yeast cell growth, the Δchs1::hisG/MRP1pCHS1 conditional mutant KWC340 was grown overnight in SMal medium, washed and resuspended into either SD or SMal at 30°C. Yeast cells of KWC340 grown in SMal were indistinguishable from wild-type cells (Fig. 3A). Under glucose-repressing conditions, aberrant morphologies were observed within one or two generations (3–4 h). Although growth continued, cell number did not increase. Instead, chains were formed with obvious constrictions that are not compartmentalized by septa (Fig. 3B). New compartments in the chains were formed at approximately 2 h intervals, comparable with the normal generation time in this medium, suggesting that each swelling may represent a cell equivalent. As growth under repressing conditions continued transiently, lateral buds appeared from within chains, and branched chains and clusters of chains were seen (Fig. 3C and I). Compartments in chains were larger than wild-type yeast cells and were multinucleate, with up to five nuclei being observed in the apical compartment (Fig. 3D). Compartments towards the ends of the chains lysed progressively (Fig. 3E), and the appearance of lysed cells corresponded with loss of viability. Lysis was observed less frequently at the constricted regions between compartments in the chain. These observations indicate a progressive loss of cell wall integrity under repressing conditions in the conditional mutant.
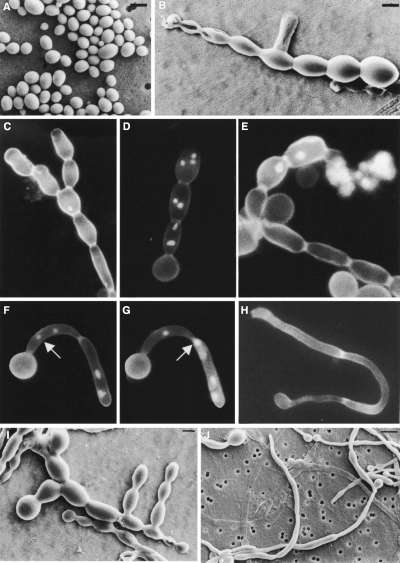
Phenotypic analysis of the Δchs1/MRP1p-CHS1 strain KWC340. Cells of strain KWC340 were grown in SMal (A), in SD (B–E, I) or in 20% (v/v) newborn calf serum supplemented with 1% glucose (w/v) (F–H, J). Cells were examined by SEM (A, B, I and J) or after Calcofluor and DAPI staining (C–H). Bars = 5 µm (A, B and I) and 10 µm J).
Calcofluor-staining of KWC340 yeast cells grown under repressing conditions showed that they still formed distinct chitin-rich rings (synthesized by Chs3p) but had no primary septa (Fig. 3C–H). The Δchs1/MRP1-CHS1 mutant also formed aseptate pseudohyphae under these conditions with visible constrictions between cell junctions (Fig. 3F). Nuclei were able to pass between adjacent compartments of such constricted chains (Fig. 3G), again demonstrating that septa were incomplete or absent. This phenotype was also observed in the Δchs1/MRP1pCHS1;Δchs3/Δchs3 mutant (strain KWC359).
CaChs1p makes chitin in the septum and is essential for the integrity of the lateral cell walls of hyphae
Hyphal development was induced in 20% serum supplemented with either glucose or maltose. The Δchs1/MRP1p-CHS1 mutant formed normal hyphae for the first three or four generations in the presence of both glucose and maltose in liquid (Fig. 3H) and on solid media (Fig. 4A and B). Germ tube emergence rates and hyphal extension rates were not significantly altered during this period when strains KWC340 and KWC359 were grown on glucose or maltose (data not shown). Cells grown on glucose exhibited a progressively more aberrant morphology, with the lateral cell walls becoming expanded and hyphae developing regular septumless constrictions reminiscent of the morphology of the mutant grown under yeast-form conditions. Calcofluor-staining wall evaginations were observed to balloon out spontaneously from the cell surface (Fig. 4C and D). These balloons were observed at different positions on the hyphae (Table 1), at the apices (Fig. 5A), at points where branches emerged (Fig. 5B) and at constricted sites in the lateral cell wall that presumably represent potential sites of septation (Fig. 5C). In some cases, lysis of the balloons occurred (Fig. 5E, Table 1). Hyphal cells grown on glucose were also multinucleate (Fig. 5F and G). The effect of depleting cells of CaChs1p was also examined in strain KWC359, which had both copies of CaCHS3 disrupted. The Δchs1/MRP1pCHS1;Δchs3/Δchs3 mutant also displayed the aberrant morphologies described above, but lysed more frequently at the hyphal apex and subapically (Fig. 5D and J, Table 1). The balloons formed in the double mutant stained with Calcofluor, indicating that chitin was still present within these regions (Fig. 5H and I). No further growth was observed after cells had formed balloons or had lysed. The addition of 1 M sorbitol as an osmotic support (Fig. 6A–F) did not remediate the ballooning or lysis phenotype. The addition of 1 mM allosamidin, an inhibitor of chitinase (Fig. 6G), did not prevent lysis or the formation of balloons, suggesting that the loss of cell integrity resulted from the loss of chitin synthesis alone and was not augmented by chitinase action.
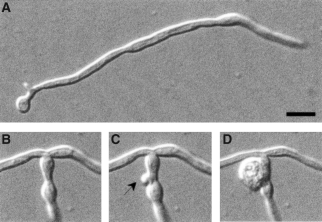
Formation of ballooning structures in the hyphal cell wall under repressing conditions for the expression of the final copy of CaCHS1 in strain KWC340. The hyphae shown had been growing on agar under repressing conditions for 7 h (A), 9 h (B) and 9.5 h (C and D). A small protrusion emerged close to the base of the branch (C) and, within a minute, this had erupted to form a balloon (D). Bar = 10 µM.
| Position of balloon or lysis | Percentagea | Strain KWC359 Δchs1/MRP1p-CHS1;Δchs3/Δchs3 |
|---|---|---|
| Strain KWC340Δchs1/MRP1p-CHS1 | ||
| Hyphal apex | 8 | 33 |
| Hyphal subapical regionb | 2 | 24 |
| Lateral cell wall | 17 | 21 |
| Branch point | 51 | 14 |
| Branch apex | 22 | 8 |
- a . One hundred hyphae were examined from three separate experiments.
- b . Subapical was defined as < 10 µm from the hyphal apex.
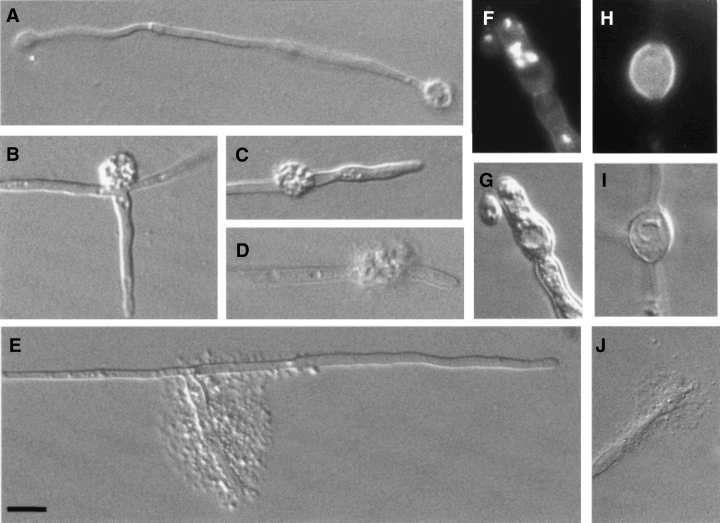
The CaCHS1 conditional mutant strains KWC340 (A–C and E–G) and KWC359 (D and H–J) were induced to form hyphae by growing on 2% agar, 20% serum and 2% glucose at 37°C. Abnormalities in cell morphology were evident by 7 h after the induction of hyphal growth (B) and were present in most hyphae by 24 h (A and C–J). Calcofluor/DAPI staining of a swollen hypha is shown in (F) with the corresponding DIC image in (G). The Δchs1/MRP1pCHS1;Δchs3/Δchs3 mutant produced balloons (I) that stained brightly with Calcofluor in contrast to the lateral cell wall (H). Bar = 10 µM.
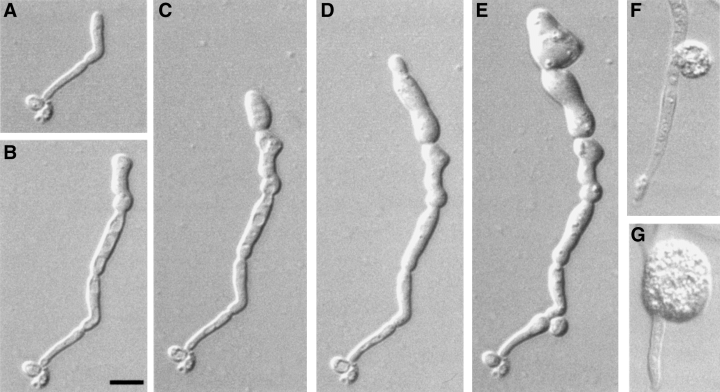
Growth of a hypha of the conditional chs1 null strain KWC359 induced on 20% serum plus 2% glucose, agar supplemented with 1 M sorbitol at 37°C (A–F) after 3.5 h (A), 5.5 h (B), 7 h (C), 8.5 h (D) and 24 h (E). In other specimens, balloons were evident by 7 h of growth (F). The addition of 1 mM allosamidin to inhibit chitinases did not remediate the abnormal cell morphology (G). Bar = 10 µM.
Ultrastructural analysis of the Δchs1/MRP1p-CHS1 mutant
Examination of thin sections of glucose-repressed KWC340 cells by transmission electron microscopy (TEM) confirmed that there were no septa separating chains of yeast cells and the compartments of hyphae (Fig. 7A and D). Yeast cells grown on SMal medium formed normal septa between the mother and bud. These septa could be labelled with wheatgerm agglutinin–colloidal gold (WGA-Au), indicating the presence of chitin (Fig. 7B). Constrictions in chains of KWC340 grown on SD medium were positive for WGA-Au labelling, showing that chitin rings were present, but abnormally thickened (Fig. 7A and C). Hyphae of strain KWC340 grown in the presence of maltose formed septa that showed a normal pattern of WGA-Au labelling (Fig. 7E). KWC340 hyphae grown under repressing conditions were labelled with WGA-Au only in the lateral walls and in areas where the chitin ring was abnormally thickened (Fig. 7F). These observations are consistent with the view that Chs1p synthesizes chitin of the primary septum in both yeast and hyphal cells.
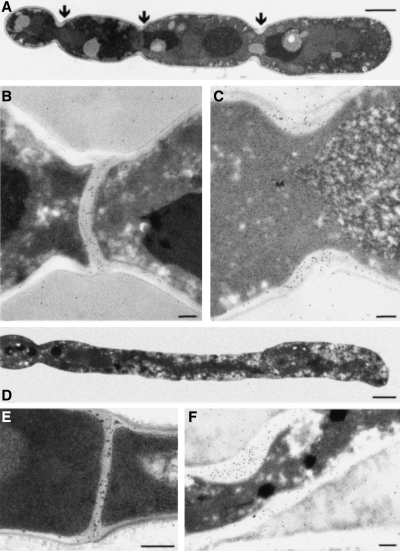
WGA-Au labelling of chitin in the conditional chs1 null mutant. Transmission electron micrographs of yeast cells of strain KWC340 grown on SD (A and C) or SMal (B) or hyphal cells grown in the presence of maltose (E) or glucose (D and F) were labelled with WGA-Au. Bars represent 2 µm (A), 200 nm (B, C, E and F) and 1 µm (D).
The conditional Δchs1/MRP1p-CHS1 mutant is avirulent
To examine the role of CaCHS1 in infection by C. albicans, strain KWC340 was assessed in a systemic murine virulence model. Three control strains were included (Table 2): SC5314 (CHS1/CHS1), the clinical isolate that is the parent of the strains used in this study; KWC352 (CHS1/MRP1pCHS1); and MRP2 (Δura3/MRP1p-URA3), a strain that has a single copy of the CaURA3 gene under the control of MRP1p. The five animals infected with SC5314 or KWC352 died within 8 days (for neutropenic mice) or 11 days (for immunocompetent mice). The wild-type virulence of KWC352 indicates that the MRP1p–CHS1 fusion was stable in vivo and that looping out of the duplicated CHS1 to create a Ura− strain did not occur at high frequency. This was unlikely because auxotrophic strains are known to be avirulent (Kirsch and Whitney, 1991; Polack, 1992). All mice injected with KWC340 or MRP2 survived for at least 20 days after inoculation (Fig. 8). Therefore, MRP2 is attenuated in virulence like Ura− auxotrophs, indicating that expression from the MRP1 promoter is switched off in vivo. To investigate further the survival of KWC340-infected animals, these mice were necropsied, and the kidneys were removed and patched onto SMal and YPD plates. No colonies were recovered, indicating that KWC340 was efficiently cleared from tissues. Thus, CaCHS1 is essential for growth in mice in systemic infections.
| Strain | Genotype | Reference |
|---|---|---|
| SGY243 | ade2/ade2;Δura3::ADE2/Δura3::ADE2;CHS1/CHS1/CHS1 | Kelly et al. (1987) |
| NGY17 | ade2/ade2;Δura3::ADE2/Δura3::ADE2;CHS1/Δchs1::hisG/Δchs1::hisG | This study |
| NGY10 | ade2/ade2;Δura3::ADE2/Δura3::ADE2;CHS1/CHS1/CHS1;Δchs2::hisG/Δchs2::hisG/Δchs2::hisG | Gow et al. (1994) |
| NGY19 | ade2/ade2;Δura3::ADE2/Δura3::ADE2;CHS1/Δchs1::hisG/Δchs1::hisG;Δchs2::hisG/Δchs2::hisG/Δchs2::hisG | This study |
| SC5314 | Wild-type clinical isolate | Fonzi and Irwin (993) |
| CAI4 | Δura3::imm434/Δura3::imm434 | Fonzi and Irwin (1993) |
| CAF2 | Δura3::imm434/URA3 | Fonzi and Irwin (1993) |
| KWC167 | Δura3::imm434/Δura3::imm434;CHS1/Δchs1::hisG-URA3-hisG | This study |
| KWC167b | Δura3::imm434/Δura3::imm434;CHS1/Δchs1::hisG | This study |
| KWC340 | Δura3::imm434/Δura3::imm434;Δchs1::hisG/Δchs1:pSK-URA3-MRP1p-CHS1 | This study |
| KWC352 | Δura3::imm434/Δura3::imm434;CHS1/Δchs1:pSK-URA3-MRP1p-CHS1 | This study |
| KWC359 | Δura3::imm434/Δura3::imm434;Δchs1::hisG/Δchs1:pSK-URA3-MRP1p-CHS1;Δchs3/Δchs3 | This study |
| MRP2 | Δura3::imm434/Δura3:pSK-MRP1p-URA3 | This study |
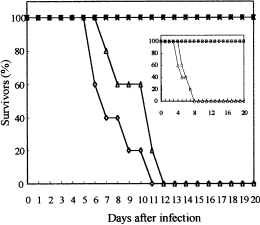
Virulence of KWC340 and related strains in immunocompetent and immunocompromised mice. The virulence of four strains was examined, as described in Experimental procedures; three strains contained MRP1 promoter fusions. For each strain, the gene regulated by the MRP1 promoter is: (A) URA3 in a Δura3 background, strain MRP2 (□); (B) CaCHS1 in a wild-type background, strain KWC352 (Δ) or (C) CHS1 in a Δchs1::hisG background, strain KWC340 (x). The clinical isolate SC5314 (◊) was used as a control. Neutropenic mice (inset graph) were injected with 1 × 10 4 cells (n = 5), and immunocompetent mice were injected with 1 × 10 6 cells (n = 5).
Discussion
In this study, we demonstrate that CaChs1p is essential for growth in vitro and in vivo and is responsible for the synthesis of primary septal chitin in C. albicans and the integrity of the lateral cell wall of both yeast cells and hyphal cells. Therefore, CaCHS1 is the first example of an essential chitin synthase gene. Although the variant gene CaCHS1A was able to complement the abnormal morphology of the S. cerevisiaeΔchs2 mutant (Sudoh et al., 1995), the phenotype of the C. albicans conditional chs1 mutant was distinctly more severe than that of the S. cerevisiae chs2 mutation. This may reflect our observations that, in C. albicans, this enzyme is required for both septum formation and integrity of the lateral cell wall.
Both CaChs1p and ScChs2p are class II enzymes, as classified by Bowen et al. (1992). ScChs2p synthesizes the chitin-rich primary septal plate and was originally thought to be essential (Silverman et al., 1988). Haploid spores lacking this gene germinated, but showed only limited growth and were aberrant in shape and lacked a primary septum. However, this phenotype was shown to be dependent on growth conditions and the strain used. Bulawa and Osmond (1990) were able to generate Δchs2 null mutants in S. cerevisiae in a different strain background and by germinating spores at a higher temperature on SD medium. However, all attempts to recover C. albicansΔchs1 null mutants using similar growth conditions were unsuccessful. The inability to perform sexual crosses in this organism (Gow et al., 2000) prevents us from formally excluding the possibility that Δchs1/Δchs1 null mutants might be viable in some genetic backgrounds, although we showed that this gene is essential in unrelated strains that were suitable for gene disruption experiments.
Expression of the CaCHS1 gene was regulated by fusing the CaCHS1 ORF to the Candida MRP1 (maltase) promoter in strain KWC340. CaCHS1 mRNA was not detected by Northern analysis 30 min after cells were switched from inducing to repressing conditions. Microscopic examination of this conditional Δchs1/MRP1pCHS1 null mutant grown under repressing conditions revealed that daughter yeast cells did not separate from their mothers and that buds emerged from the distal pole of the daughter leading to the formation of chains. Hyphae either developed balloons that ruptured from the cell wall or lysed at sites of weakness. Growth in the yeast and hyphal forms continued for several generations, and growth rates in liquid cultures were initially similar before growth ultimately slowed and cells underwent cell lysis. Because growth was able to continue after CaCHS1 transcription was repressed, some recycling of Chs1p may have occurred before the ultimate arrest and death of cells. Under repressing conditions, the chains formed by the Δchs1/MRP1pCHS1 mutant yeast cells tended to lyse preferentially at the sites of constrictions between cells at the newly formed end. This may reflect dilution of the previously synthesized CaChs1p pool of enzyme after repression of de novo CaChs1p synthesis in the conditional mutant. However, in S. cerevisiae, the equivalent ScChs2p is turned over rapidly via proteolysis in the vacuole, whereas ScChs1p and ScChs3p are more stable and are expressed throughout the cell cycle (Chuang and Schekman, 1996). If CaChs1p is turned over in a similar way to ScChs2p, this would support the observation that CaChs1p enzyme is rapidly diluted out in the conditional mutant under repressing conditions. Ultimately, depletion of CaChs1p was therefore a lethal event.
CaChs1p synthesizes chitin in the primary septum
Calcofluor staining and WGA-Au labelling of the cell wall in TEM thin sections suggested that CaChs1p is responsible for synthesis of the chitinous primary septal plate in both yeast and hyphal forms of C. albicans. Therefore, septum formation in yeast and hyphal cells shares at least some of the same biosynthetic machinery. The implication that septal chitin was synthesized by CaChs1p was also evident in analyses of the C. albicansΔchs2/Δchs2;Δchs3/Δchs3 double null mutant, in which Calcofluor still stained septal plates (Mio et al., 1996). The ballooning phenotype of depleted CaChs1p hyphal cells at variable locations including the apex and subapex suggested that CaChs1p also contributes to lateral cell wall synthesis. This could not be predicted from studies of the ΔScchs2 mutant, which does not exhibit these dramatic phenotypes. The sites of ballooning had a different distribution between CaChs1p-depleted Chs3+ and Chs3− hyphal cells. In Chs3− cells, a higher percentage of cells had defects at the apex or subapically, whereas in the Chs3+ cells, 50% of the cells had defects at the point where a branch emerges. These observations suggest that CaChs1p synthesizes some chitin in the lateral cell wall, and that this chitin is essential for maintaining the integrity of the cell wall. Chitinase enzymes may be active at some of the cellular sites at which these defects were apparent, and chitinases are thought to be responsible for cell wall softening to allow branches to emerge in most filamentous fungi (Gooday et al., 1992). We tested the hypothesis that chitinases may antagonize the Δchs1 phenotype by growing the cells in the presence of the chitinase inhibitor allosamidin. Neither allosamidin nor osmotic support using sorbitol was able to remediate the ballooning phenotype, suggesting that C. albicans has no compensatory mechanism for the loss of CaChs1p. The inability to rescue the mutant with sorbitol suggests that CaChs1p is required for more than just cell wall integrity. The expression of ScCHS2 is cell cycle regulated, and Δchs2 mutants had multiple nuclei (Bulawa and Osmond, 1990; Pammer et al., 1992; Choi et al., 1994). Similarly, depletion of CaChs1p resulted in the loss of co-ordination between the budding and nuclear cycles. Compartments formed by yeast cells of the Δchs1/MRP1pCHS1 mutant under repressing conditions were larger, elongated and nuclear division was uncoupled from cytokinesis. Often, the terminal compartment in a chain had multiple nuclei, greater in number than the number of compartments in the chain. Hyphal cells were also multinucleate. Therefore, nuclear division and segregation were also affected by loss of CaChs1p. It is not known whether CaChs1p has a direct effect on nuclear division or whether these effects are a consequence of the inability to undergo cytokinesis. Mutants in S. cerevisiae with cytokinesis defects, e.g. Δshs1 andΔhof1, also have increased cell size and are multinucleate (Mino et al., 1998; Vallen et al., 2000). The increased cell size may indicate that CaChs1p-deficient cells have a prolonged G1 phase. The budding pattern was also affected in the conditional Cachs1 mutant, in which the normal bipolar budding pattern was abolished, and growth occurred at the distal pole of the daughter cell.
The fact that Chs1p-deficient cells undergo cell lysis suggests that this mutation results in significant weakening of the integrity of the cell wall. In the yeast form, lysis occurred at the constricted neck region between two compartments. In the hyphal form, sites of constrictions were also most prone to lysis, suggesting that the absence of the primary septal plate increased the fragility of the cell wall at these junctions. Chitin in the lateral cell wall was often thickened at these constricted regions, suggesting that an alternative chitin synthase, possibly Chs3p, may have been activated locally. A range of S. cerevisiae mutants has been shown to elevate chitin synthesis in response to cell wall weakening to restore the structural integrity of the wall (Popolo et al., 1997; Kapteyn et al., 1999a, b). This may be mediated via the cell wall integrity MAP kinase cascade (Smits et al., 1999). Homologues of this pathway have been identified in C. albicans (Navarro-Garcia et al., 1998). Therefore, chitin ring formation may be regulated by the presence or absence of the primary chitinous septum, perhaps through local induction of the cell wall integrity response. In the Δchs1/MRP1pCHS1;Δchs3/Δchs3 strain, the swollen balloons still stained with Calcofluor, suggesting that another chitin synthase, e.g. CaChs2p, may be responsible for chitin synthesis in these structures. Mutant analyses show that this enzyme makes around 10% of the chitin in the hyphal form (Gow et al., 1994; Munro et al., 1998).
Hyphae of C. albicans normally branch behind positions of septa. It was evident from analysis of the Δchs3/Δchs3 mutant that septum formation can proceed without the presence of the chitin ring synthesized by CaChs3p and, in the Δchs1/MRP1pCHS1;Δchs3/Δchs3 strain, branch formation occurs without the septal plate synthesized by CaChs1p and the CaChs3p-derived chitin ring. Loss of CaChs1p results in weakening of the cell wall at these locations and, although branches are formed, many of the cells lyse or balloon at the point where branches emerged. Therefore, CaChs1p must play a vital role in maintaining cell integrity at the hyphal–branch junction and at the tips of growing cells.
Shadow-cast TEM analysis revealed that chitin in the primary septum of C. albicans is more fibrillar than the short rodlets of chitin in the lateral cell wall (Gow et al., 1980; Gooday and Gow, 1983). This suggests that the chitin synthesized by CaChs1p differs in length and crystallinity from the chitin synthesized by CaChs3p. Regulation of the hydrogen bonding and crystallization of nascent chitin microfibrils in different locations in the cell wall is not understood, but may lead to chitin microfibrils being formed with significantly different structural and tensile properties.
Chitin synthase isoenzymes are differentially sensitive to chitin synthase inhibitors such as the nikkomycins and polyoxins (Cabib, 1991; Gaughran et al., 1994). In S. cerevisiae, septum-synthesizing ScChs2p is the least sensitive chitin synthase isoenzyme to both these classes of antifungals. Our studies of CaChs1p demonstrate that this isoenzyme is the most important enzyme for viability of C. albicans in vitro and in infected animals. Many antifungal screens have been based on the inhibition of total cellular chitin synthase activity, the majority of which results from ScChs1p/CaChs2p, and CaChs3p, neither of which are required for viability (Gow et al., 1994; Bulawa et al., 1995; Mio et al., 1996; Munro et al., 1998). This suggests that CaChs1p is the most important target for screens for novel Chs inhibitors directed against Candida species. One such inhibitor was recently reported (Masubuchi et al., 2000) that selectively inhibits CaChs1p and shows in vitro antifungicidal activity against a number of Candida spp. and mild in vivo efficacy in a murine systemic candidiasis model. Treatment of C. albicans cells with this inhibitor resulted in growth arrest, with the yeast cells forming chains indicative of loss of septa (Sudoh et al., 2000). This compound was only weakly active against Aspergillus fumigatus. CaCHS1 homologues have not yet been identified in filamentous fungi, predicting that such compounds may be most active against ascomycetous yeast-like pathogens. The finding that individual members of CHS gene families may be essential for viability highlights the need to understand the specific roles of Chs isoenzymes in fungal growth and their relative importance as potential drug targets.
Experimental procedures
Strains, media and growth conditions
The C. albicans strains used in this study are listed in Table 2. C. albicans Ura− strains were grown in YPD [1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) glucose] at 30°C (Sherman, 1991). Ura+ strains were grown in either SD [0.67% (w/v) yeast nitrogen base without amino acids, 2% (w/v) glucose] or SMal [0.67% (w/v) yeast nitrogen base, 2% (w/v) maltose] at 30°C (Sherman, 1991). Solid medium contained 2% (w/v) purified agar (Oxoid). Counterselection of Ura− segregants was on SD medium containing 1 mg ml−1 5-fluoroorotic acid (5-FOA) and 100 µg ml−1 uridine (Fonzi and Irwin, 1993).
Transformation of C. albicans
Ura−C. albicans cells were transformed after treatment with lithium acetate (Bulawa et al., 1995) or using a spheroplast method (Kurtz et al., 1986). Ura+ transformants were streaked on SD plates, single colonies were picked and grown in 5 ml of SD medium, and genomic DNA was extracted for Southern analysis.
Construction of plasmids and C. albicans strains
A 9.2 kb SalI–NheI fragment containing CaCHS1 and 200 bp of YEp13 sequence was subcloned from plasmid pJA-IV (Au-Young and Robbins, 1990) into the SalI–XbaI sites of pBluescript SK– producing PKW013. Plasmid pKW014 was generated from plasmid pKW013 by cutting with XhoI and ligating the digested DNA in a large volume to promote intramolecular ligations. pKW014 contained 3.5 kb of downstream CaCHS1 sequence with a 5.5 kb upstream XhoI fragment deleted.
The 4.5 kb PstI–AflIII fragment of pMB-7 containing the ura-blaster cassette (Fonzi and Irwin, 1993) was inserted into the 5′NsiI and 3′NcoI sites of the CaCHS1 ORF in plasmid pKW014 to create plasmid pKW015, which was used for the disruption of the CaCHS1 gene. This resulted in the deletion of nucleotides 921–2726 of CaCHS1 (GenBank accession no. X52420). Plasmid pKW015 was cut with XhoI and AspI, leaving 540 bp of upstream and 613 bp of downstream CaCHS1 sequence for targeted integration, and the linearized cassette was introduced into C. albicans Ura− strain CAI4 and a Ura− derivative of CACB8B-5 (Δchs3/Δchs3) by electroporation.
For the construction of the conditional CaCHS1 mutant, a 1.7 kb PCR product (nucleotides 271–2030; accession no. X52420) was first amplified from the CaCHS1 locus of strain CAI4 using primers CaCHS1-1 5′-GATGATAACAATCACACC (nucleotides 271–288) and CaCHS1-2 5′-GACACCAATGAACCTGTC (nucleotides 2013–2030). The amplified product was cut with Asp718 and EcoRI, and the resulting 0.6 kb fragment was ligated into the Asp718–EcoRI sites of pBluescript SK– to generate plasmid pKW024.
The selectable marker, CaURA3, was amplified by PCR from plasmid pKW015 using primers 5′-ATATCCGCGGTACTAATAGGAATTGATTTG (nucleotides 3–22 of GenBank accession no. X14198; KspI site underlined) and (5′-CTAGAGCTCCCACCTTTGATTGTAA (nucleotides 1355–1340; SacI site underlined). The resulting 1.35 kb SacI–KspI fragment was subcloned into the SacI–KspI sites of pKW024 to generate plasmid pKW025. The same SacI–KspI fragment was subcloned into pBluescript SK to form plasmid pKW016.
To recover the 5′ end of CHS1, plasmid pKW025 was cut with ClaI and transformed into CAI-4. Transformants were analysed by Southern analysis of genomic DNA, and strain CAI-4A, which contained pKW025 integrated at the ClaI site of CHS1, was identified. CAI-4A DNA was cut with HindIII, diluted, ligated and transformed into Escherichia coli. Plasmids were screened for CHS1 sequence by PCR using primer CaCHS1-1b, 5′-GGCGCAGCACACCAAGAGCAC-3′ (base numbers 1840–1861) and the 3′ primer CaCHS1-3 5′-GGCGTGAGCACAAATGAGCG-3′ (the complement of base numbers 2598–2617). Clones producing the expected 780 bp product were confirmed as having CHS1 sequence. Plasmid pKW030 was one such clone identified.
The entire CHS1 locus was reconstructed by ligating the 3.6 kb HindIII–PstI fragment of pKW030 and the 3.5 kb BstEII–NotI fragment from plasmid pKW013 into pBluescript SK. The resulting plasmid, pKW035, contains a 5.3 kb fragment of C. albicans DNA including the entire CHS1 ORF.
A 1 kb portion of the maltose-regulatable promoter MRP1p was amplified by PCR with primers Malp-1 (BamHI site underlined), 5′-ATAGGATCCTCACCAATTCCATCAC, and Malp-2 (with an RcaI site at the ATG), 5′-CTTAGTGTTGACTGTCATGACGATC.
The MRP1pCHS1 cassette was finally constructed using a three-way ligation that included: (i) a 2 kb CaCHS1 RcaI–Asp718 fragment (nucleotides −1 to 2084) from plasmid pKW035; (ii) a 4.4 kb BamHI–Asp718 fragment from pKW016 carrying CaURA3 and pBluescript; and (iii) a 1 kb BamHI–RcaI-cleaved MRP1p PCR product. This generated plasmid pKW044, containing the MRP1 promoter fused at the CaCHS1 start codon to a 3′ truncated CaCHS1 lacking 1610 bp of the ORF, in pBluescript SK– with URA3 as the selectable marker. Plasmid pKW044 was cut with XhoI before transformation into the CHSI/Δchs1::hisG Ura− strain to direct integration into the corresponding XhoI site of the chromosomal copy of CaCHS1, thus generating the conditional mutant.
To examine the effect of strain background on the viability of CaCHS1 disruptants, a further series of heterozygous CHS1/Δchs1::hisG mutants was created. This alternative CaCHS1 disruption cassette was constructed from a 4 kb XbaI–PstI fragment containing the CaCHS1 gene derived from plasmid pJA16 (Au-Young and Robbins, 1990). This was ligated into XbaI- and PstI-cut pUC18 to form plasmid pAB1. The hisGURA3hisG cassette was excised from plasmid p5921 (Gow et al., 1994) with BamHI and BglII and blunt ended using mung bean nuclease (Boehringer Mannheim, Roche Diagnostics). Plasmid pAB1 was cleaved with ClaI and the ends rendered blunt by treatment as before. The hisGURA3hisG fragment was ligated into the ClaI-cut and blunted pAB1 to form plasmid pAB9 (co-ordinates 1158–2555 deleted). The CaCHS1 disruption cassette was linearized with PvuII before transformation into strains CAI4, SGY243 and NGY10 (see Table 2 for genotypes) by the spheroplast method (Kurtz et al., 1986).
Northern analyses
Total RNA was extracted from C. albicans, and Northern blots were prepared according to the protocol of Hube et al. (1994). A 533 bp RcaI–XhoI fragment corresponding to positions −1 to 532 of the CaCHS1 ORF from plasmid pKW035 was radiolabelled with [α-32P]-dCTP using the random-primed labelling kit (Boehringer) to give the CHS1-specific probe N. A 0.7 kb fragment representing co-ordinates 126–829 bp of the C. albicans TEF3 coding region cloned in pUC19 (Colthurst et al., 1992) was used as a positive control for Northern analysis. The hybridization conditions described by McCreath et al. (1995) were used.
Light microscopy
Yeast cells were examined either directly or after fixation in 10% (v/v) neutral-buffered formalin (Sigma). Hyphal cells were grown on poly-l-lysine-coated microscope slides (Crombie et al., 1990) placed in Petri dishes containing 20% (v/v) newborn calf serum (Sigma) plus 2% (w/v) maltose or glucose. Cells were mixed with a drop of 50 µg ml−1 Calcofluor (American Cyanamid) or 2 µg ml−1 DAPI (Sigma) after permeabilization in 70% (v/v) ethanol and examined by fluorescence microscopy. A slide culture technique was used for time-lapse microscopy of hyphal cells. A sterile microscope slide was placed in a Petri dish and overlaid with 9.5 ml of 20% (v/v) serum agar containing 2% (w/v) glucose or maltose. The agar was inoculated with 1 × 105C. albicans cells, a coverslip placed on the slide and excess agar removed. The coverslip was then sealed to the slide with 1:1:1 Vaseline–lanolin–paraffin (Kron et al., 1994). The slide was incubated at 37°C on the heated stage of a Zeiss microscope, and the cells were examined using Nomarski differential interference optics over a 24 h period.
WGA-colloidal gold staining of chitin
Cells were grown as described above, harvested after 6 h and fixed in 2.5% (v/v) glutaraldehyde in 0.1 M sodium phosphate, pH 7.6, and stored at 4°C. The cells were washed three times in 0.1 M sodium phosphate, pH 7.6, and fixed with 1% osmium tetroxide in the same buffer for 1 h. Cells were washed three times in phosphate buffer and embedded in 1% (w/v) molten agarose. Blocks of samples were dehydrated first in graded ethanol solutions and then in polypropylene oxide before embedding in Araldite resin (Agar Scientific). Thin sections (60–80 nm) were prepared, mounted on 300 mesh gilded grids (Agar Scientific) and labelled with 10 nm WGA-gold particles (British Biocell International). The grids were immersed for 1 h in WGA-gold, diluted 1:5 or 1:10 with Tris-buffered saline (TBS). To control for non-specific binding of colloidal gold, grids were incubated with a 1:10 dilution of goat F(ab′)2 anti-mouse IgG/IgM conjugated to colloidal gold (British Biocell International). Grids were dipped in TBS, rinsed in TBS and then washed in dH2O. The thin sections were post-stained with 5% (w/v) aqueous uranyl acetate and lead citrate (Reynolds, 1963) and examined in a Jeol 1200ExB Temscan electron microscope operated at 120 kV.
Cryogenic scanning electron microscopy (SEM)
Stationary phase yeast cultures were washed in sterile dH2O, spread over the surface of sterile polycarbonate Nucleopore membranes (Whatman; diameter 47 mm, pore size 2 µm) that had been overlaid on agar plates and incubated overnight. Membranes were lifted from the plate, submerged in 10% (v/v) neutral-buffered formalin (Sigma), rinsed in phosphate-buffered saline (PBS) and then dH2O. Samples of membrane with cells attached were prepared using the Oxford CT1500C Cryotrans system according to the manufacturer's recommendations. Each sample was cooled in nitrogen slush and transferred under vacuum to the cold stage of the SEM. Samples were coated with platinum and examined on the cryomicroscope stage at −180°C in a Jeol JSM-35CF SEM operated at an accelerating voltage of 10 kV.
Virulence tests
Candida albicans strains were grown in SMal at 30°C, harvested, washed and resuspended in sterile water to the required density. Five male ICR mice (18–22 g; Harlan–Sprague–Dawley) per test were injected with 106 cells via the lateral tail vein. Neutropenic mice were also tested. These were injected intraperitoneally with 150 mg kg−1 cyclophosphamide 96 and 24 h before challenge with 104C. albicans cells and subsequently at 3 day intervals. Neutropenia was confirmed by comparing the ratio of neutrophils with the total number of leucocytes before and after administration of cyclophosphamide. Checks were made for dead or moribund mice three times daily for 20 days after infection. Moribund mice were euthanized by cervical dislocation and necropsied.
Acknowledgements
We thank Diane Slater and Tim King (Rowett Institute, Aberdeen) for help with TEM, and Debbie Marshall (Department of Medical Microbiology, Aberdeen) for help with SEM. We also acknowledge and thank Dr Tanya Parkinson, Dr Chris Hitchcock and Dr Yigal Koltin for helpful advice. N.A.R.G. acknowledges the award of a BBRSC Studentship and ACT(R) Fellowship for C.A.M. and the financial support of the Wellcome Trust (grants 039643 and 055015).




