The partition system of multidrug resistance plasmid TP228 includes a novel protein that epitomizes an evolutionarily distinct subgroup of the ParA superfamily
Abstract
The segregational stability of bacterial, low-copy-number plasmids is promoted primarily by active partition. The plasmid-specified components of the prototypical P1 plasmid partition system consist of two proteins, ParA (44.3 kDa) and ParB (38.5 kDa), which, in conjunction with integration host factor, form a nucleoprotein complex at the plasmid partition site, parS. This complex is the probable substrate for the directed temporal and spatial intracellular movement of plasmids before cell division. The genetic organization of the partition cassette of the multidrug resistance plasmid TP228 differs markedly from that of the P1 paradigm. The TP228 system includes a novel member (ParF; 22.0 kDa) of the ParA superfamily of ATPases, of which the P1 ParA protein is the archetype. However, the ParF protein and its immediate relatives form a discrete subgroup of the ParA superfamily, which evolutionarily is more related to the MinD subgroup of cell division proteins than to ParA of P1. The TP228 and P1 partition modules differ further in that the former does not include a parB homologue, but does specify a protein (ParG; 8.6 kDa) unrelated to ParB. Homologues of the parF gene are widely disseminated on eubacterial genomes, suggesting that ParF-mediated partition may be a common mechanism by which plasmid segregational stability is achieved.
Introduction
The accurate distribution of low-copy-number plasmids to daughter cells at bacterial cell division is mediated primarily by active partition (Nordström and Austin, 1989; Hiraga, 1992; Williams and Thomas, 1992). A number of plasmid partition systems have been analysed in detail, including those of the F, P1, RK2 and R1 plasmids of Escherichia coli (e.g. Macartney et al., 1997; Jensen and Gerdes, 1999; Kim and Wang, 1999; Rodionov et al., 1999). These studies have revealed that active partition is a highly organized spatial and temporal process, which probably involves the interaction of nucleoprotein complexes assembled on the plasmid partition site with specialized intracellular host structures that direct the ordered movement of plasmids within the cell.
One of the best-studied partition systems is that of the P1 plasmid. The plasmid-located components of the P1 segregation system are organized as a module that consists of two genes, parA and parB, which are arranged as an autoregulated operon, and a downstream partition site, parS (Abeles et al., 1985; see Fig. 7). Each of these loci is necessary for partition, although the parS site (≈100 bp) is the only element that is required in cis (Abeles et al., 1985; Friedman and Austin, 1989; Davis et al., 1996). ParB is a bifunctional DNA-binding protein, which recognizes a complex set of heptameric and hexameric motifs at the outer ends of parS (Davis and Austin, 1988; Funnell and Gagnier, 1993; Hayes and Austin, 1993; Radnedge et al., 1996). The interaction of ParB with parS is enhanced by the binding of the host-encoded, DNA-bending protein, integration host factor between the parS arms, resulting in a topologically complex nucleoprotein structure (Davis and Austin, 1988; Funnell, 1988a; Hayes and Austin, 1994).
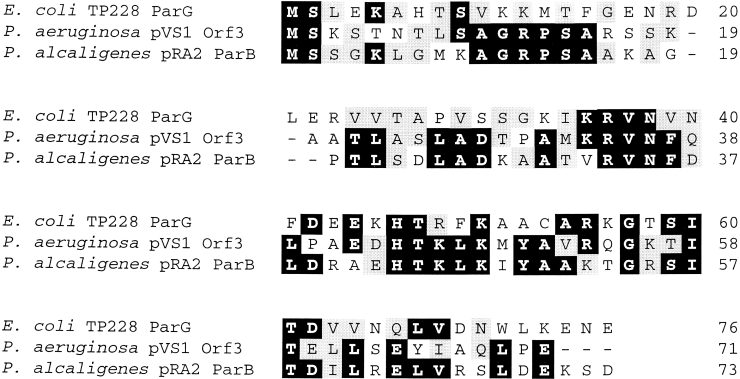
Amino acid sequence alignment of ParG homologues. Identical and similar residues that are conserved in a majority (more than two) of homologues are outlined in black and grey respectively. Database accession numbers: E. coliTP228 ParG, AAF74218; P. aeruginosa pVS1 Orf3, AAD19679; P. alcaligenes pRA2 ParB, AAD40335.
The ParA protein also associates with the nucleoprotein complex formed at parS, but does so via an interaction with ParB rather than directly contacting parS (Youngren and Austin, 1997; Bouet and Funnell, 1999; Erdmann et al., 1999). ParA is the prototype of a widespread and diverse superfamily of evolutionarily related proteins involved in a variety of functions, including plasmid and chromosome segregation, and cell division (Motallebi-Veshareh et al., 1990; Koonin, 1993). Members of the ParA superfamily are unified further by the presence of a conserved ATP-binding motif, which is a distinctive variant of the type I nucleotide-binding motif (Motallebi-Veshareh et al., 1990). The nucleotide hydrolysis activity of ParA may be implicated in the directed movement of P1 plasmid copies associated with a host-encoded partition apparatus, which rapidly guides the plasmids to specific intracellular locations soon after P1 DNA replication has been completed, or may be involved in some other aspect of the assembly of an active partition complex (Davis et al., 1992; 1996; Davey and Funnell, 1994; 1997; Gordon et al., 1997; Rodionov et al., 1999).
In addition to its role at parS, ParA is a DNA-binding protein: a helix–turn–helix motif near the C-terminal end of the protein is required for the recognition by ParA of an operator site that overlaps the parAB operon promoter (Davis et al., 1992; Hayes et al., 1994; Radnedge et al., 1998). Binding of ParA to the operator sequence, which is enhanced by DNA, ADP and ParB, substantially represses parAB operon transcription, thereby maintaining the concentrations of the ParAB proteins at appropriate levels for partition (Friedman and Austin, 1989; Davis et al., 1992; Davey and Funnell, 1994; Hayes et al., 1994). Amino acid residues in the C- and N-terminal domains of ParA and ParB, respectively, are involved in the contacts between these proteins at both the operator and the parS sites (Radnedge et al., 1996; 1998).
Most plasmid partition cassettes that have been analysed to date have been identified fortuitously as accessory stabilizing sequences flanking the plasmid replicon. Nevertheless, those partition systems that have been tested can also promote the segregational stability of heterologous replicons in a variety of genetic contexts, indicating that there is no a priori reason to presume that all partition cassettes are closely associated with the replicon of the plasmid on which they reside. Furthermore, the analysis only of partition genes that are physically associated with their cognate replicons may limit the range of partition systems that are characterized. In this study, plasmid TP228 was screened for the presence of an active partition system without regard to the location of the TP228 plasmid replicon. The conjugative plasmid TP228 (72 kb) was originally identified in Salmonella newport; it specifies resistance to a range of antibiotics (kanamycin, neomycin, spectinomycin, streptomycin, sulphonamides and tetracycline) and mercuric ions and is a member of plasmid incompatibility group X1 (Bradley, 1980; Couturier et al., 1988; Jones et al., 1993). TP228 and other multiply resistant IncX plasmids are thought to have arisen through the acquisition of mobile antibiotic resistance elements by preantibiotic era precursor plasmids (Jones et al., 1993). The TP228 segregation system described here includes a novel member of the ParA superfamily, which exemplifies a group of putative partition proteins whose genes are widely disseminated on eubacterial genomes.
Results
Isolation of the partition cassette of plasmid TP228
The multidrug resistance plasmid TP228 replicates at low copy number but exhibits no detectable loss during 25 generations of unselected growth in E. coli. It is reasonable to presume that the segregational stability of TP228 is promoted by one or more accessory stability systems, as is the case with other large, low-copy-number plasmids (Nordström and Austin, 1989; Williams and Thomas, 1992). Plasmid pALA136 is a plasmid that replicates conditionally at low copy number via the P1 replicon but contains no additional stabilizing sequences (Martin et al., 1987). This plasmid can be used as a partition probe vector by constructing a library of target plasmid fragments in the vector with subsequent testing for members of the library that exhibit enhanced segregational stability at low copy number (Hayes, 1998). The TP228 partition cassette was isolated in this manner by screening a library of plasmid fragments generated by partial Sau3AI digestion of TP228 followed by cloning in the BamHI site of pALA136 (for further details, see Experimental procedures). Approximately 10% of the recombinant plasmids in this library exhibited a 15- to 50-fold increase in segregational stability compared with pALA136 itself. This frequency of stabilizing inserts was similar to that obtained recently in analogous experiments with another IncX plasmid, R485 (Hayes, 1998). Examination of the restriction patterns of selected recombinant plasmids with enhanced stability revealed the presence of two apparently unrelated TP228-derived insert fragment types (data not shown). One recombinant plasmid (pFH263) with a 12 kb insert and a high level of segregational stability was chosen for further study.
Molecular organization of the TP228 partition cassette
A 5.0 kb NsiI subfragment of pFH263, which was implicated in the partition phenotype by a combination of transposon Tn5 mutagenesis (data not shown) and subcloning in pALA136 or its derivative pFH450, promoted partition as effectively as did the 12 kb fragment present in pFH263 without any apparent increase in plasmid copy number (Fig. 1). Further subcloning and testing refined the partition locus to a 1472 bp StuI–ScaI fragment. This region contains two complete open reading frames (ORFS), parF and parG, which potentially specify proteins of 206 and 76 amino acids respectively (Fig. 1). ParF has a predicted molecular weight of 22.0 and a pI of 5.65, whereas ParG has an expected molecular weight of 8.6 and a pI of 9.25. Protein species with these approximate molecular weights were visualized by in vitro transcription–translation experiments using a DNA template harbouring the parFG genes (Fig. 2).
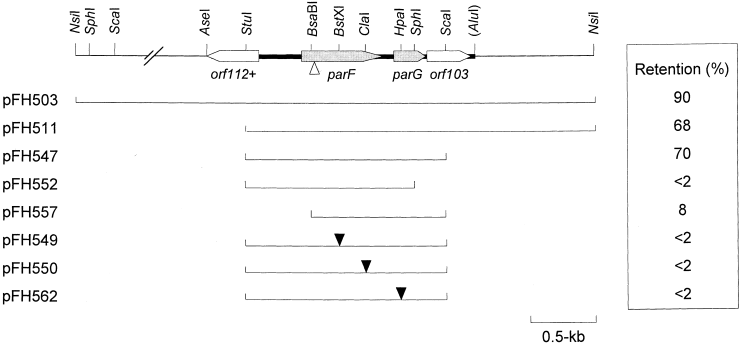
Genetic organization of the parFG genes and flanking regions from plasmid TP228. Arrows indicate the length and orientation of ORFs. The thick line indicates the extent of the region whose nucleotide sequence was determined. The positions of relevant restriction enzyme sites are indicated. The AluI site is shown in parentheses to indicate that additional Alu sites are present. Lines beneath the map denote regions cloned in the stability probe vector pFH450. The levels of retention conferred by these fragments after approximately 25 generations in the absence of selective pressure in a polA strain are shown. Filled and open triangles indicate the respective insertion positions of a 2.1 kb (pFH549 and pFH550) or 4.3 kb (pFH562) spectinomycin cassette within the 1472 bp StuI–ScaI fragment and of Tn5 within a 12 kb fragment, which included the 1472 bp StuI–ScaI fragment.
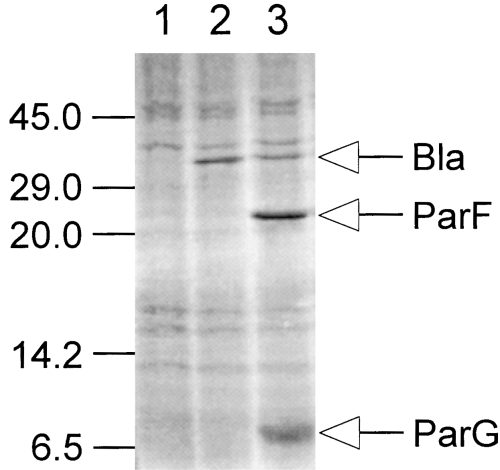
In vitro transcription–translation studies of the parFG genes of TP228. Plasmids pMPM-A3 and pFH553, which consists of the parFG genes cloned in pMPM-A3, were linearized with BamHI and used as templates in in vitro transcription–translation experiments. The positions of the Bla protein specified by both plasmids and the ParF and ParG proteins produced by pFH553 are indicated. The positions of molecular weight markers (in kDa) are indicated on the left. Lanes: 1, no template DNA added to the reaction; 2, pMPM-A3 template; 3, pFH553 template.
The parF and parG genes are apparently transcribed in the same direction but are separated by a 54 bp intergenic spacer (Fig. 1). The region immediately upstream of parG includes a sequence with a 5/9 match to the consensus ribosome binding site motif of E. coli, suggesting that parG may be transcribed and translated independently of parF. The putative ribosome binding site upstream of the latter is less readily identifiable than that of parG.
The parF and parG genes were both necessary for partition (Fig. 1): insertion of a 2.1 kb spectinomycin resistance cassette after either codon 109 or codon 175, a Tn5 insertion after codon 45 or deletion of the N-terminal 38 codons and upstream sequences of parF reduced the segregational stability of the test plasmid to background levels. Similarly, deletion of the C-terminal 23 codons or the introduction of a 4.3 kb spectinomycin resistance cassette after codon 37 of parG abolished the stabilization effect. An ORF downstream of parG and apparently transcribed in the same direction and an incomplete ORF upstream of parF but transcribed on the opposite DNA strand were not required for stability (Fig. 1).
Identification of ParF homologues from a diversity of sources
Database searching identified ParF homologues specified by plasmids ece1 from Aquifex aeolicus (AA35; Deckert et al., 1998), pCL1 from Chlorobium limicola (Orf2.4; GenBank accession number AAB36936), pCLP from Mycobacterium celatum (ParA; Picardeau et al., 2000), pVS1 from Pseudomonas aeruginosa (StaA; GenBank accession number AAD19678), pRA2 from Pseudomonas alcaligenes (ParA; Kwong et al., 2000), p4180A from Pseudomonas syringae (Par; GenBank accession number AAD50907), pRmeGR4a from Sinorhizobium meliloti (ParA; Herrera-Cervera et al., 1998), pTAR from Agrobacterium tumefaciens (ParA; Gallie and Kado, 1987), pB171 from E. coli (Orf69; Tobe et al., 1999), by the virulence plasmids of Agrobacterium sp. and Rhizobium etli (VirC1; Close et al., 1987) and by the chromosomes of Helicobacter pylori (ParA; Tomb et al., 1997) and Zymomonas mobilis (ParA; GenBank accession number AAF23806). (Fig. 3). These homologues are of similar size (209–240 amino acids) to the ParF protein encoded by TP228. The homology among members of the ParF subgroup extends through the lengths of the proteins, but the N-terminal halves are more highly conserved than the C-terminal segments. The VirC1 proteins specified by Agrobacterium sp. virulence plasmids pTiC58, pTiA6Nc, pRiA4B and R. etli plasmid pa are closely related [> 85% identity; as described previously by Close et al. (1987)], but share a comparatively low level of identity with other ParF family members (Table 1). Excluding the VirC1 proteins, the ParF homologues encoded by plasmids pVS1, pRA2, pCLP, pCL1 and ece1 are most alike (30–78% identity) (Table 1). The ParF protein of TP228 exhibits most similarity to the homologues specified by plasmids pCL1, pRA2 and pVS1. The similarities between certain ParF homologues and members of the ParA superfamily have been noted previously (Motallebi-Veshareh et al., 1990; Koonin, 1993; Dominy et al., 1998; Herrera-Cervera et al., 1998; Tobe et al., 1999; Kwong et al., 2000). Furthermore, the ParF homologue of pTAR promotes plasmid partition in a variety of Rhizobiaceae (Gallie et al., 1985; Gallie and Kado, 1987), further supporting a role for ParF family members in plasmid partition.
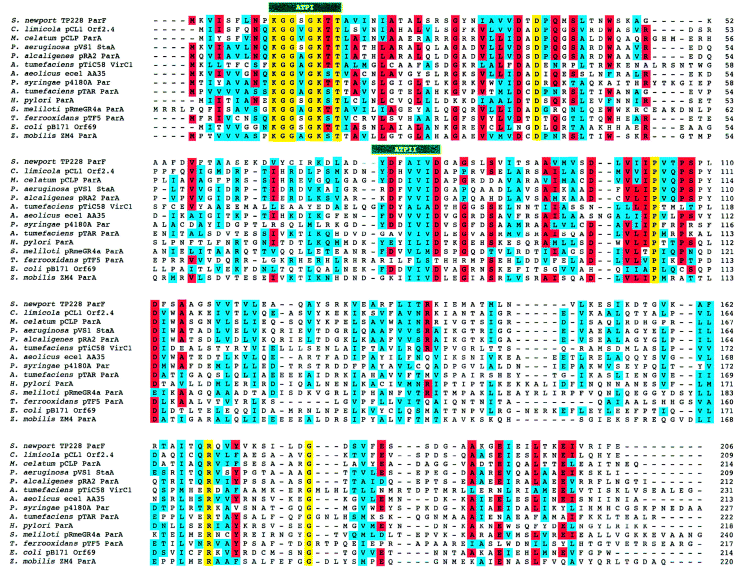
Amino acid sequence alignment of ParF homologues. Identical and similar residues that are conserved in a majority (more than eight) of homologues are outlined in red and blue respectively. Amino acid residues conserved in all proteins are indicated in yellow. The locations of the two patches (ATPI and ATPII) that constitute the ATP-binding motif are shown. Note that the ParF family includes four VirC1 homologues, which share > 85% identity, but that, for clarity, only one of these homologues is included in the alignment.
| TP228ParF | pCL1Orf2.4 | pVS1StaA | pRA2ParA | ece1AA35 | H. pylori ParA | pTAR ParA | pTF5ParA | pRmeGR4a ParA | pTiC58VirC1 | pCLP ParA | pB171Orf69 | p4180A Par | Z. mobilis ParA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP228 ParF | 100 | 32 | 28 | 29 | 25 | 22 | 23 | 20 | 19 | 19 | 27 | 21 | 21 | 22 |
| pCL1 Orf2.4 | 100 | 44 | 45 | 30 | 22 | 18 | 23 | 19 | 16 | 45 | 19 | 24 | 18 | |
| pVS1 StaA | 100 | 78 | 33 | 19 | 24 | 25 | 20 | 17 | 35 | 22 | 22 | 22 | ||
| pRA2 ParA | 100 | 31 | 19 | 24 | 25 | 22 | 18 | 34 | 20 | 22 | 21 | |||
| ece1 AA35 | 100 | 23 | 20 | 18 | 21 | 16 | 25 | 25 | 25 | 19 | ||||
| H. pylori ParA | 100 | 21 | 17 | 17 | 17 | 19 | 24 | 21 | 20 | |||||
| pTAR ParA | 100 | 21 | 20 | 18 | 19 | 17 | 22 | 63 | ||||||
| pTF5 ParA | 100 | 19 | 18 | 19 | 15 | 18 | 19 | |||||||
| pRmeGR4a ParA | 100 | 19 | 21 | 18 | 17 | 18 | ||||||||
| pTiC58 VirC1 | 100 | 17 | 14 | 15 | 22 | |||||||||
| pCLP ParA | 100 | 21 | 27 | 18 | ||||||||||
| pB171 Orf69 | 100 | 25 | 16 | |||||||||||
| p4180A Par | 100 | 20 | ||||||||||||
| Z. mobilis ParA | 100 |
The ParF homologues form a discrete subgroup of the ParA superfamily
The ParF homologues are members of the diverse ParA superfamily of bacterial ATPases, which also includes the ParA protein of the P1 plasmid and related partition proteins, the Soj group of chromosome partition proteins, the MinD group of cell division proteins and a number of other proteins implicated in plasmid replication or stability (see Fig. 4; Motallebi-Veshareh et al., 1990; Ogasawara and Yoshikawa, 1992; Williams and Thomas, 1992; Koonin, 1993). Members of the ParA superfamily are characterized in part by the presence of an ATP-binding motif, which is a variant of the archetypical type I nucleotide-binding motif (Motallebi-Vesherah et al., 1990; Koonin, 1993). This motif is composed of two patches with the amino acid consensus KGGvgK[ST] and dXdXXXXDXXp. A further variation of this motif with the consensus [NS]XKGGXGK[ST]T and dXXXXdXXg is present in the putative ATP-binding motif in ParF family members (Fig. 3).
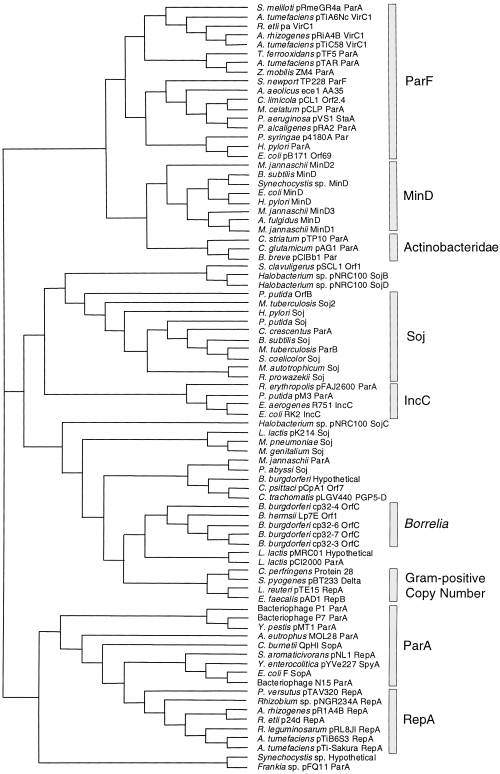
Unrooted cladogram of a non-exhaustive set of 83 members of the ParA superfamily. The major identifiable subgroups are indicated on the right. The MinD and Soj subgroups each currently contain > 20 proteins, of which representative members only are included. The relationships among the MinD and Borrelia OrfC proteins presented here parallel those described previously (Gerard et al., 1998; Stevenson et al., 1998). Note that a number of ParA superfamily members may have been misnamed, e.g. the Caulobacter crescentus ParA and Mycobacterium tuberculosis ParB proteins are members of the Soj subgroup. Note that the lengths of the horizontal bars do not reflect evolutionary distance. Database accession numbers: Aquifex aeolicusAA35, AAC07976; Alcaligenes eutrophusMOL28 ParA, S61914; Archaeoglobus fulgidus MinD, AAB90541; Agrobacterium rhizogenes pRiA4B RepA, P05682; A. rhizogenes pRiA4B VirC1, P13459; Agrobacterium tumefaciens pTAR ParA, P07175; A. tumefaciens pTiA6Nc VirC1, P06665; A. tumefaciens pTiB6S3 RepA, A32812; A. tumefaciens pTiC58 VirC1, P07165; A. tumefaciens pTi-Sakura RepA, BAA25270; Bacteriophage N15 ParA, AAC19064; Bacteriophage P1 ParA, P07620; Bacteriophage P7 ParA, S06099; Bifidobacterium breve pCIBb1, AAD34708.1; Bacillus subtilis MinD, Q01464; B. subtilis Soj, P37522; Borrelia burgdorferi cp32-3 OrfC, AAC35443; B. burgdorferi cp32-4 OrfC, AAC35447; B. burgdorferi cp32-6 OrfC, AAC35451; B. burgdorferi cp32-7 OrfC, AAC35455; B. burgdorferi Hypothetical, AAC66805; Borrelia hermsii LpTE Orf1, AAB17955; Coxiella burnetii QpHI SopA, CAA75819; C. crescentus ParA, AAB51267; Corynebacterium glutamicum pAG1 ParA, AAD25059; Chlorobium limicola pCL1 Orf2.4, AAB36936; Clostridium perfringens Protein 298, I40880; Chlamydia psittaci pCpA1 Orf7, CAA44339; Corynebacterium striatum pTP10 ParA, AAC95478; Chlamydia trachomatis pLGV440 PGP5-D, P10559; E. coli F SopA, P08866; E. coli MinD, P18197; E. coli pB171 Orf69, BAA84904; E. coliRK2 IncC, P07673; Enterobacter aerogenesR751IncC, Q52312; Enterococcus faecalis pAD1 RepB, B47092; Frankia sp. pFQ11 ParA, AAB88413; Halobacterium sp. pNRC100 SojB, AAC82813; Halobacterium sp. pNRC100 SojC, AAC82849; Halobacterium sp. pNRC100 SojD, AAC82871; Helicobacter pylori MinD, AAD07400; H. pylori ParA, AAD08045; H. pylori Soj, AAD08185; Lactococcus lactis pCI2000 ParA, AAF27301; Lactococcus lactis pK214 Soj, CAA63500; Lactobacillus lactis pMRC01 Hypothetical, AAC56023; Lactobacillus reuteri pTE15 RepA, AAC02983; Methanococcus autotrophicum Soj, AAB85931; Mycobacterium celatum pCLP ParA, AAD42964; Mycoplasma genitalium Soj, AAC72491; Methanococcus jannaschii MinD1, AAB98539; M. jannaschii MinD2, AAB98570; M. jannaschii MinD3, Q57633; M. jannaschii ParA, Q60283; Mycoplasma pneumoniae Soj, S62837; M. tuberculosis Soj2, CAB08303; M. tuberculosis ParB, CAA16231; Pyrococcus abyssi Soj, CAB50199; Pseudomonas aeruginosa pVS1 StaA, AAD19678; Pseudomonas alcaligenes pRA2, AAD40334; Pseudomonas putida pM3 ParA, AAD46128; P. putida OrfB, AAC08068; P. putida Soj, P31856; Pseudomonas syringae p4180A Par, AAD50907; Paracoccus versutus pTAV320 RepA, AAC83387; Rhodococcus erythropolis pFAJ2600 ParA, AAC45808; Rhizobium etli pa VirC1, AAD55073; R. etli p24d RepA, AAB69096; Rhizobium sp. pNGR234A RepA, P55393; Rhizobium leguminosarum pRL8JI RepA, CAA61616; Rickettsia prowazekii Soj, CAA14529; Sphingomonas aromaticivorans pNL1 RepA, AAD03874; Streptomyces clavuligerus pSCL1 Protein 1, S30400; Streptomyces coelicolor Soj, AAC03484; Sinorhizobium meliloti pRmeGR4a ParA, CAA11245; S. newportTP228 ParF, AAF74217; Streptococcus pyogenes pBT233 Delta, S68604; Synechocystis sp. Hypothetical, BAA10648; Synechocystis sp. MinD, Q55900; Thiobacillus ferrooxidans pTF5 ParA, AAC80179; Yersinia enterocolitica pYVe227 SpyA, AAD16853; Yersinia pestis pMT1 ParA, AAC82736; and Zymomonas mobilisZM4 ParA, AAF23806.
ParA superfamily members are unified by evolutionary relatedness and, in part, by shared functionality (Motallebi-Veshareh et al., 1990; Koonin, 1993). Recent advances in genome sequencing have increased the number of known ParA family members to > 100, thereby facilitating a re-evaluation of evolutionary relationships within the family (Fig. 4). A number of distinct subgroups are readily identifiable. The ParA partition protein of the P1 plasmid is the prototype of the ParA superfamily, and the P1 parA gene forms an operon with the parB partition gene (Abeles et al., 1985). The P1 ParA protein is most closely related to homologues, e.g. the P7 plasmid ParA protein (Ludtke et al., 1989) and the F plasmid SopA protein (Ogura and Hiraga, 1983), which similarly are elements of two-component partition systems. These ParA homologues and a set of proteins (RepA) implicated in plasmid copy number control and segregational stability (Bartosik et al., 1998) form a single clade within the ParA superfamily (Fig. 4). The repA genes are also physically associated with parB homologues. Furthermore, although members of the ParF and ParA/RepA subgroups are both involved in plasmid partition, they form discrete branches of the ParA superfamily (Fig. 4).
The ParF and ParA/RepA subgroups show strong conservation of different amino acid residues: only six of 15 amino acids absolutely conserved in 16 members of the ParA/RepA group are present in all 17 members of the ParF group (K10, G11, G12, K15, D39 and P104 in TP228 ParF) (Fig. 5). Of these six commonly conserved residues, four are located in the ATP-binding motif. Four other amino acids (G14, T17, R169 and G179 in TP228 ParF) are uniquely conserved in the ParF subgroup, whereas nine other residues are conserved only in the ParA/RepA subgroup. Furthermore, the spacing between the ATP patches in the primary sequence of ParF subgroup members is shorter compared with that in members of the ParA/RepA subgroup (Fig. 5).
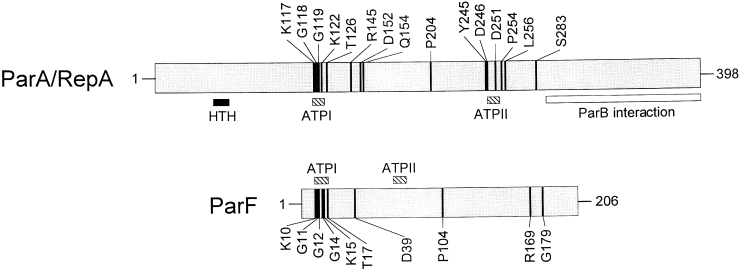
Comparison of fully conserved residues in the ParA/RepA (16 proteins) and ParF (17 proteins) subgroups of the ParA superfamily. The positions of residues conserved in all subgroup members are indicated using the ParA protein of P1 and the ParF protein of TP228 as examples. The two proteins are aligned from the conserved lysine residues (K117 in P1 ParA and K10 in TP228 ParF) in the ATPI patches of the ATP-binding motifs. The positions of the ATP-binding motifs are indicated by hatched boxes. Filled and open boxes beneath P1 ParA denote the locations of the helix–turn–helix (HTH) motif, which is necessary for ParA binding to the parAB operon operator site (Hayes et al., 1994; Radnedge et al., 1998), and the region that interacts with ParB (Radnedge et al., 1998) respectively.
In addition to the ParF and ParA/RepA subgroups, a number of other subgroups were distinguishable in the ParA superfamily: (i) the Soj chromosome partition proteins that are present in a range of eubacteria and archaebacteria (Ogasawara and Yoshikawa, 1992; Gal-Mor et al., 1998); (ii) the MinD cell division proteins that are similarly widely dispersed (de Boer et al., 1989; Gerard et al., 1998); (iii) proteins that are implicated in plasmid copy number control in Gram-positive bacteria (Weaver et al., 1993); (iv) proteins related to the IncC partition protein of plasmid RK2 (Macartney et al., 1997); and (iv) separate groups of putative partition proteins encoded by linear and circular plasmids of Borrelia sp. (Barbour et al., 1996; Zückert and Meyer, 1996; Stevenson et al., 1998) and Actinobacteridae (O'Riordan and Fitzgerald, 1999) (Fig. 4).
Homologues of the parG gene of TP228 are present on plasmids of Pseudomonas sp.
The parG gene is necessary for plasmid segregation mediated by the TP228 partition system (Fig. 1). Homologues of parG are also located downstream of the parF homologues on plasmids pVS1 and pRA2 from Pseudomonas sp. (Fig. 6). The parF and parG homologues on plasmids TP228, pVS1 and pRA2 are separated by 54, 20 and 21 bp respectively. The putative ParG proteins encoded by these three plasmids are 20–43% identical (Fig. 7), but exhibit no homology to either the ParB protein specified by the P1 plasmid or other protein sequences in the GenBank database. Other parF homologues, e.g. on plasmid pTF5 and the H. pylori chromosome, are accompanied by short, downstream ORFs unrelated to parG (Fig. 6), but the function of these ORFs remains to be elucidated.
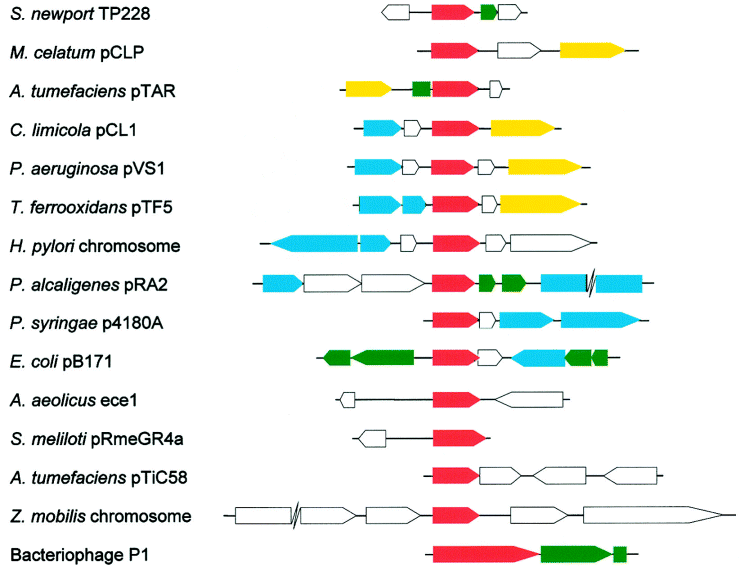
Comparative genomic organization of parF genes and flanking regions. Regions are aligned from the N-termini of the parF homologues (indicated by red arrows) present in each genome. Putative replication genes, genes for transposases or site-specific recombinases and genes other than parF which are or may be implicated in segregational stability, are denoted by yellow, blue and green arrows respectively. Other ORFs are indicated by open arrows. For simplicity, the genomic organization of sequences flanking only one of four known virC1 genes is included. The parABS partition cassette of P1 is included for comparison purposes.
Genomic organization of sequences flanking parF homologues
The genetic organization of sequences flanking the 17 known parF homologues is variable (Fig. 6). Of the 10 parF-containing genomes for which sufficient flanking sequence information (> 2 kb upstream and downstream of parF) is present in the GenBank database, five harbour a putative gene for DNA replication in close proximity to parF. The replication gene is positioned downstream of the parF homologue in pCL1, pVS1, pCLP and pTF5 but, in contrast, is located upstream of the parF homologue in pTAR. In all five instances, the parF homologue and the putative replication gene are apparently transcribed in the same direction. The five putative replication proteins share no extensive homology. Other plasmid partition modules, e.g. the P1, F and R1 partition genes, are linked closely to the replication initiation genes of their respective plasmids. The structural organization of plasmid genomes is dynamic. The common physical linkage between genes for plasmid replication and partition may minimize the frequency with which these critical modules become separated during plasmid genome rearrangements. Nevertheless, it is apparent that many parF genes are not closely associated with the replicon of the plasmid on which they reside (Fig. 6).
Seven of the 10 parF homologues for which sufficient flanking sequence information (> 2 kb upstream and downstream of parF) is available are situated near one or more genes specifying putative DNA cut-and-paste enzymes (transposases or site-specific recombinases), which potentially induce genome reorganizations. It remains to be determined whether this common genetic organization among parF-containing genomes is coincidental and reflects the frequent occurrence of genes for DNA cut-and-paste enzymes on plasmid genomes, or whether parF genes are associated with active or degenerate mobile genetic elements.
Incompatibility properties of the TP228 partition cassette
Plasmid partition sites are incompatibility determinants. This is most easily explained if plasmids are considered as a pool from which individuals are withdrawn randomly for pairwise segregation. Plasmids that harbour identical partition sites cannot be distinguished from each other during the partition process, and random assortment ultimately gives rise to populations that have one or other plasmid but not both (Austin and Nordström, 1990). Plasmids based on the p15A moderate-copy-number replication origin and the P1 low-copy-number replicon are compatible and, therefore, can be used in assessing the incompatibility properties of relevant sequences that are cloned in pairs of vectors replicating via these replicons. Plasmids were constructed that harboured the parFG genes and identical flanking regions and that replicated via the p15A (pFH553) or P1 (pFH554) origins. Plasmid pFH554 was strongly incompatible with pFH553 (Fig. 8). In contrast, pFH554 was segregationally stable in the presence of the p15A-based vector without the cloned TP228 partition cassette. Therefore, the TP228 partition cassette contains an incompatibility locus.
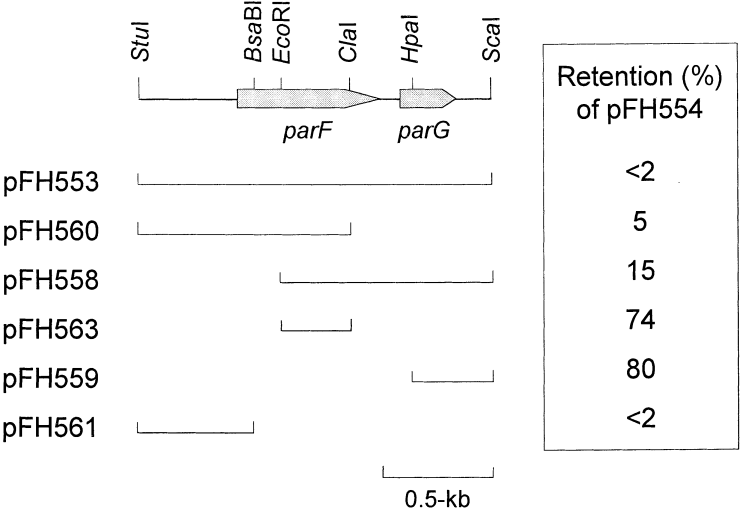
Incompatibility determinants in the TP228 partition cassette. Arrows indicate the length and orientation of the parFG genes. The positions of relevant restriction enzyme sites are indicated. Lines beneath the map denote regions cloned in the pMPM-A3 vector. The levels of retention of plasmid pFH554, which replicates via the P1 replicon and harbours the parFG genes and flanking sequences, are shown in the presence of the relevant pMPM-A3-based plasmid after approximately 25 generations in the absence of selective pressure for pFH554. Plasmid pFH554 is retained at a level of ≈ 75% in the presence of pMPM-A3 without a cloned insert.
A set of p15A-based plasmids that contained subfragments of the TP228 partition region were tested for incompatibility towards pFH554 (Fig. 8). Plasmids harbouring either the N-terminal 175 codons of parF and 405 bp upstream of parF (pFH560) or the C-terminal 138 codons of parF, the intergenic sequences between parF and parG, the complete parG gene and 164 bp sequences downstream of parG (pFH558) expressed strong but incomplete incompatibility towards pFH554. The region of overlap between the insert DNA in these plasmids resides in the 225 bp EcoRI–ClaI fragment within the parF gene (Fig. 8). However, this fragment was insufficient for incompatibility (pFH563). One possible explanation for this apparent discrepancy is that expression of the parG and truncated parF genes separately in pFH558 and pFH560, respectively, may perturb partition and result in the incompatibility effects observed with these plasmids. It is known that inappropriate expression of plasmid partition genes, e.g. the parAB genes of the P1 plasmid (Abeles et al., 1985; Funnell, 1988b; Hayes et al., 1994; Lobocka and Yarmolinsky, 1996), can dramatically impair partition.
A 518 bp fragment that consisted of the upstream sequences and the first 37 codons of parF exerted incompatibility towards the complete partition region of TP228 present in pFH554 (pFH561; Fig. 8). As the 518 bp fragment is largely non-coding, it is tempting to speculate that the TP228 partition site is located within this fragment. Further analysis of the incompatibility properties of the TP228 partition cassette will illuminate these observations.
The parS site of the P1 partition cassette is an incompatibility determinant (Austin and Abeles, 1983). However, the TP228 and P1 partition modules do not exert mutual incompatibility, as assessed by incompatibility experiments similar to those described in the preceding section (data not shown).
Discussion
The plasmid-specified components of the active partition cassette of plasmid TP228 and the prototypical partition systems of the F, P1, RK2 and R1 plasmids exhibit pronounced dissimilarities. Each of the latter group includes two proteins (ParAB in the P1 system), which are produced from an autoregulated operon and interact with the cis-acting partition site (parS in the P1 system) situated near the partition genes (Nordström and Austin, 1989; Williams and Thomas, 1992). The TP228 partition system also requires two plasmid-encoded proteins, one of which (ParF) is a novel but widely disseminated member of the ParA superfamily. The ParF protein (206 amino acids) is approximately half the size of ParA (398 amino acids) and, apart from residues in their ATP-binding motifs, the subgroups of which the two proteins are representative exhibit different patterns of highly conserved amino acids (Fig. 5). The ParA protein of the P1 plasmid is an ATPase whose nucleotide hydrolysis activity is required for partition (Davis et al., 1992; 1996). ATP hydrolysis by ParA may provide the energy required for the directed movement of P1 plasmid copies from the cell centre to the one- and three-quarter cell length positions, which occurs soon after plasmid replication has been completed, or may be involved in some other aspect of the partition process (Gordon et al., 1997). ParF is also a putative ATPase, which suggests that this protein may play a role in the TP228 partition system similar to that performed by the ParA protein in the segregation of P1. This notion is strengthened by the observation that ParF is a member of the superfamily of which the ParA protein is the archetype. This superfamily includes a number of distinct subgroups that are implicated in different but related cellular processes concerned with genome replication and stability, and cell division (Fig. 4; Motallebi-Veshareh et al., 1990; Koonin, 1993). The ParF protein and its immediate relatives form a discrete clade of the ParA superfamily, which is more closely related to the MinD subgroup of cell division proteins than to other plasmid or chromosome partition proteins (Fig. 4).
The second protein (ParG) in the TP228 partition system is evolutionarily unrelated to ParB of the P1 plasmid and has only two obvious homologues in the sequence databases. These homologues are specified by plasmids pRA2 and pVS1 of Pseudomonas sp. and are of unknown function. However, the genes for these homologues are located downstream of parF genes (as is the case with the parG gene of TP228), which suggests that these parG homologues may be components of the partition systems of these plasmids. The ParB protein is a DNA-binding protein that recognizes an array of motifs in the partition site arms (Davis and Austin, 1988; Funnell and Gagnier, 1993; Hayes and Austin, 1993). ParB binds the partition site in co-operation with integration host factor, which induces a bend approximately in the centre of the site (Davis and Austin, 1988; Funnell, 1988a; Hayes and Austin, 1994). ParA associates with the resulting nucleoprotein complex, which in turn is thought to interact with a host-specified partition apparatus (Youngren and Austin, 1997; Bouet and Funnell, 1999). The ParG protein of TP228 or a host-encoded protein may play a role analogous to that of ParB in the P1 system. However, the absence of a parB homologue in the TP228 partition cassette and the observation that the TP228 and P1 partition modules do not exert mutual incompatibility suggest that the architectures of the nucleoprotein complexes at the TP228 and P1 partition sites may differ. Furthermore, the markedly different physical and genetic organizations of the TP228 and ‘classical’ partition cassettes may reflect mechanistic differences in how active partition is promoted by these systems. Further analysis of the TP228 system, including identification of the partition site and elucidation of the functions of the ParF and ParG proteins, will address these questions.
The parF homologue and flanking sequences of pTAR from A. tumefaciens promote plasmid partition in a variety of Rhizobiaceae (Gallie et al., 1985; Gallie and Kado, 1987). The indispensable region downstream of parF in pTAR harbours neither a parG homologue nor any other essential ORF, and the role of this region in partition remains to be clarified, although recent evidence indicates that a nucleotide sequencing error is present in the published sequence of this region of pTAR (K. Kalnin, unpublished observations). The indispensable region upstream of the parF homologue of pTAR contains 12 copies of a heptanucleotide sequence, which are separated by integral turns of the DNA helix (Gallie and Kado, 1987). These repeat motifs may either be bound by a protein that is involved in the regulation of parF homologue expression (parF homologue expression is autoregulated in pTAR) and/or constitute the partition site. The equivalent region upstream of parF in TP228 contains no obvious comparable repeat motifs, although, like the region upstream of the parF homologue in pTAR, it appears to harbour an incompatibility determinant. Thus, although the partition modules of both TP228 and pTAR include parF homologues, the different genetic organizations of the critical elements that flank these homologues suggest that partition of TP228 and pTAR may be dissimilar, which might partly reflect the different hosts from which these plasmids originated. In this regard, it is interesting that the pTAR partition cassette does not promote plasmid segregational stability in E. coli (F. Hayes, unpublished data).
Complete sequence information is currently available for six parF-containing genomes. In the cases of four of these genomes (ece1, pCL1, pRA2 and pTF5), parF is the only evident segregational stability gene, indicating that this system alone may be sufficient for accurate plasmid distribution in at least some instances. In contrast, the H. pylori chromosome apparently harbours the soj/spo0J segregation system in addition to a parF homologue (Tomb et al., 1997), and the parF homologue on plasmid pB171 is embedded in a region that includes three other putative segregational stability systems, viz. homologues of the stbAB partition system of plasmid R1, of the ccdAB post-segregational cell-killing system of the F plasmid and of a resolvase implicated in the monomerization of F plasmid multimers (Tobe et al., 1999; Fig. 6). Other plasmids are also known to specify multiple mechanisms that co-operatively promote plasmid segregational stability (Nordström and Austin, 1989; Williams and Thomas, 1992). Nevertheless, as parF genes are widely disseminated on the genomes of eubacteria, the use of a ParF homologue may be a common strategy by which plasmids promote their segregational stability.
Experimental procedures
Bacterial strains and growth conditions
E. coli DH5α (endA1 hsdR17 (rk− mk−) supE44 thi1 recA1 gyrA96 (Nalr) relA1 Δ(lacZYA-argF)U169 deoR (φ80dLacδ (lacZ)M15)) (Woodcock et al., 1989) was used for plasmid propagation, cloning and preparation of plasmid templates for sequencing reactions. Strain BR825 is polA (Ludtke et al., 1989) and was used in plasmid partition assays. Strains were grown in Luria–Bertani medium at 37°C with antibiotics for plasmid selection when appropriate. Antibiotics were used at the following concentrations (µg ml−1): ampicillin, 100; chloramphenicol, 10; tetracycline, 5.
Plasmids
Plasmids used in this study are described in Table 2.
| Plasmid | Descriptiona | Reference |
|---|---|---|
| pALA136 | 7.8-kb cloning vector containing P1 and ColE1 replicons; Cmr | Martin et al. (1987) |
| pALA1557 | P1 parABS cassette cloned in pALA136 | Radnedge et al. (1997) |
| pFH263 | 12-kb partial Sau3A1 fragment of TP228 inserted in BamHI-cleaved pALA136 | This study |
| pFH450 | pALA136 containing a synthetic multiple cloning site | Hayes (1998) |
| pFH503 | 5.0-kb NsiI fragment of pFH263 cloned in NsiI-cleaved pFH450 | This study |
| pFH511 | pFH503 digested with StuI and religated; equivalent to pFH450 containing 2.6-kb subfragment of pFH503 | This study |
| pFH547 | 1472-bp SalI-ScaI fragment of pFH511 cloned in SalI-EcoRV-cleaved pFH450 | This study |
| pFH549 | 2.1-kb SmaI Spr fragment of pHP45Ω inserted in BstXI-cleaved pFH547 | This study |
| pFH550 | 2.1-kb SmaI Spr fragment of pHP45Ω inserted in ClaI-cleaved and end-filled pFH547 | This study |
| pFH552 | 1239-bp StuI-SphI fragment of pFH547 cloned in StuI-SphI-cleaved pFH450 | This study |
| pFH553 | 1472-bp StuI-ScaI fragment of pFH511 cloned in EcoRV-cleaved pFH450 | This study |
| pFH554 | pFH547 digested with AseI and StuI, end-filled and religated resulting in deletion of the ColE1 origin sequence | This study |
| pFH557 | 951-bp BsaBI-SalI fragment of pFH553 cloned in StuI-SalI-cleaved pMPM-A3 | This study |
| pFH558 | pFH553 digested with EcoRI and religated | This study |
| pFH559 | pFH553 digested with SmaI and HpaI and religated | This study |
| pFH560 | pFH553 digested with ClaI and religated | This study |
| pFH561 | pFH553 digested with HincII and BsaBI and religated | This study |
| pFH562 | SmaI-linearized pHP45Ω inserted in HpaI-cleaved pFH547 | This study |
| pFH563 | pFH560 digested with EcoRI and religated | This study |
| pHP45Ω | Source of Spr fragment for insertion mutagenesis | Prentki and Krisch (1984) |
| pMPM-A3 | 3.4-kb cloning vector containing p15A replicon; Apr | Mayer (1995) |
| TP228 | 72-kb naturally-occurring plasmid; Kmr Nmr Spr Smr Sur Tcr Hgr | Bradley (1980) |
- a . Ap r, ampicillin resistance; Cmr, chloramphenicol resistance; Hgr, mercuric ion resistance; Kmr, kanamycin resistance; Nmr, neomycin resistance; Spr, spectinomycin resistance; Smr, streptomycin resistance; Sur, sulfonamide resistance; Tcr, tetracycline resistance.
DNA techniques
Plasmid DNA was prepared, manipulated and transformed by standard procedures (Sambrook et al., 1989). Plasmid templates for sequencing were prepared by alkaline lysis followed by precipitation with PEG-8000. Sequencing was by the dideoxynucleotide chain termination method (Sanger et al., 1977) using dye terminator chemistry, after which samples were run on an ABI Prism 377 DNA sequencer (Applied Biosystems). The sequence of the TP228 partition region and flanking regions was determined by primer walking on both template strands. Enzymes were obtained from New England Biolabs, Life Technologies or Helena Bioscience. Reagent-grade chemicals were supplied by Sigma Chemical.
Cloning of the TP228 partition system
To isolate segregational stability determinants from TP228, an analogous strategy was used to that described recently for the isolation of a stability cassette from plasmid R485 (Hayes, 1998). Briefly, a library of 4 to 6 kb fragments generated by partial Sau3AI digestion of TP228 was constructed by insertion into the BamHI site of the stability probe vector pALA136, followed by transformation of strain DH5α. In a PolA+ host, pALA136 replicates by the ColE1 origin and can be isolated and manipulated with ease. However, in a polA host, the ColE1 origin is non-functional, and replication switches to a low copy number under the control of the P1 replicon. As pALA136 does not possess accessory stability genes, it is unstable in this host in the absence of selective pressure. Insertion of a stability locus will restabilize the plasmid (Martin et al., 1987; Radnedge et al., 1997; Hayes, 1998). To identify recombinant pALA136 plasmids harbouring the TP228 partition system, the library of TP228 fragments in pALA136 was transformed into the polA strain BR825 with selection for pALA136-encoded chloramphenicol resistance. Colonies from this transformation were replica plated once on solid medium containing chloramphenicol and then successively 10 times on medium without antibiotic selection. At the end of this procedure, approximately 10% of colonies retained chloramphenicol resistance. Control experiments with pALA136 resulted in < 1% chloramphenicol resistance (Hayes, 1998). The apparent increase in the stability of plasmids isolated from chloramphenicol-resistant colonies was retested by retransforming candidate plasmids into the polA strain. After growth for approximately 25 generations in the absence of selective pressure (see the next section), the test plasmids were found to be maintained at levels of 30–95% compared with pALA136, which was maintained at a frequency of < 2%. One plasmid with a high level of segregational stability that was chosen for further study contained an approximately 12 kb insert. This relatively large insert resulted from contamination of the 4–6 kb purified fragments with higher molecular weight TP228 species.
Partition assays
The efficacy of the TP228 partition cassette and its subfragments were tested after growth for approximately 25 generations in the absence of selective pressure in a low-copy-number context essentially as described by Martin et al. (1987). Briefly, the pALA136- or pFH450-based plasmid containing the fragment to be tested was transformed into strain BR825 (polA) with selection for chloramphenicol resistance on LB medium. Eight randomly chosen colonies from this transformation were purified by streaking on chloramphenicol-containing LB medium and incubating at 37°C for 16 h. Individual colonies from each of these streaks were streaked on non-selective medium followed by 16 h incubations at 37°C, thereby providing approximately 25 generations of non-selective growth. The proportion of cells in these colonies that retained the plasmid was determined by streaking for single colonies on non-selective LB medium and testing eight colonies from each of these eight streaks, i.e. a total of 64 colonies, for retention of the test plasmid by stabbing to plates containing chloramphenicol. In this assay system, pALA136 or pFH450 without a cloned insert is retained at a frequency of < 2%. Assays were performed at least in triplicate. Standard deviations were < 10%.
Incompatibility assays
Plasmids pFH554 and pFH553 contain the parFG genes on a chloramphenicol resistance, low-copy-number plasmid that replicates via the P1 replicon and on an ampicillin resistance, moderate-copy-number plasmid that replicates via the ColE1 replicon (pMPM-A3) respectively. Incompatibility assays were performed by transforming pFH553 or its derivatives into strain DH5α pretransformed with pFH554 with selection only for ampicillin resistance specified by the incoming plasmid. Eight randomly chosen colonies from this transformation were purified by streaking on ampicillin-containing medium and incubating at 37°C for 16 h. The proportion of cells that retained pFH554 during the approximately 25 generations of growth in the absence of its selection was determined by testing eight colonies from each of these eight streaks, i.e. a total of 64 colonies, by stabbing to plates containing chloramphenicol. In this assay system, pFH554 is retained at frequencies of ≈ 75% and < 2% in the presence of pMPM-A3 and pFH553 respectively. Assays were performed at least in duplicate. Standard deviations were < 10%.
In vitro transcription–translation studies
An E. coli S30 extract system for linear templates (Promega UK) was used according to the manufacturer's instructions for in vitro transcription–translation studies of the parFG genes of TP228. Templates consisted of either the pMPM-A3 vector or the pMPM-A3 vector containing the parFG genes (pFH553). Templates were linearized with BamHI before use. Proteins were labelled with L-[35S]-methionine (Amersham Pharmacia Biotech UK). Reactions were analysed on 15% polyacrylamide gels run in Tris-borate buffer (Sambrook et al., 1989) at 25 mA for 3.5 h. Gels were dried and exposed to Kodak X-OMAT AR film (IBI).
Nucleotide and protein sequence analysis
Nucleotide sequences were analysed and translated using dna strider (Douglas, 1995). Homology searches were performed with the blast search programs (Altschul et al., 1997) using the National Center for Biotechnology Information World Wide Web interface. Protein sequences were aligned with the jalview World Wide Web-based multiple sequence alignment editor and manipulated further with boxshade. Cladograms were constructed from jalview alignments using the phylip (Felsenstein, 1989) and treeview (Page, 1996) packages. The nucleotide sequence of the parFG genes and flanking regions has been deposited in the GenBank database with accession number AF204292.
Acknowledgements
I thank Stuart Austin, Martine Couturier and Matthias Mayer for generous gifts of strains and plasmids and Daniela Barillà, Matt Bright and Ruth Grady for encouragement. This work was initiated while the author was the recipient of Wellcome Trust Research Career Development Fellowship 040822/Z/94/Z in the Department of Biochemistry, University of Oxford, Oxford, and was supported subsequently by Biotechnology and Biological Sciences Research Council research grants 36/G11604 and 36/G13032.




