Individual functions of the HAK and TRK potassium transporters of Schwanniomyces occidentalis
Abstract
We have cloned the gene encoding the TRK transporter of the soil yeast Schwanniomyces occidentalis and obtained the HAK1 trk1Δ and the hak1Δ TRK1 mutant strains. Analyses of the transport capacities of these mutants have shown that (i) the HAK1 and the TRK1 potassium transporters are the only transporters operating at low and medium K+ concentrations (< 1 mM); (ii) the HAK1 transporter is functional at low pH but fails at high pH; and (iii) the TRK1 transporter functions at neutral and high pH and fails at low pH. At neutral pH, both transporters are functional, but HAK1 is not expressed, except at very low K+ concentrations (< 50 µM) where HAK1 is very effective. TRK1 is also involved in the control of the membrane potential.
Introduction
Potassium is an important cellular constituent, which is normally maintained at concentrations ranging from 50 mM to 500 mM in the cytoplasm of all living cells. Because of this requirement, K+ must be taken up from the external medium, in which K+ may be at a fairly high and constant concentration (1–5 mM), as in sea water and in the milieu bathing animal cells, or at low and highly variable concentrations, as in many terrestrial environments. In animal cells, K+ uptake has been studied extensively, but much less is known about this process in eukaryotic non-animal cells. Saccharomyces cerevisiae was the first fungal species in which the molecular basis of K+ uptake was completely clarified (Ko and Gaber, 1991; Gaber, 1992). This clarification was an important breakthrough. However, S. cerevisiae is adapted to fruit products with high K+ contents, and its K+ transporters may be different from those existing in other fungi adapted to the more nutritionally limiting life in soil. Probably because of its environmental adaptation, S. cerevisiae is furnished exclusively with transporters of one sole type, TRK1 and TRK2 (Rodríguez-Navarro, 2000). The same exception applies to Schizosaccharomyces pombe, in which the K+ transporters also seem to be of the TRK type only (Calero et al., 2000).
Soil is probably the environment in which the K+ concentration varies most, as do the concentrations of other cations, such as H+, Na+ and Ca2+, which affect K+ uptake in fungi and plants. Probably as a means of adaptation to these complex conditions, K+ uptake in plants and in soil fungi is mediated by at least three different types of transport systems, TRK-HKT and HAK transporters and K+ channels (Rodríguez-Navarro, 2000). Neurospora crassa is the soil fungus in which K+ uptake has been studied most extensively. N. crassa is furnished with a TRK transporter similar to the S. cerevisiae transporters, but also with a HAK transporter, which belongs to a family of K+ transporters that are apparently better adapted than the TRK transporters to operate at low K+ concentrations (Haro et al., 1999). In Arabidopsis thaliana and probably in other plants, root K+ uptake is thought to be mediated by HAK and HKT transporters (HKT transporters belong to the TRK family) co-existing with inward-rectifying K+ channels (Rodríguez-Navarro, 2000). Although the genes and cDNAs encoding these transporters have been cloned, expressed in heterologous systems and studied quite extensively, the understanding of the overall process of K+ uptake in plants and soil fungi is still conditioned by incomplete knowledge of the contribution of each transport system to the overall process.
To establish each individual contribution, the disruption of the genes encoding the transporters is the most powerful approach. These disruptions have been attained in S. cerevisiae, S. pombe and Arabidopsis thaliana, but a general picture of K+ uptake in fungi and plants has not yet been deduced from these experiments, except in S. cerevisiae. Making the problem even more difficult, in all the cases studied so far, the disruption of K+ transporters resulted in a hyperpolarization of the cells, both in fungi (Madrid et al., 1998; Calero et al., 2000) and in A. thaliana (Hirsch et al., 1998; Spalding et al., 1999).
Schwanniomyces occidentalis was the first eukaryotic organism in which a HAK K+ transporter was identified (Bañuelos et al., 1995). The HAK transporter of S. occidentalis is very active and apparently makes the existence of other K+ transporters unnecessary. However, the identification of HAK and TRK K+ transporters in N. crassa suggested that other soil fungi may also need transporters of the two families for reasons that are presently unknown (Haro et al., 1999). In this study, we report the cloning of the TRK1 gene of S. occidentalis and the properties of the HAK1 and TRK1 disruptants. Our results suggest that HAK transporters are specialized for low K+ and low pH, whereas the TRK transporter dominates in all other conditions. The TRK transporter is involved in the control of the membrane potential, but not the HAK transporter.
Results
Cloning of the TRK1 gene
In a previous investigation of the K+ transporters of S. occidentalis, which was carried out by complementing a trk1 trk2 S. cerevisiae mutant, only the gene encoding a HAK transporter was found (Bañuelos et al., 1995). However, the cloning of the trk-1 and hak-1 genes in N. crassa (Haro et al., 1999) suggested to us that S. occidentalis could also be furnished with a TRK K+ transporter. Therefore, with the purpose of isolating a SoTRK gene, we attempted the polymerase chain reaction (PCR) amplification of a gene fragment that could encode the part of a TRK transporter located between two conserved regions, using the degenerate primers deduced from these regions. Afterwards, the amplified fragment was used for colony hybridization in the library. Using this procedure, we identified a unique plasmid (pRM1) containing an insert of approximately 5 kb. The sequence of this insert revealed the presence of an open reading frame (ORF) of 3057 bp, which could encode a protein of 1018 amino acids and 116 kDa, with all the sequence characteristics of TRK transporters. These included the typical four sequential M1PM2 elements of TRK–HKT transporters, made up of an N-terminal outer membrane helix (M1), a P-loop region (P) and a C-terminal inner transmembrane helix (M2), and the glycine residues conserved in the four P fragments, in M2b, and immediately after Pd (Durell and Guy, 1999; Durell et al., 1999) (Fig. 1). The cloned gene was named TRK1, and Southern blotting analysis indicated that additional copies of TRK1 or other highly homologous genes do not exist in the genome of S. occidentalis. The phylogenetic analysis of SoTRK1 and the other fungal transporters of the family (Fig. 2) revealed that SoTRK1 is most closely related to the S. cerevisiae transporters (46.9% and 48.4% identity with ScTRK1 and ScTRK2 respectively) and least related to the transporters of S. pombe (39.0% and 35.1% identity with SpTRK1 and SpTRK2 respectively).

Alignment of the four M1PM2 fragments of SoTrk1p. Hydrophobic segments of the Trk1 protein are shown in grey boxes. Glycine residues conserved in all TRK–HKT transporters are highlighted in black boxes (see Durell and Guy, 1999).
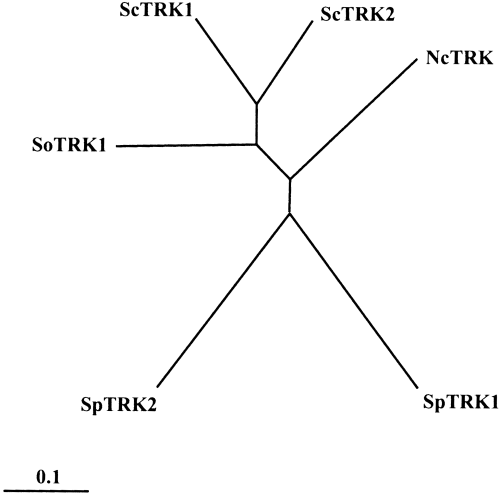
Phylogenetic tree of the TRK transporters. To avoid the introduction of long gaps, the sequences used for constructing the phylogenetic tree started 30 amino acid positions before M1b and ended at the end of M1d (Rodríguez-Navarro, 2000). The tree was constructed using the clustal X program. The scale bar corresponds to a distance of 10 changes per 100 amino acid positions. Sc, S. cerevisiae; Sp, S. pombe; Nc, N. crassa; So, S. occidentalis. Accession numbers: ScTRK1, P12685; ScTRK2, P28584; SpTRK1, P47946; SpTRK2, Q10065; NcTRK1, AJ009758; SoTRK1, AJ290453.
Transformation of pRM1 into the trk1 trk2 S. cerevisiae mutant weakly suppressed the growth defect of this strain at low K+[normal growth in minimal media requires a K+ influx of 3.5 nmol mg−1 min−1 (Rodríguez-Navarro, 2000); in arginine phosphate medium, the trk1 trk2 mutant does not present appreciable growth below 1 mM K+ and presents poor growth up to 10 mM K+]. Analysis of Rb+ influx in the transformant strain revealed two kinetic components, a low-affinity uptake identical to that described for the trk1 trk2 mutant (Ramos et al., 1994) and a high-affinity influx exhibiting a Rb+Km of 0.25 mM and a Vmax of 2.2 nmol mg−1 min−1, which had to result from the expression of the SoTRK1 gene. The transformant also took up K+ and Cs+ from much lower concentrations than the trk1 trk2 mutant, but a similar enhancement of Na+ uptake was not observed, probably because the pRM1-dependent Na+ influx exhibits a high Km and could not be distinguished from the endogenous influx, which exhibits a much higher Vmax. The Rb+ influx mediated by SoTRK1 was competitively inhibited by K+, Cs+ or Na+, exhibiting Kis of 0.1 mM, 0.5 mM and 10 mM respectively.
Disruption of SoHAK1 and SoTRK1
To establish the individual functions of the HAK1 and TRK1 transporters in S. occidentalis, we constructed the corresponding gene-disrupted strains. Because gene disruption in S. occidentalis can be carried out by homologous recombination using the adenine auxotrophy of strain RKA-7 (Klein and Favreau, 1988; Bañuelos and Rodríguez-Navarro, 1998), we constructed two DNA fragments in which we substituted the SoADE2 gene for central fragments of SoHAK1 and SoTRK1 (1372 bp in HAK1 and 1165 bp in TRK1). The resulting fragments were then transformed into RKA-7, and the clones growing in an adenine-free medium were analysed by Southern hybridization. Approximately 5% of the clones growing in the adenine-free medium carried out the gene disruptions.
The kinetic analyses of Rb+ influx in K+-starved cells of several trk1Δ disruptant strains show that these strains kept only one transport system (Fig. 3), which was kinetically coincident (Km and Vmax) with that found in K+-starved cells from the wild strain (Bañuelos et al., 1995). The Km was also coincident with the Km of the high-affinity Rb+ influx exhibited by the trk1 trk2 S. cerevisiae mutant transformed with SoHAK1 (Bañuelos et al., 1995). In the hak1Δ disruptants, the kinetics of Rb+ influx exhibited high- and low-affinity phases (Fig. 3). The Rb+Km and the K+, Cs+ and Na+Kis of the high-affinity Rb+ influx of the disruptants were not significantly different from the corresponding constants in the high-affinity Rb+ influx exhibited by the trk1 trk2 S. cerevisiae mutant transformed with SoTRK1. Because no differences could be found among different disruptants of the same gene, only two strains, MAB17 (trk1Δ) and MAB115 (hak1Δ), were selected for further study.
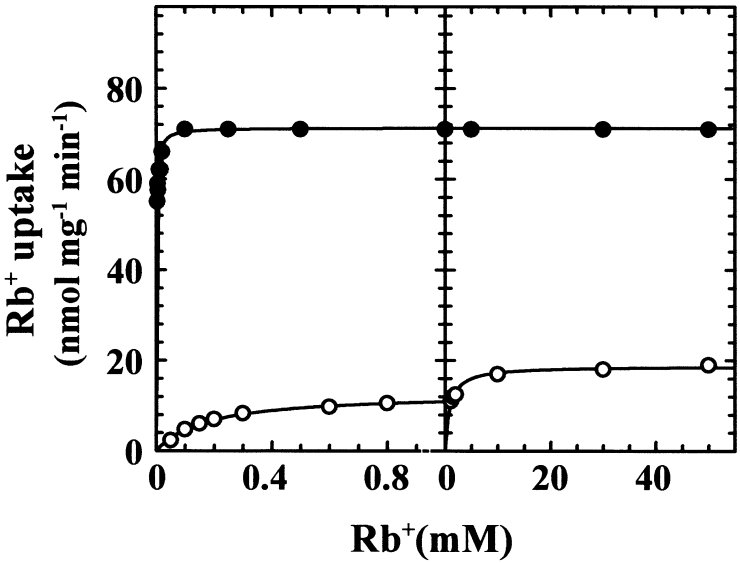
Rb+ influx kinetics in the HAK1 trk1Δ and hak1Δ TRK1 strains. Cells were grown in standard medium and K+ starved for 4 h in K+-free arginine phosphate medium. Rb+ influx was tested in 10 mM MES-Ca2+, pH 6.0. The data are shown in two parts in order to increase the details at low (left) and high (right) Rb+ concentrations. Closed circles, HAK1 trk1Δ (strain MAB17); open circles, hak1Δ TRK1 (strain MAB115).
Comparative growth tests with the HAK1 trk1Δ, hak1Δ TRK1 and wild-type strains carried out by dropping samples of cell suspensions in solid media (pH 6.0) revealed that the wild-type strain and the HAK1 trk1Δ strains grew well in low-K+ media (50–100 µM), whereas the hak1Δ TRK1 mutant displayed defective growth. This defective growth could not be expected from the kinetic data obtained for hak1Δ TRK1 (calculated K+Km of 100 µM and Vmax of 12 nmol mg−1 min−1) and reflected the fact that the amount of K+ required for the growth of the high number of cells inoculated with the drops produced a strong depletion of the K+ content in the proximity of the growth spots, and this depletion could not be brought about by the SoTRK1 transporter. In agreement with this notion, when growth was tested in a liquid medium at 50 µM K+ (pH 6.0) and low cell concentrations to avoid a significant K+ depletion, the three strains grew at the same rate (see the growth of the mutants in liquid medium in Fig. 5).
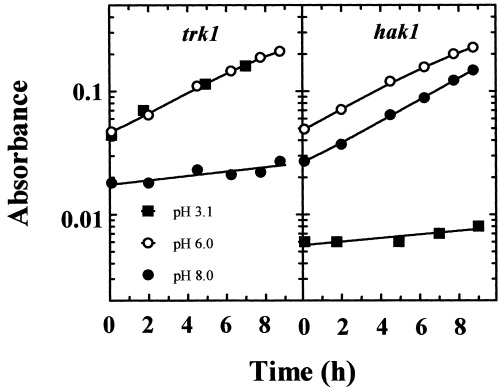
Growth of the HAK1 trk1Δ and hak1Δ TRK1 strains at 50 µM K+ at three pH values. Samples of cells grown in standard medium were inoculated in the testing media at a cell density of 2 µg ml−1 (approximately 103 cells ml−1) and incubated for approximately 15 h before starting to record the apparent absorbance of the cultures at 550 nm. Growth was recorded in arginine phosphate medium supplemented with 50 µM KCl and 5 mM tartaric acid, 10 mM MES or 10 mM TAPS and adjusted to pH 3.1, 6.0 and 8.0, respectively, with arginine.
pH profiles of the activities of SoHAK1 and SoTRK1
Micromolar K+ uptake in S. cerevisiae, which only has TRK transporters, is very sensitive to low pH values (Rodríguez-Navarro and Ramos, 1984). This suggested that a fungus that grows at low pH and low K+ may need a different type of transporter that is better adapted to low pH, and this, in view of the present findings, could be the HAK transporter. We tested this possibility with the HAK1 trk1Δ and hak1Δ TRK1 strains and found that, in fact, the pH profiles of K+ uptake of both strains at 100 µM K+ were completely different (Fig. 4). The results demonstrated that, in HAK1 trk1Δ, the uptake was barely affected at pH 3.3, but was inhibited about fivefold at pH 8.1. In contrast, in hak1Δ TRK1, the uptake was inhibited a mere 30% at pH 8.1 and completely at pH 3.3. Growth tests at 50 µM K+ confirmed that the wild type exhibited good adaptation to the whole range of pH values, whereas the mutants failed at the extreme values. The HAK1 trk1Δ mutant failed at high pH and the hak1Δ TRK1 mutant at low pH (Fig. 5). These results demonstrated that, at 50 µM K+, S. occidentalis depended upon HAK1 for K+ uptake at pH 3.1 and upon TRK1 at pH 8.0.
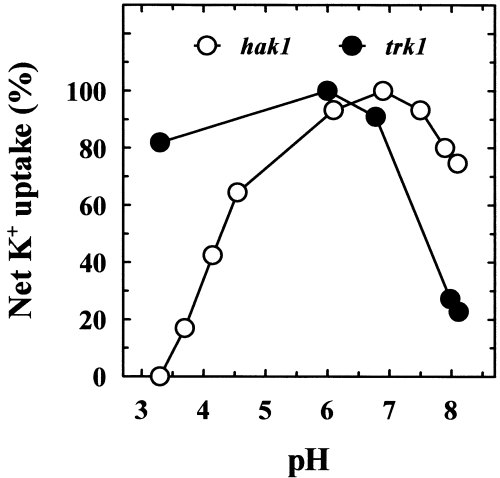
pH profiles of net K+ uptake in the HAK1 trk1Δ and hak1Δ TRK1 strains. Cells were grown in standard medium and K+ starved for 4 h in K+-free arginine phosphate medium. Samples of cells were suspended in testing buffers with 100 µM K+, and the initial rates of decrease in the K+ concentration were recorded. Buffers were prepared with 5 mM tartaric acid, 10 mM MES or 10 mM TAPS and Ca(OH)2 for adjusting the pH. K+ concentration was determined by atomic emission spectrophotometry. Results are expressed as percentages of the maximum rates of uptake, which were 44 and 12 nmol mg−1 min−1 for the HAK1 trk1Δ and hak1Δ TRK1 strains respectively.
HAK1 and TRK1 are the most significant K+ transporters in S. occidentalis
The kinetic analysis of Rb+ influx in the HAK1 trk1Δ and hak1Δ TRK1 strains suggested that only HAK1 and TRK1 made a significant contribution to Rb+ uptake at low Rb+ (Fig. 3, left), but that a third system could exist at high Rb+ (Fig. 3, right). However, the great difference between the Vmaxs exhibited by the HAK1 trk1Δ and hak1Δ TRK1 strains at low Rb+ (70 and 12 nmol mg−1 min−1 respectively) made it difficult to determine whether Rb+ influx in the hak1Δ TRK1 strain was mediated by two systems with similar Kms, one of which was still present, although undetectable, in the HAK1 trk1Δ strain. Therefore, we tried to construct the trk1Δ hak1Δ double mutant by disrupting the HAK1 gene with the kanr marker gene in both the wild and the HAK1 trk1Δ strains (Wach et al., 1994) (the disruption of the TRK1 gene cannot be tried with this marker gene because the disruption has the intrinsic effect of increasing the sensitivity to geneticin, as described below for hygromycin). Unfortunately, neither of the two mutants was isolated. In our experience, S. occidentalis showed a high capacity to develop several types of geneticin-resistant phenotypes, and the selection of the transformants with the kanr gene was impossible.
Because we could not isolate the double mutant, it could not be demonstrated whether the second phase (Fig. 3, right) in the hak1Δ TRK1 strain was mediated by a third system. Bearing this in mind, we tried to find out whether HAK1 and TRK1 were the only operative transporters at low K+ or Rb+ (< 1 mM) (Fig. 3, left) by kinetic analysis. To make the kinetic analysis possible, it was necessary to reduce the difference between the Vmaxs exhibited by the HAK1 trk1Δ and hak1Δ TRK1 mutants, and we took advantage of the decrease in the activity of SoHAK1 at high pH (Fig. 4). Testing Rb+ influx at pH 8.0, it was found that Rb+ influx in the HAK1 trk1Δ and hak1Δ TRK1 strains exhibited pure Michaelis–Menten kinetics with the Kms described for pH 6.0 (Fig. 3, left) and Vmaxs of 22 and 10 nmol mg−1 min−1 respectively. Because the Vmaxs of the two strains were more similar in these conditions, it can be assumed that, if the kinetics exhibited by the hak1Δ TRK1 strain had been the addition of two systems with similar Kms, the kinetics of the HAK1 trk1Δ strain would have shown two phases with very different Kms and not very different Vmaxs.
These results indicate that nothing but TRK1 contributes significantly to Rb+ influx at high pH values ([Rb+] < 1 mM) in the hak1Δ TRK1 strain. This conclusion also applies to pH 6.0, because the Vmaxs of Rb+ influx at pH 6.0 and 8.0 were very similar (12 and 10 nmol mg−1 min−1 respectively). Therefore, if a third system existed (e.g. SoTRK2), active at pH 6.0 but not at pH 8.0, to keep the addition of the activities of SoTRK1 and the hypothetical SoTRK2 almost constant at pH 6.0 and 8.0, SoTRK1 should significantly decrease its activity at pH 6.0. This possibility was tested for SoTRK1 expressed in S. cerevisiae; we found that SoTRK1 did not decrease its activity at pH 6.0.
Functions of the SoHAK1 and SoTRK1 transporters
We have shown that SoHAK1 dominates K+ uptake at very low K+ concentrations (growth tested by spotting drops of the HAK1 trk1Δ and hak1Δ TRK1 strains in a low-K+ solid medium) and that, at 50–100 µM K+, SoHAK1 dominates at low pH and SoTRK1 at high pH (4, 5). The question is now which transporter dominates at higher K+ concentrations, for example at 1 mM. We addressed this question by taking advantage of the high sensitivity to Cs+ of the HAK1 trk1Δ mutant compared with the hak1Δ TRK1 mutant (HAK and TRK transporters mediate Cs+ uptake, and the different tolerance of the mutants can be explained if SoHAK1 is a better Cs+ transporter than SoTRK1). Figure 6 shows that, at 1 mM K+ and pH 6.0 and 8.2, the wild-type strain showed the same tolerance to Cs+ as the hak1Δ TRK1 strain. In contrast, at pH 3.1, the wild strain became more similar to the HAK1 trk1Δ mutant. This suggested that SoTRK1 was the dominant transporter at neutral and high pH. At low pH, the dominant transporter was SoHAK1, but SoTRK1 still kept a significant activity.
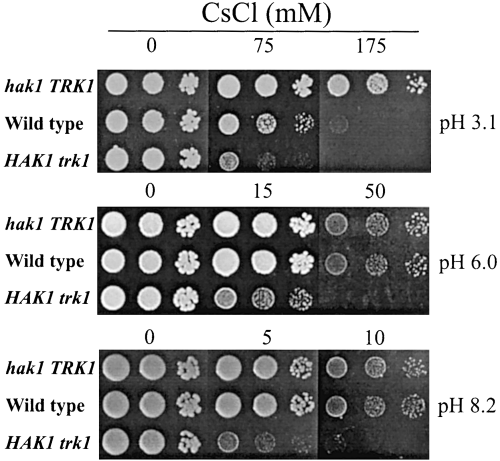
Growth inhibition by CsCl of wild-type, HAK1 trk1Δ and hak1Δ TRK1 strains. Serial 10-fold water dilutions of YPD-grown cells were spotted onto solid arginine phosphate medium buffered to the indicated pH values as described in the legend to Fig. 5. The medium contained 1 mM KCl and CsCl.
To investigate further the alternation of the two transporters in different conditions, we analysed the expression of the SoHAK1 transcripts. First, we determined the level of SoHAK1 transcripts associated with normal growth, exemplified by the HAK1 trk1Δ strain growing at pH 6.0 and 1 mM K+. From this experiment, we learned that the level of SoHAK1 transcripts necessary for normal growth at 1 mM K+ was barely detected by Northern analysis (Fig. 7). Then, we analysed the expression levels in the wild type in different conditions (Fig. 7). The conclusions can be summarized as follows: (i) at pH 6.0 and 1 mM K+, SoHAK1 transcripts are not expressed; (ii) K+ starvation triggers an expression that may be 100 times the expression required for normal growth; (iii) pH 8.0 notably decreased the transcript levels in K+-starved cells; (iv) pH 3.1 increased the transcript levels, even in cells with K+ in excess. These results and those obtained with Cs+ lead to the conclusion that SoHAK1 dominates uptake at low K+ and low pH and that SoTRK1 dominates in all other conditions. Interestingly, for the expression of the HAK1 transcripts, the pH dominates over the K+ status of the cells, because a high pH repressed expression when K+ was limiting, and a low pH enhanced expression when K+ was in excess.
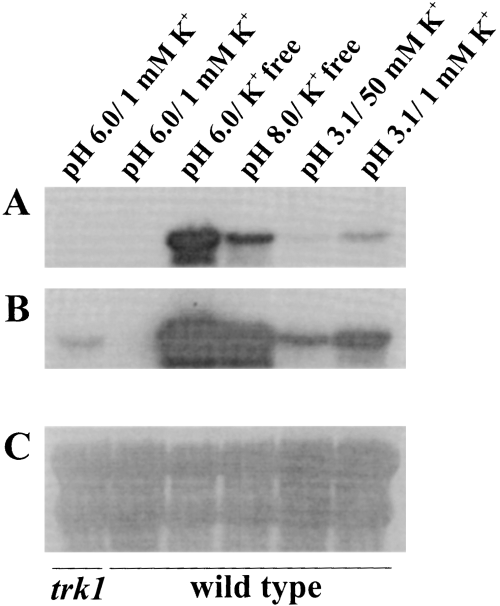
Northern analyses of HAK transcripts in HAK1 trk1Δ and wild-type cells. Cells grown in standard medium were incubated for 3 h in arginine phosphate medium supplemented with the recorded KCl concentrations and buffered as described in the legend to Fig. 5. Total RNA of cell samples was extracted and analysed as described in Experimental procedures. In (B), the film was exposed for a greater length of time than in (A). In (C), the membrane was stripped and stained with 0.04% methylene blue in 0.5 M Na+-acetate to illustrate the relative amounts of RNA loaded in each lane (12 µg). The K+ contents of the cells at the moment of sampling were 750, 780, 430, 400, 730 and 730 nmol mg−1, recorded from left to right as shown in the figure.
Similar experiments could not be carried out for the SoTRK1 transcripts, because they were not detected by Northern blot hybridizations.
SoTRK1 is involved in the control of the membrane potential
In S. cerevisiae (Madrid et al., 1998) and S. pombe (Calero et al., 2000), it has been suggested that the TRK transporters participate in the control of the membrane potential and that the disruption of these genes hyperpolarizes the plasma membrane. To answer the question of whether the HAK or the TRK transporters of S. occidentalis control the membrane potential, we compared the sensitivity of the wild-type, HAK1 trk1Δ and hak1Δ TRK1 strains to hygromycin and found that the hak1Δ mutation did not affect the hygromycin sensitivity, whereas the trk1Δ mutation increased it dramatically (Fig. 8). We then tested whether plasmid pRM1 containing SoTRK1 suppressed the hygromycin hypersensitivity of the trk1 trk2 S. cerevisiae mutant and found no effect. These results demonstrated that, although SoTRK1 participated in the control of the membrane potential in S. occidentalis and weakly suppressed the K+ uptake defect of the trk1 trk2 S. cerevisiae mutant, it did not show any effect on the control of the membrane potential in S. cerevisiae.
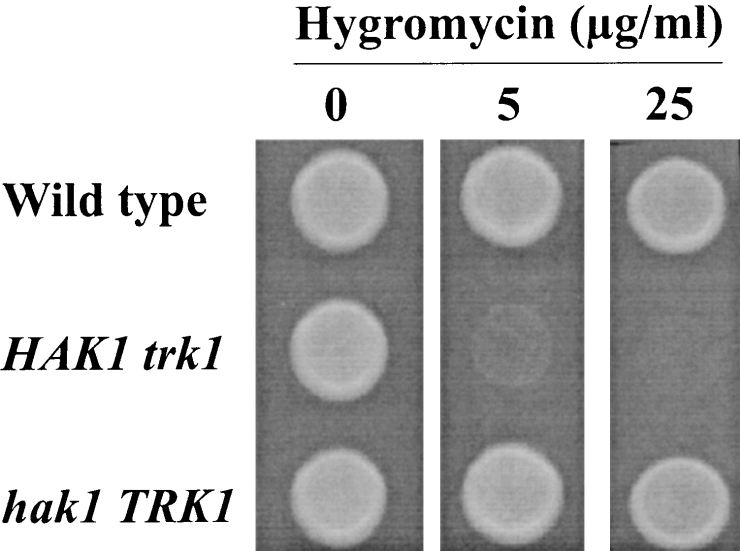
Hygromycin sensitivity of wild-type, HAK1 trk1Δ and hak1Δ TRK1 strains. Samples of cells grown in YPD medium were spotted onto solid YPD medium containing the indicated concentrations of hygromycin.
Discussion
As found previously in N. crassa (Haro et al., 1999), S. occidentalis is furnished with HAK and TRK transporters, and this report gives a functional explanation of the co-existence of these two types of transporters. We propose that HAK transporters are especially adapted to mediating K+ uptake at low K+ concentrations (even lower than 1 µM) and at any K+ concentration if the pH is low, whereas TRK transporters show excellent activities and dominate K+ uptake at higher K+ concentrations (50–100 µM) if the pH is not acidic. Furthermore, the TRK transporters are probably necessary for a normal physiology of the cell, because they play a role in the control of the membrane potential. In contrast, the HAK transporters are not necessary and are not expressed, if the cells do not meet extreme conditions of low K+ or low pH. The absence of HAK transporters in S. cerevisiae (Madrid et al., 1998) and S. pombe (Calero et al., 2000) supports the notion that fungi do not require the HAK transporters, except for growth at low K+ and low pH. Certainly, S. cerevisiae and S. pombe grow at low pH values, but probably always in plant products, in which, because of their very high K+ concentrations (must is one example), the low efficiency of the TRK transporters is compensated for by the high K+ content.
The superior performance of fungal HAK transporters over TRK transporters at low pH can be connected to a previous finding, which indicates that, in Escherichia coli, the Kup system is the major uptake system at low pH (Trchounian and Kobayashi, 1999). Taking both findings together and considering that bacterial Kup and fungal HAK transporters belong to the same type of K+ transporters (Rodríguez-Navarro, 2000), the hypothesis that Kup–HAK transporters are the universal transporters for low-pH media comes to the fore.
The huge expression of the SoHAK1 transcript in response to K+ starvation (compare the expression of the HAK1 trk1Δ strain growing at 1 mM K+ with the expression of K+-starved cells in the wild type in Fig. 7) is consistent with the very high Vmax of Rb+ influx observed in K+-starved cells (70 nmol mg−1 min−1). If the amount of transcript is proportional to the amount of protein, it is very likely that the measured Vmax underestimates the capacity of the transporter, because the observable Vmax may be limited by the incapacity of the H+-ATPase to deliver more current. Taking all this into consideration, we can conclude that S. occidentalis can probably grow at less than 0.03 µM K+, which is consistent with the capacity of S. occidentalis to deplete the K+ content down to detectable concentrations (< 0.03 µM) (Bañuelos et al., 1995). It is worth observing that N. crassa does not have this outstanding capacity (Rodríguez-Navarro et al., 1986). The interesting consideration is that the only environment in which S. occidentalis may encounter such a low K+ concentration is the depletion zone around plant roots (Marschner, 1995). Remarkably, the Km of the plant root HAK transporters (Santa-María et al., 1997; Rubio et al., 2000) is not low enough to suggest that plant roots can take up K+ effectively at 0.03 µM K+. S. occidentalis is probably one of the organisms able to grow in the root depletion zone.
Regarding the number of K+ transporters in S. occidentalis, our results strongly suggest that only HAK1 and TRK1 have significant capacity for transporting K+ at low and medium concentrations (< 1 mM). We failed to construct the double hak1 trk1 mutant, but what has been learned from S. cerevisiae is that the question of the number of K+ transporters does not have a simple answer, even when all the disruptions can be obtained. In S. cerevisiae, the disruption of the trk1 gene creates an ectopic K+ uptake (Madrid et al., 1998), which cannot be distinguished easily from the uptake mediated by a genuine transporter. In S. occidentalis, the disruption of HAK1 triggers a second phase of low-affinity Rb+ influx at high Rb+, which might be an ectopic uptake or a second phase of SoTRK1.
An interesting finding from this study was the low Vmax and the low expression of SoTRK1, which excludes SoTRK1 as an adequate transporter for very low K+ concentrations. This is consistent with its dual function for transporting K+ and controlling the membrane potential, because if SoTRK1 reached the level of expression of SoHAK1, the cell would be completely depolarized [the membrane potential can be controlled in different ways (Madrid et al., 1998) but, for a simple understanding of the problem, it may be assumed that the membrane potential is controlled by allowing the passage of a H+ current (Bihler et al., 1999)]. On the contrary, the single function of SoHAK1 as a K+ transporter allows for its high expression without affecting the membrane potential, except as a consequence of the K+ current that it mediates. It is possible that the existence of two TRK transporters in the fungi furnished only with TRK transporters, S. cerevisiae and S. pombe, is a way to make the enhancement of K+ uptake at low K+ and the control of the membrane potential compatible. Interestingly, the disruption of the two TRK genes has a synergistic hyperpolarizing effect in both S. cerevisiae and S. pombe (Madrid et al., 1998; Calero et al., 2000), which suggests that both transporters co-operate in the control of the membrane potential, although both transporters are not necessary for a competent K+ uptake (Ramos et al., 1994; Calero et al., 2000).
The present results also illustrate the function of the HAK and HKT transporters in plant cells. Regarding the control of the membrane potential in root cells, the role of the fungal TRK K+ transporters in its control suggests that plant HKT transporters may play the same role, because they belong to the TRK family (Durell and Guy, 1999; Durell et al., 1999). This notion is further supported by the observation that HKT1 strongly depolarizes trk1 trk2 S. cerevisiae mutants (Madrid et al., 1998). However, two observations are pertinent regarding this point. The first is that the transcription of HKT1 is considerably enhanced in K+-starved plants (Wang et al., 1998), which is not consistent with the notion that a K+ transporter controlling the membrane potential must be slightly upregulated. The second is that a K+ channel is an obvious candidate for that function. Interestingly, the Arabidopsis AKT1 K+ channel shows an almost constant expression (Basset et al., 1995), and an akt1 mutant of A. thaliana shows hyperpolarization, especially at non-limiting K+ (Hirsch et al., 1998; Spalding et al., 1999).
Experimental procedures
Strains and culture conditions
The S. cerevisiae trk1 trk2 mutant WΔ3 (Mata ura3 his3 leu2 trp1 trk1Δ::LEU2 trk2Δ::HIS3) (Haro et al., 1999) and the S. occidentalis strain RKA-7 (ade2) (Klein and Favreau, 1988) have been described previously. The strains were routinely grown in YPD medium (1% yeast extract, 2% peptone, 2% glucose). The K+- and Na+-free arginine phosphate medium (Rodríguez-Navarro and Ramos, 1984) was supplemented with 10 mM K+ when used as standard mineral medium or supplemented with other K+ concentrations, as indicated in each case. Yeast transformants were grown in minimal medium lacking the nutritional requirement whose synthesis in the recipient strain depends on the marker gene in the plasmid. The growth temperature was 28°C. K+-starved cells were prepared by incubating standard-grown cells in K+-free arginine phosphate medium for 4 h.
Rb+ uptake experiments
Cells were suspended in either 10 mM MES or 10 mM TAPS brought to the corresponding pH with Ca(OH)2. In all cases, the buffer contained 0.1 mM MgCl2 and 2% glucose. Cells were collected on Millipore membrane filters and rapidly washed with 20 mM MgCl2 solution. Filters and cells were treated overnight with 0.1 M HCl, and Rb+ was determined by atomic emission spectrophotometry (Rodríguez-Navarro and Ramos, 1984). All experiments were repeated at least three times. Standard deviations are not recorded, but they were always lower than 10% of the corresponding mean.
Recombinant DNA techniques
Manipulation of nucleic acids was by standard protocols (Sambrook et al., 1989) or, where appropriate, according to the reagent manufacturer's instructions. Polymerase chain reactions (PCRs) were performed in a Perkin-Elmer thermocycler, and the PCR products were first cloned into the PCR2.1 vector using the TA cloning kit (Invitrogen). Sequencing was carried out using an automated ABI Prism 377 DNA sequencer (Perkin-Elmer). DNA sequence data for comparative analyses were obtained from blast (NCBI, Bethesda, MD, USA) (Altschul et al., 1990). The protein sequence alignment and phylogenetic tree was obtained using the clustal X program (Thompson, 1997). For colony hybridization, the colonies of the S. occidentalis gene library were immobilized on nylon membranes (Hybond-N; Amersham Pharmacia Biotech) and screened using a SoTRK1 fragment as a digoxygenin (DIG)-labelled probe, which was prepared using the DIG system (Boehringer Mannheim) according to the manufacturer's instructions. Membranes were hybridized in the presence of 50% formamide at 42°C. For Southern blot hybridization, genomic DNA was digested with restriction enzymes. Then, after electrophoresis and blotting onto a nylon membrane, it was hybridized in the presence of 50% formamide at 42°C to DIG-labelled probes. For Northern blots, total RNA was extracted (Carlson and Botstein, 1982), fractionated through formaldehyde–agarose gels and blotted onto a nylon membrane. Membranes were hybridized, as described above, with DNA probes that were labelled with [α-32P]-dATP by the random priming method (Feinberg and Vogelstein, 1983).
Cloning of the TRK1 gene
Total DNA from S. occidentalis RKA-7 strain was used for amplification by PCR using the Expand High Fidelity PCR System (Boehringer Mannheim) with a sense degenerate primer (5′-TWYATNNTNATGATGTA-3′) and an antisense degenerate primer (5′-CCNACNGTNCCRTANGC-3′), both deduced from the conserved sequences YM(L/V/F/I)MMY and AYGTVG in the TRK transporter family (Rodríguez-Navarro, 2000). This procedure yielded one DNA fragment of approximately 300 bp. The PCR fragment was then used as a probe for screening the S. occidentalis gene library (Klein and Favreau, 1988). A clone isolated by this procedure harboured a plasmid (pRM1) whose insert contained an ORF that could encode a typical TRK transporter. The library is constructed in the episomal plasmid pYcDE8, which contains the S. cerevisiae ADH1 promoter preceding the cloning site. Although we did not investigate the individual effects of the ADH1 and SoTRK1 promoters on the expression of the TRK1 protein in S. cerevisiae, the long distance between the ADH1 promoter and the start of the ORF, approximately 1 kb, suggests little influence of the ADH1 promoter. The GenBank accession number for SoTRK1 is AJ290453.
Disruption of the SoHAK1 and SoTRK1 genes
For the disruption of SoHAK1, we constructed a lineal fragment of DNA from position −502 to position 3418 of the SoHAK1 gene, in which we substituted the 4.4 kb SacI–BamHI fragment from plasmid pADE (Klein and Favreau, 1988), containing ADE2, for an internal fragment of SoHAK1 (from positions 286 to 1657). The resulting fragment was then transformed into RKA-7 strain. For the disruption of SoTRK1, we constructed a lineal fragment of DNA from positions 47 to 2448 of the SoTRK1 gene, in which we substituted the 4.4 kb EcoRI–BamHI fragment from plasmid pADE (Klein and Favreau, 1988) for an internal fragment of SoTRK1 (from positions 788 to 1952). Then, this fragment was transformed into RKA-7 strain. In both cases, transformants were selected in adenine-free medium.
Acknowledgements
We thank R. D. Klein, Pharmacia and Upjohn Co., for providing the S. occidentalis gene library and the RKA-7 strain. This work was supported by the European Commission DG XII Biotechnology Programme, contract number BIO4 CT960775, and by grant PB97-0560 from Dirección General de Enseñanza Superior e Investigación Científica, Spain.




