Antisense inhibition of expression of the light subunit (35 kDa) of the Gal/GalNac lectin complex inhibits Entamoeba histolytica virulence
Abstract
One of the under-represented genes identified by cDNA representational difference analysis (RDA) between avirulent Entamoeba histolytica strain Rahman and virulent strain HM-1:IMSS was the amoebic light (35 kDa) subunit of the Gal/GalNac lectin complex. This lectin complex, which mediates the adhesion of the parasite to the target cell, also contains a heavy (170 kDa) subunit, which has the carbohydrate-binding domain. Stable transfectants of the virulent strain in which the expression of the 35 kDa subunit was inhibited by antisense RNA were not significantly affected in their adhesion activity to mammalian or bacterial cells but were strongly inhibited in their cytopathic activity, cytotoxic activity and in their ability to induce the formation of liver lesions in hamsters. These findings suggest that the 35 kDa subunit may have a specific function in the pathogenic pathway and provides a new insight into the role of this component of the Gal/GalNac lectin complex in amoebic virulence.
Introduction
The human intestinal protozoan parasite Entamoeba histolytica is the causative agent of the disease amoebiasis. A number of toxic molecules of the protozoan parasite E. histolytica that cause damage to the host cells and tissues have been identified and characterized. (i) Cell surface molecules engaged in recognition and adhesion to distinct receptors on host cells. These consist of a number of lectin-like molecules that recognize different carbohydrate components (Ravdin et al., 1985; Petri, 1996). (ii) Several cysteine and acidic proteinases that are released by the amoebae and degrade a variety of host components, such as matrix proteins, mucins and IgA (Bruchhaus et al., 1996; Que and Reed, 1997). (iii) Amoebapores — small protein molecules that form pores in the membranes of target cells and cause depolarization and cell death (Leippe, 1997). Virulent as well as avirulent strains of E. histolytica, which apparently cannot cause symptoms in humans, have been identified, but little is known about their difference in gene expression. In order to identify genes specifically expressed in a highly virulent strain of E. histolytica, we used the recently described, polymerase chain reaction (PCR)-coupled subtractive procedure of cDNA representational difference analysis known as cDNA RDA (Hubank and Schatz, 1994). This technique was used to identify differences in gene expression between two strains of E. histolytica : the highly virulent strain HM1:IMSS and the avirulent strain Rahman. Surprisingly, one of the genes found to be under-represented in the avirulent strain Rahman was the 35 kDa subunit of the Gal/GalNac-specific lectin. The Gal/GalNAc lectin is a heterodimeric molecule composed of a transmembrane heavy (170 kDa) subunit and glycosylphosphatidylinositol-anchored light 31 kDa and 35 kDa subunits, which are linked to the heavy subunit by disulphide bonds (Petri, 1996). Recently, the carbohydrate-binding domain has been shown to be localized on the 170 kDa heavy subunit (Dodson et al., 1999), and the cytoplasmic domain of the heavy subunit has been proposed to be involved in the regulation of adherence (Vines et al., 1998). Until now, no specific role has been attributed to the 35 kDa subunit. Using the antisense technology developed recently in our laboratory to inhibit the expression of cysteine proteinases (Ankri et al., 1998), we have managed to significantly inhibit the expression of the 35 kDa subunit in the virulent strain HM-1:IMSS. The results show that this inhibition results in a decrease in virulence of the amoeba.
Results
cDNA representational difference analysis
Using the technique described by Hubank and Schatz (1994), after three rounds of amplification–hybridization, transcripts more abundant in the ‘tester’ than in the ‘driver’, identified in the second and third round of amplification-hybridization, were gel purified and cloned in pGEM-5Zf(+) vector. According to the insert size, two independent inserts (clones 2.1 and 2.2) were isolated from the second round of amplification–hybridization, and two independent inserts (clones 3.1, 3.2) were isolated from the third round of amplification–hybridization. Southern blots of the starting representations (PCR product stage) were probed with the inserts from cloned subtracted products to confirm their uprepresentation in the HM-1:IMSS strain. As a control of basal expression, we used a probe for actin. Figure 1A shows that clone 2.1 is significantly more abundant (three times) in the HM-1:IMSS representation. To confirm that this uprepresentation reflects a higher expression of the corresponding mRNA in the HM-1:IMSS strain, dot-blot quantification and comparison of mRNA transcripts in HM-1:IMSS and Rahman strains was performed using the 2.1 insert as a probe. Figure 1B shows that mRNA of clone 2.1 is more abundant (two times) in the HM-1:IMSS strain compared with the Rahman strain. The insert of clone 2.1 (380 bp) was sequenced, and the resulting sequence was compared with the GenBank database using the blast program (Altschul et al., 1990) in which it was 100% homologous to the mRNA of the E. histolytica 35 kDa subunit of the Gal–lectin complex (accession no. L06065) (McCoy et al., 1993).
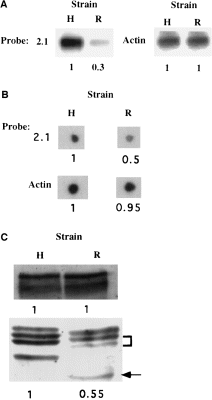
. A. Southern blots of representations probed with (i) insert 2.1 obtained from cloned difference products and (ii) actin. R, DNA from Rahman representation. H, DNA from HM-1:IMSS representation. Identical amounts of DNA (1 μg) were loaded in each lane. B. Dot-blot hybridization of amoebic RNA with labelled DNA probes prepared from actin or from insert 2.1 obtained from cloned difference products. R, RNA from strain Rahman. H, RNA from strain HM-1:IMSS. Identical amounts of total RNA (1 μg) were placed in each blot. C. Western blot analysis of heavy and light subunits of Gal/GalNAc lectin from E. histolytica trophozoites strain HM-1:IMSS (H) and Rahman (R). Identical amounts of protein (25 μg) were loaded in each lane. Numbers in bold represent densitometric quantification and are normalized by taking the value of densitometric quantification for HM-1:IMSS as 1.0 in each case.
Analysis of the 35 kDa subunit expression in avirulent strain Rahman
In order to find out whether the lower level of transcription of the 35 kDa subunit found in the avirulent strain Rahman is correlated with a lower level of 35 kDa subunit expression, we determined and compared by Western blot the intensity of the band patterns in strains HM-1:IMSS and Rahman. After SDS–PAGE of total lysates from Rahman and HM-1:IMSS trophozoites performed under reducing conditions, Western immunoblotting was performed with a polyclonal monospecific antibody raised against a recombinant 35 kDa subunit. As shown previously by Western immunoblots of SDS–PAGE with a monoclonal antibody against the 35 kDa subunit (McCoy et al., 1994), three distinct bands can be seen in the 35 kDa region in lysates of HM-1:IMSS trophozoites (Fig. 1C). An additional band of around 31 kDa is also recognized by the polyclonal antibody used in this study. In strain Rahman, two of the three bands around 35 kDa have lower intensity, and all three bands have a slightly lower molecular mass. In addition, the fourth band is strongly shifted to a lower molecular weight (Fig. 1C). The differences between the band patterns of the 35 kDa isoforms found in Western blots of strains HM-1:IMSS and Rahman could be the result of some structural differences, such as post-translational modifications. The lower intensity of two out of three bands in Rahman trophozoites compared with HM-1:IMSS trophozoites indicates that strain Rahman also has a deficiency in the expression of the 35 kDa subunits. This deficiency is in agreement with the lower transcription level of 35 kDa subunits seen in strain Rahman compared with HM-1:IMSS (Fig. 1B). Western blots reacted with a polyclonal antibody against the Gal–lectin 170 kDa subunit revealed that the levels of Gal–lectin heavy subunit in trophozoites of strains Rahman and HM-1:IMSS were similar (Fig. 1C). Under non-reducing conditions, Western blots reacted with antibody against the Gal–lectin 170 kDa subunit or against the 35 kDa subunit revealed that the level of Gal–lectin 260 kDa heterodimers in strains Rahman and HM-1:IMSS trophozoites were similar (Fig. 4).
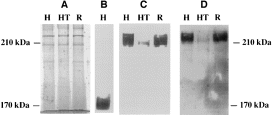
. A. Coomassie blue staining of SDS–PAGE gel under non-reducing conditions. B. Western blot analysis under reducing conditions of the Gal/GalNAc lectin complex from E. histolytica trophozoites probed with the anti-170 kDa gal–lectin subunit. C. Western blot analysis under non-reducing conditions of the Gal/GalNAc lectin complex from E. histolytica trophozoites probed with the anti-170 kDa gal–lectin subunit. D. Western blot analysis under non-reducing conditions of the Gal/GalNAc lectin complex from E. histolytica trophozoites probed with the anti-35 kDa subunit. Proteins from whole E. histolytica were resolved for 60 min at 200 V on 8% polyacrylamide gels (25 μg lane−1). Lanes: H, HM-1:IMSS; HT, HM-1:IMSS pSA20 transfectant grown with G418 (60 μg ml−1); R, Rahman.
Antisense inhibition of the 35 kDa subunit of the E. histolytica Gal/GalNac lectin complex
In order to find out whether the deficiency in the synthesis of the 35 kDa subunit is an important factor responsible for amoebic avirulence, we used the antisense technology, developed previously to inhibit cysteine proteinase expression in E. histolytica (Ankri et al., 1998), to inhibit the expression of the 35 kDa subunit in virulent strain HM1:IMSS. A plasmid construct pSA20 (Fig. 2A) containing a segment of the coding region (842 bp) of the 35 kDa subunit gene (obtained from plasmid gEh-35/1 kindly provided by Dr E. Tannich, Hamburg, Germany) was inserted in the opposite orientation between the 5′ and 3′ untranslated regions of the E. histolytica gene coding for ribosomal protein RP-L21 (ehg34 gene copy) (Petter et al., 1994). pSA20 was transfected into E. histolytica strain HM-1:IMSS and, after a gradual increase in the resistance to G418, the transfected trophozoites were subcultured continuously in the presence of 6 μg ml−1 or up to 150 μg ml−1 G418. Plasmid DNA was rescued from pSA20 trophozoites grown in the presence of 6 μg ml−1 or 60 μg ml−1 G418 and introduced into Escherichia coli cells to yield ampicillin-resistant transfectants. Restriction patterns of the rescued plasmid and the pSA20 plasmid were similar, showing that the plasmid remained intact in E. histolytica trophozoites (data not shown). The transformation efficiency between E. coli transformed with plasmid DNA rescued from pSA20 trophozoites grown in the presence of 6 μg ml−1 or 60 μg ml−1 G418 was ≈10 times, and this is in agreement with the finding of Haman et al. (1995) that the plasmid copy number is proportional to the level of resistance of the cultures to G418. In order to determine whether the antisense mRNA of the 35 kDa subunit is transcribed, the blot was probed with an enriched fraction of the sense single strand or double strand of the 35 kDa subunit gene (Fig. 2B).The mRNA bands in the antisense-transfected trophozoites were more intense for both probes, indicating that they transcribe an antisense mRNA. As a control, the blot was also probed with a double-stranded neo fragment and probed with a double-stranded actin fragment. As shown in 2Fig. 2B, the pSA20-transfected trophozoites grown with 60 μg ml−1 G418 specifically transcribe Neo mRNA. The basal level of transcription of the actin genes is similar in pSA20-transfected trophozoites and in the untransfected strain HM-1:IMSS.
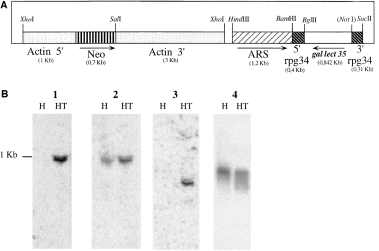
. A. Schematic depiction of plasmid pSA20. pSA20 is derived from plasmid pSA8 (Ankri et al., 1998). The size of plasmid pSA20 is 10.3 kbp. Arrows indicate the orientation of transcription. Numbers in brackets represent the size of each segment. Restriction site NotI was blunt ended for the purpose of the construction. B. Transcription of antisense 35 kDa subunit mRNA in E. histolytica HM-1:IMSS pSA20 transfectants. Total cellular RNA (10 μg) was size fractionated under denaturing conditions on agarose gels containing formaldehyde, transferred to a nylon membrane and hybridized under stringent conditions with probes of 35 kDa subunit sense strand (lane 1) and double-stranded (lane 2) as well as probes of Neo (lane 3) and actin (lane 4) coding regions. Lanes: H, HM-1:IMSS strain; HT, HM-1:IMSS pSA20 transfectant grown with G418 (60 μg ml−1).
The level of 35 kDa subunit gene expression in the pSA20-transfected trophozoites was determined by Western immunoblot under reducing conditions by enhanced chemiluminescence (Fig. 3), as well as by radioactivity determination after the interaction of blots with 125I-labelled protein A (data not shown). In both determinations, trophozoites transfected with pSA20 and grown in the presence of 60 μg ml−1 G418 had a significant decrease in the intensity of the three 35 kDa bands (≈60% less) compared with the intensity of the bands found in the wild-type strain HM-1:IMSS. This result indicates that the expression of the 35 kDa subunit is significantly inhibited in the pSA20 transfectant. On the other hand, the levels of expression of the Gal–lectin 170 kDa in pSA20 transfectant grown in the presence of 60 μg ml−1 G418 were similar to that present in HM-1:IMSS trophozoites. This result is in agreement with the above-mentioned observation made on the levels of light and heavy Gal–lectin subunits in strain Rahman (see above) and again confirm that a deficiency in 35 kDa subunit expression does not affect the level of expression of the heavy 170 kDa Gal–lectin subunit. Western blots reacted under non-reducing conditions with antibody against the Gal–lectin 170 kDa subunit (Fig. 4C) or against the 35 kDa subunit (Fig. 4D) revealed in both cases that the amounts of the 260 kDa Gal–lectin heterodimers present in the pSA20 transfectant were lower than those present in strain HM-1:IMSS. Monomeric forms of the 170 kDa Gal–lectin subunit were not detected under these conditions in the pSA20 transfectant (Fig. 4C).
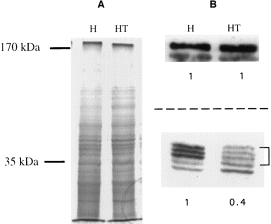
. A. Coomassie blue staining of SDS–PAGE gel. Lanes: H. HM-1:IMSS; HT, HM-1:IMSS pSA20 transfectant grown with G418 (60 μg ml−1). B. Western blot analysis of heavy (upper side) and light (lower side) subunits of Gal/GalNAc lectin from E. histolytica trophozoites strain HM-1:IMSS (H) and HM-1:IMSS pSA20 transfectant grown with G418 (60 μg ml−1) (HT). The same amounts of protein (25 μg of protein per line) were loaded in each gel as shown in (A). Numbers in bold represent densitometric quantification and are normalized by taking the value of densitometric quantification for HM-1:IMSS as 1.0. Identical results were obtained in three independent experiments.
As an additional control, the levels of enzymatic activity of alcohol dehydrogenase were determined in lysates of pSA20-transfected trophozoites (60 μg ml−1 G418) and untransfected trophozoites and found to be very similar (49.3 and 49.6 U), indicating that general protein synthesis was not affected in the transfected cells. Furthermore, the growth rate in Diamond's TYI-S-33 medium of pSA20 transformants growing in the presence of 60 μg ml−1 G418 was very similar (generation time of 8 h) to various pEhAct-Neo or other transfected trophozoites grown at the same concentration of G418 (Ankri et al., 1998), suggesting that the 35 kDa subunit is not a critical component for growth in culture.
Effects of antisense inhibition of the 35 kDa subunit on cytopathic activity, cytotoxic activity and adherence of the trophozoites
Intact trophozoites of strain HM-1:IMSS are known to destroy baby hamster kidney (BHK) monolayer cells (Bracha and Mirelman, 1984). As shown previously (Burchard and Mirelman, 1988), no significant cytopathic activity was measured for avirulent strain Rahman (Fig. 5A). A 65% decrease in the cytopathic activity was observed for the pSA20-transfected trophozoites of strain HM-1:IMSS compared with untransfected trophozoites or with pSA8-transfected trophozoites that are deficient in cysteine proteinase (Ankri et al., 1998) (Fig. 5A). Interestingly, pSA20 transfectants that were grown for 3 months in the absence of G418 recovered 90% of their cytopathic activity (Fig. 5A). Examination of the amounts of pSA20 plasmid present inside the trophozoite population that was grown in the absence of G418 for 3 months revealed that it was significantly lower (90%), and these trophozoites had higher expression levels of the 35 kDa subunit (data not shown). This indicates that the observed phenotype in the pSA20 transfectants correlates with the levels of antisense transcript. The fact that the pSA8 transfectants, as shown previously (Ankri et al., 1998), are able to destroy BHK monolayers but not the pSA20 transfectant suggests that inhibition of expression of the 35 kDa subunit in the pSA20 transfectant is responsible for the loss of cytopathic activity. This also proves that the inhibition of cytopathic activity is not caused by a squelching effect of the plasmid 5′ and 3′ Ehrpg34 flanking sequences that are present at high copy number.
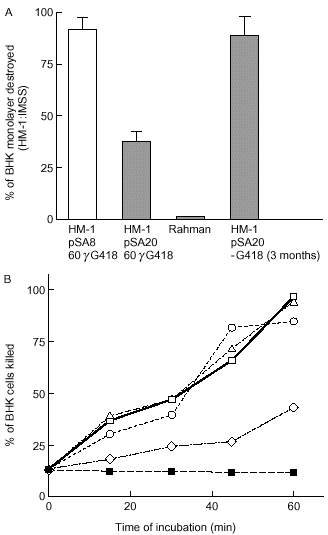
. A. Cytopathic activity of trophozoites (2 × 105) from: E. histolytica strains HM-1:IMSS, Rahman, HM-1:IMSS pSA8 and pSA20 transfected trophozoites as well as a revertant of transfectant pSA20 obtained after 3 months of growth in the absence of G418. After 1 h of interaction, the destruction of the BHK tissue-cultured monolayer was determined as described in Experimental procedures. Data represent the mean and standard deviation of three independent experiments performed in triplicate. Cytopathic activity of HM-1:IMSS (80% of monolayer destroyed) was taken as 100% cytopathic activity. B. Cytotoxic activity of E. histolytica trophozoites (1.5 × 105) incubated in suspension with BHK cells (9 × 105). (□) Parent strain HM-1:IMSS; (▵) strain HM-1:IMSS in the presence of polyclonal monospecific anti-35 kDa subunit antibody (40 μg ml−1); (○) transfectant pSA8 (Ankri et al., 1998); (◊) transfectant pSA20 and (▪) BHK cells alone. Viability was determined by trypan blue exclusion as described in Experimental procedures.
Incubation of E. histolytica trophozoites in suspension with mammalian cells resulted in killing of the mammalian cells as analysed by trypan blue uptake (Ravdin et al., 1980). A 55% decrease in the cytotoxic activity to BHK cells was observed for the pSA20-transfected trophozoites of strain HM-1:IMSS compared with untransfected trophozoites or with pSA8-transfected trophozoites that are deficient in cysteine proteinase (Ankri et al., 1998) (Fig. 5B). Interestingly, incubations performed in the presence of the polyclonal monospecific antibody (40 μg ml−1) against the recombinant 35 kDa subunit did not inhibit the cytotoxic activity of trophozoites of strain HM-1:IMSS to BHK cells (Fig. 5B).
The Gal-inhibitable adhesion of the trophozoites to the target cells is an event preceding the cytopathic and cytotoxic effect (Petri, 1996). In order to test whether the low cytopathic and cytotoxic activity in the pSA20-transfected trophozoites results from a defect in adherence, we measured the adhesion of pSA20-transfected trophozoites to BHK cells (Fig. 6A) as well as to 14C-labelled Escherichia coli serotype 055 (Fig. 6B). No significant difference was found between the levels of adhesion of strain Rahman, pSA20-transfectant, pSA8-transfectant and strain HM-1:IMSS to BHK cells (Fig. 6A). The adhesion was inhibitable for all the strains at 200 mM galactose (≈50–70% of inhibition) (Fig. 6A), which indicates that most of the adhesion in all the tested trophozoites continues to be Gal–lectin mediated (Petri, 1996). Furthermore, no significant difference was found in the adherence of E. coli serotype 055, a bacteria known to attach to the amoebic Gal-surface lectin (Bracha et al., 1982), to the strain Rahman, pSA20 transfectant, pSA8 transfectant or HM-1:IMSS untransfected trophozoites (Fig. 6B).
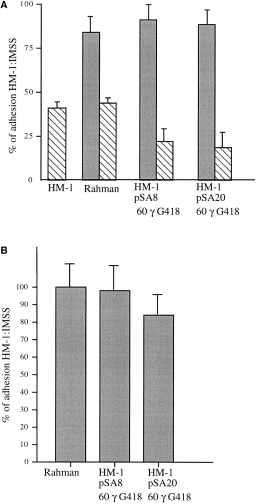
. Adhesion activity of E. histolytica trophozoites (2 × 105) of strains HM-1:IMSS, Rahman and HM-1:IMSS pSA8 and pSA20 transfectants. A. Adhesion to BHK fixed monolayers. Data represent the mean and standard deviation of three independent experiments. Adhesion activity of untransfected strain HM-1:IMSS was taken as 100% of activity. Hatched boxes: adhesion experiments performed in the presence of galactose (0.2 M). B. Adhesion to 14C-labelled E. coli serotype 055. Adhesion of E. coli to trophozoites of strain HM-1:IMSS (110 bacteria/trophozoite) was taken as 100% of adhesion activity. Data represent the mean and standard deviation of three independent experiments.
Effect of antisense inhibition of the 35 kDa subunit on cysteine protease activity and amoebapore
Cysteine proteinase and amoebapores have been described as amoebic molecules that cause damage to the host cells (Leippe, 1997; Que and Reed, 1997). In order to see whether the inhibition of expression of the 35 kDa subunit has an effect on these amoebic molecules, we compared the cysteine proteinase activity as well as the amount of amoebapore A and B between the pSA20-transfected trophozoites and the HM-1:IMSS strain. No difference was found between these types of trophozoites in the level of cysteine proteinase (± 45 U) or in the amounts of amoebapore A and B detected on Western blots by specific antibodies obtained from Dr M. Leippe (Hamburg, Germany) (data not shown).
Effect of antisense inhibition of the 35 kDa subunit on erythrophagocytosis activity and resistance of trophozoites to lysis by serum
The Gal/GalNac-inhibitable amoebic lectin activity is involved in the attachment and agglutination of human erythrocytes (Ravdin et al., 1985). In order to see whether the inhibition of expression of the 35 kDa subunit in the transfectant has an effect on the rates of erythrophagocytosis of human red blood cells, we compared the erythrophagocytosis activity between the pSA20-transfected trophozoites and the HM-1:IMSS strain. No difference between the level of erythrophagocytosis was found between each type of trophozoites (± 12 erythrocytes per amoeba after 10 min of incubation at 37°C).
Virulent amoebae activate the complement system but are able to evade this host defence mechanism (Hamelmann et al., 1992). This evasion has been shown to be mediated by the ability of the Gal–lectin to bind complement components C8 and C9, thereby abrogating assembly of the membrane attack complex (Braga et al., 1992). No significant difference was found in the susceptibility of pSA20 transfectants to human serum in comparison with the control of pEhAct-Neo or untransfected strain HM-1:IMSS. In all cases, lysis was approximately ± 70%.
Effect of antisense inhibition of the 35 kDa subunit on liver abscess formation in the hamster
In order to test whether the decrease in cytopathic activity of pSA20 is correlated with a general decrease in virulence that can also be observed in liver abscess formation, hamsters were inoculated intrahepatically with 5 × 105 trophozoites/animal of non-transfected HM-1:IMSS strain, pTS-4 transfectants or pSA20 transfectants grown in the presence of 150 μg ml−1 G418. All the hamsters inoculated with HM-1:IMSS strain or with pTS-4 transfected amoebae presented extensive necrotic lesions (> 2 cm). Out of the six hamsters injected with pSA20 transfectants, only one showed a very minor lesion (< 0.2 cm). As a control, we rescued and determined in transformed E. coli cells the amounts of pSA20 plasmid that were present in transfected trophozoites grown in TYI-33 medium in the presence of 150 μg ml−1 G418 and then cultivated for 1 week in TYI-S-33 medium in the absence of G418. The numbers of E. coli colonies obtained before or after the removal of G418 was approximately the same (data not shown).
Discussion
The under-representation of the 35 kDa subunit of the Gal/GalNac lectin complex in avirulent E. histolytica strain Rahman identified by the cDNA RDA subtraction procedure aroused our interest in the possible role of this 35 kDa subunit in amoebic virulence. For this purpose, we used our recently developed antisense technology (Ankri et al., 1998) to inhibit the expression of the 35 kDa subunit gene in the virulent strain HM-1:IMSS. Transfectants in which the expression of their 35 kDa subunit gene was significantly inhibited have a very low cytopathic and cytotoxic activity, but retain their normal levels of adherence activity to mammalian cells or bacteria such as E. coli 055, which are known to attach to the amoebic surface lectin (Bracha et al., 1982). These findings suggest that the 35 kDa subunit has an important role in the virulence mechanism. Our findings are interesting in view of the numerous reports assigning a predominant role in virulence to the 170 kDa subunit (Petri, 1996). The 170 kDa heavy subunit is responsible for the carbohydrate recognition and adhesion to target cells (Dodson et al., 1999). Monoclonal antibodies that bind to this molecule also prevent adherence (Mann et al., 1993). In the absence of adherence, the toxic activity against the target cells is also inhibited. The 170 kDa subunit has a multidomain that includes a single transmembrane span near the carboxy-terminus with a short putative cytoplasmic tail (Mann et al., 1993). The large subunit is encoded by a family of five hgl genes (Ramakrishnan et al., 1996), and the deduced amino acid sequences of the putative cytoplasmic domains of the sequenced genes revealed several potential phosphorylation sites (Tannich et al., 1991), suggesting that the lectin could be involved in cell signalling, and that the signal transduction may occur by way of phosphorylation. Recently, interference and competition with the cytoplasmic domain of the 170 kDa molecule has been shown to have a dominant negative effect, which caused a decrease in the Gal-inhibitable adhesion and cytopathic activity of the trophozoites without affecting the structure and protein level of the Gal–lectin 170 kDa subunit (Vines et al., 1998).
Until now, the only function that the 35 kDa subunits were assumed to have was their S–S linkage to the heavy 170 kDa subunit in the assembly of the heterodimeric 260 kDa Gal–lectin molecules on the trophozoite membrane (Petri, 1996). The light subunit is encoded by a family of lgl genes located at six loci in the genome (Purdy et al., 1993; Ramakrishnan et al., 1996) and consists of several polypeptide chains with considerable antigenic homology. Two light (31/35 kDa) subunits of the lectin are present in two isoforms: the 31 kDa isoform is glycerolphosphatidylinositol (GPI) anchored; and the 35 kDa isoform is more highly glycosylated (McCoy et al., 1993). Monoclonal antibodies raised against the 35 kDa subunit have been shown to be incapable of inhibiting adhesion of the parasite to CHO cells (McCoy, 1994). Interestingly, a carbohydrate-binding activity has recently been reported for the 35 kDa light subunit of the lectin molecules of the closely related Entamoeba invadens (Cho and Eichinger, 1998). It is not yet known whether the 35 kDa subunit of E. histolytica may have a carbohydrate-binding capacity or any other function. Our present results show that the inhibition of expression of the 35 kDa subunit did not affect the Gal-inhibitable adherence to target cells, the rate of erythrophagocytosis, the sensitivity to complement lysis or the activity of cysteine proteinases or amoebapore expression. The deficiency in the 35 kDa subunit of the pSA20 transfectants did, however, affect their cytopathic activity and cytotoxic activity as well as their ability to induce the formation of liver abscesses in hamsters. This is the first indication of a possible independent role of the 35 kDa protein in amoebic virulence. The reduced expression of the 35 kDa subunit in the transfectants seems to affect the proper assembly of the heterodimeric 260 kDa native lectin molecules. However, the reduced amount of the heterodimers in the transfectants does not affect the Gal–lectin recognition and binding properties of the trophozoites. This result is not surprising in view of the recent report showing that the 170 kDa subunit by itself contains the Gal–lectin recoginition and binding activity (Dodson et al., 1999). The structural organization of the 170 kDa heavy subunits in the absence of 35 kDa light subunits is currently under investigation.
Our present findings that a deficiency in the expression of the 35 kDa light subunit causes a significant decrease in virulence are supported by another recent observation in our laboratory in which both the expression of the 35 kDa subunit and the cytopathic activity were inhibited when trophozoites were grown for several weeks in monoxenic cultures with bacteria (E. coli serotype 055) that bind to the amoebic Gal–lectin (Padilla-Vaca et al., 1999). The present study also enables us to understand better the defects of the avirulent E. histolytica strain Rahman. Previous studies have shown that strain Rahman has a deficiency in the cell surface lipophosphoglycan structure (Moody et al., 1998). Our present finding that strain Rahman has some structural modification and lower expression of its 35 kDa subunits suggest that this may be an additional reason for its lack of virulence. Interestingly, these structural modifications do not seem to affect the amount of Gal–lectin heterodimers formed in strain Rahman significantly. Preliminary results with transfected trophozoites of strain Rahman in which the gene coding for the 35 kDa subunit of strain HM-1:IMSS under the control of amoebic actin promoter was slightly expressed revealed that such transfectants did not recover their cytopathic activity (data not shown). This suggests that additional defects in strain Rahman, such as the above-mentioned lack of high-molecular-weight lipophosphoglycan molecules on the trophozoite surface, could contribute to its lack of virulence. Studies are under way to identify and characterize the possible roles that the additional genes identified in the subtractive representation libraries may have on the avirulence of strain Rahman.
In conclusion, the use of a subtractive representation cDNA library between a highly virulent and an avirulent strain of E. histolytica coupled with antisense technology is enabling us to selectively investigate and dissect the complex process of interaction between the parasite and the host cell. It is hoped that such an approach may lead to the identification of additional gene products that are crucial for the pathogenesis of the amoeba and will enable the development of novel therapeutic strategies.
Experimental procedures
E. histolytica strains
Trophozoites of E. histolytica strains HM-1:IMSS and Rahman were cultured under axenic conditions in Diamond's TYI-S-33 medium (Diamond et al., 1978). Trophozoites in log phase of growth were used in all experiments. Avirulent strain Rahman is a well-characterized E. histolytica strain that has been known for quite a number of years (Sargeaunt et al., 1980; Burchard and Mirelman, 1988). It was originally isolated in England in 1964 by Dr Robinson from the stool of a sailor arriving from Calcutta and suffering from dyspepsia. It was subsequently cultured by the late Dr Ralph Neal in London and axenized in the laboratory of Dr L. S. Diamond (personal communication). In the beginning, the strain had virulent properties but, after axenization, an avirulent culture was obtained, and the original virulent parental strain is no longer available.
E. histolytica strains HM-1:IMSS and Rahman were tested before use for their ability to produce liver abscesses in hamsters (Burchard et al., 1988; Navarro-Garcia et al., 1995) and for their cytopathic activity on baby hamster kidney (BHK) cell monolayers (Bracha and Mirelman, 1984). E. histolytica strain HM-1:IMSS was fully virulent, whereas strain Rahman had no cytopathic activity (Fig. 5A) and did not induce the formation of liver abscesses in hamsters (data not shown). E. histolytica pSA8 transfectant (ehcp5 antisense RNA; Ankri et al., 1998) was used in this study as a positive control for the cytopathic activity assay. E. histolytica pTS4 transfectant (CAT reporter gene flanked by the upstream and downstream regions of Ehg34; Moshitch-Moshkovitch et al., 1998) was used in this study as a positive control for the induction of amoebic liver abscesses in hamsters.
cDNA representational difference analysis (cDNA RDA)
For the cDNA RDA, we used the same protocol as that described by Hubank and Schatz (1994). Briefly, total RNA was prepared using an RNA isolation kit RNAZOL B (Cinna/BioTech Laboratories). mRNA from strains HM-1:IMSS and Rahman was isolated twice by purification over an oligo(dT) cellulose column (Ausubel et al., 1987). Double-stranded cDNA was prepared by reverse transcription of the poly(A) RNA using an oligo(dT) primer (Ausubel et al., 1987). To reduce its complexity, we restricted each cDNA with the 4 bp cutting enzyme DpnII. After ligation of the synthetic oligonucleotides (referred to in the report by Hubank and Schatz, 1994) to restricted cDNAs and filling in of the ends, cDNAs were amplified by PCR (Taq DNA polymerase; Boehringer Mannheim) to obtain the HM-1:IMSS and Rahman representations. Assuming that E. histolytica strain HM-1:IMSS specifically expressed some additional genes that are involved in virulence compared with the avirulent strain Rahman, strain HM-1:IMSS was chosen as the ‘tester’ and strain Rahman as the ‘driver’. Three rounds of amplification–hybridization were performed. The overabundant selected products were cloned in the pGEM-5Zf(+) vector using the pGEM-T vector systems (Promega).
Construction of hybrid plasmids for transfection
All common techniques and routine DNA manipulations, including transformation, plasmid preparation and gel electrophoresis, were carried out according to standard procedures (Sambrook et al., 1989). Restriction and modifying enzymes (Boehringer Mannheim and New England Biolabs) were used in accordance with the manufacturer's recommendations.
Plasmid pSA20 (Fig. 3A) was constructed as follows: the 35-kDa subunit of the E. histolytica Gal-inhibitable lectin was amplified by PCR from the plasmid gEh-35/1 (a kind gift from Dr E. Tannich, Hamburg, Germany) using the sense primer catgccatggttatattagtcttattgata and the antisense primer gcgcgtcgacttatgcaaacacaggaataa. The 867 bp PCR product obtained was sequenced and was fully homologous to the E. histolytica Gal–lectin 35 kDa subunit. This fragment was digested with Bgl II and ligated to our previously reported plasmid pSA8 (Ankri et al., 1998) after removal of its EhCP5 insert by Bgl II and NotI digestion. After blunt ending with T4 DNA polymerase, the plasmid was closed by ligation.
Transfection of E. histolytica cells
Exponentially growing trophozoites of E. histolytica strain HM-1:IMSS were transfected as described previously (Vines et al., 1995). The cells were allowed to recover for 2–3 days, and the G418 concentration was gradually increased until all the trophozoites in the control tubes, which underwent electroporation in the absence of plasmid, died. The media was removed carefully every 24–48 h to reduce the amount of debris in the culture until the transfected trophozoites recovered. The G418 concentration was then raised gradually to 150 μg ml−1 within a period of 2–3 weeks.
Southern and Northern blot hybridization
For Northern blot hybridization, total RNA was prepared using an RNA isolation kit RNAZOL B (Cinna/BioTech Laboratories), and 10 μg of total RNA was size fractionated under denaturing conditions on agarose gels containing formaldehyde. The DNA or RNA was transferred to a nylon membrane and hybridized under stringent conditions as described previously (Bracha et al., 1995). Probes were prepared from the appropriate PCR fragments. For preparation of the single-strand probe, used in the Northern blot hybridization, asymmetric PCR was performed using the E. histolytica galactose-inhibitable lectin (g-35/1) gene (accession number M96024) as template. A labelled double strand probe was prepared with the Ready Prime II kit (Amersham) using the same template.
SDS–PAGE and Western immunoblotting
E. histolytica trophozoites were solubilized with 1% NP-40 in PBS in the presence of 50 μM protease inhibitor E-64. Proteins from whole E. histolytica were resolved for 35 min at 200 V on 10% polyacrylamide gels (25 μg lane−1) under reducing conditions (Laemmli, 1970) or for 60 min at 200 V on 8% polyacrylamide gels (25 μg lane−1) under non-reducing conditions (without β-mercaptoethanol) and transferred electrophoretically to nitrocellulose membranes. The blots were reacted with a suspension of polyclonal anti-Gal–lectin 170 kDa (1:5000) (a gift from Dr S. L. Stanley, St Louis, MO, USA) or with a suspension of polyclonal monospecific anti-35 kDa subunit (1:250) (see below) and then subjected to interaction with an HRP-conjugated goat anti-rabbit antibody (1:5000) and developed by enhanced chemiluminescence. Detection was done by autoradiography. The amount of bound polyclonal monospecific anti-35 kDa subunit antibody was determined by incubating the blots with 125I-labelled protein A (10−6 Ci ml−1; Amersham) for 1 h. Detection and quantification was carried out using a phosphorimager.
For Western immunoblotting of the amoebapore, trophozoites were resuspended in saline buffer containing protease inhibitors (10 μM E-64, 6.4 mM benzamidine, 0.5 mM PMSF) at a concentration of 107 trophozoites ml−1 and disrupted by freeze–thaw (three times). Homogenates were sedimented at 100 000 × g at 4°C for 15 min. Proteins from the soluble fraction (5 μg lane−1) were resolved on 20% polyacrylamide gels under non-reducing conditions and transferred electrophoretically to nitrocellulose membranes. The blot was reacted with a suspension of polyclonal anti-amoebapore A or B (1:1000) (a gift from Dr M. Leippe, Hamburg, Germany) and then subjected to interaction with an HRP-conjugated goat anti-rabbit antibody (1:5000).
Preparation of a polyclonal monospecific anti-35 kDa subunit antibody
A recombinant 35 kDa subunit was prepared from the prokaryotic expression vector system pGEX-2T (Pharmacia). The 35 kDa subunit of the E. histolytica Gal-inhibitable lectin was amplified by PCR from the plasmid gEh-35/1 (a kind gift from Dr E. Tannich, Hamburg, Germany) using the sense primer cgcggatccaagactcaagacggaaaagat and the antisense primer gcgcgtcgacttatgcaaacacaggaataa. The PCR product obtained was digested with Bgl II and BamHI and cloned into the pGEX-2T plasmid at the BamHI site of the polylinker. This allowed for the inducible expression of a glutathione-S-transferase–35 kDa subunit. Intracellular fusion protein was recovered from E. coli lysates and purified by affinity chromatography using a glutathione Sepharose gel matrix by direct exchange with reduced glutathione (1 mM). The glutathione-S-transferase (GST)–35 kDa subunit fusion protein was used to inoculate rabbits for the generation of polyclonal anti-35-kDa subunit antibodies. Monospecific anti-35 kDa subunit antibodies were obtained by affinity chromatography of the rabbit antiserum over a Sepharose-4B column (Pharmacia) conjugated to the GST–35 kDa subunit fusion protein. The specific recognition of the polyclonal monospecific anti-35 kDa subunit antibodies to the GST–35 kDa subunit fusion protein (data not shown) or to the E. histolytica 35 kDa Gal–lectin subunits (Fig. 3) was verified by Western blot analysis and compared with the specific recognition of a monoclonal antibody for the 35 kDa subunit (a kind gift from Dr B. Mann, VA, USA) as a positive control. Preimmune serum was not able to detect either the GST–35 kDa fusion protein or the E. histolytica 35 kDa subunit (data not shown).
Cytopathic activity
The destruction rate of tissue-cultured monolayers of baby hamster kidney (BHK) cells by transfected and untransfected viable trophozoites (2 × 105) was performed essentially as described previously (Bracha et al., 1984). After 60 min of interaction between the trophozoites and BHK cells, the experiment was stopped by placing the plates on ice and washing off the trophozoites with cold saline. The percentage of living BHK cells remaining in the wells was determined using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide]-based assay (Mosmann, 1983).
Cytotoxic activity
The activity was determined by the vital dye exclusion assay (Ravdin et al., 1980). Freshly harvested BHK cells (9 × 105) with and without amoebae (1.5 × 105) were placed in 450 μl of TYI-S-33 media without serum and incubated with rotation at 37°C for 1 h. Samples (30 μl) were taken at different time points, treated with trypan blue (0.1% final concentration) and examined microscopically in a haemocytometer chamber. Viability was calculated by counting the BHK cells that included or excluded trypan blue. Three separate counts were made for each sample, and the experiment was repeated twice. Standard deviation of the mean was calculated and found to be in the range of ± 5%. The cytotoxic activity of strain HM-1:MMS was also determined in the presence of added polyclonal antibodies, monospecific against the 35 kDa subunit (40 μg ml−1).
Adhesion assay to BHK cells
For adhesion assays, the BHK monolayers were fixed with 5% formaldehyde, washed twice with PBS, incubated with 250 mM glycine for 30 min and washed with PBS. Trophozoites (2 × 105) were added to wells containing fixed monolayers in 1 ml of Dulbecco's modified Eagle medium (DMEM) without serum and incubated at 37°C for 30 min. The number of trophozoites adherent to BHK cells was determined by counting under the microscope the trophozoites that remained adhered to cell monolayers after gentle decanting (twice) of the non-adhered trophozoites with 37°C warmed DMEM. Inhibition of adhesion was determined in the presence of 200 mM galactose.
Adhesion assay of E. coli serotype O55
The assay was performed according to Bracha et al. (1982). [14C]-E. coli (109) (300 c.p.m. 10−6 bacteria) were incubated with trophozoites (106) in 1 ml of saline solution for 30 min at 4°C. Separation between bacteria that attached to the trophozoites and those that did not was performed by discontinuous density gradient centrifugation with Percoll (Pharmacia). Unattached bacteria layered between 100% and 60% Percoll, whereas amoebae layered between 60% and 20%. The bacteria attached to trophozoites were counted in a scintillation fluid using a liquid scintillation counter.
Erythrophagocytosis assay
Erythrophagocytosis assay was measured as described previously (Mora-Galindo et al., 1997). HRBC (108) and trophozoites (106) were mixed and incubated for 10 min at 37°C. Phagocytosis was stopped by the addition of distilled water. The average number of erythrocytes inside the trophozoites was quantified using a calibration curve and by reading the absorbance at 397 nm after resuspending the pellet of parasites in 90% formic acid.
Alternative pathway killing of E. histolytica by non-immune human sera
Determination of alternative pathway killing of E. histolytica was as described previously (Hamelmann et al., 1992). Human sera (0.1 ml) was mixed with 5 × 105 trophozoites in 0.15 ml of 20 mM phosphate buffer, pH 7.4, containing 135 mM NaCl, 10 mM EGTA and 2.5 mM MgCl2. After incubation for 15 min at 37°C, the number of dead amoebae was determined (sum of those lysed and stained with 0.1% eosin). The control group contained heat-inactivated sera (56°C for 30 min).
Cysteine proteinase activity
CP activity was measured in total lysates of trophozoites (106) lysed in 1 ml of Nonidet P-40 (1% in PBS). CP activity was determined by following the digestion of the chromophoric substrate benzyloxycarbonyl-l-arginyl-l-arginine-p-nitroanilide (Z-Arg-Arg-pNA; Bachem) (Leippe, 1995). One unit (U) of activity is defined as the number of μmol of substrate digested min−1 mg−1 protein.
Alcohol dehydrogenase (ADH) activity
ADH activities were determined as described previously (Leippe, 1995). One unit (U) is defined as the number of μmol of substrate digested min−1 mg−1 protein.
Induction of amoebic liver abscesses
Syrian golden hamsters (6 weeks old) were inoculated intrahepatically with 5 × 105 (i) E. histolytica HM1:IMSS trophozoites; (ii) E. histolytica HM1:IMSS trophozoites transfected with pSA20 and grown in the presence of 150 μg ml−1 G418; and (iii) E. histolytica HM1:IMSS trophozoites transfected with pTS-4 (Moshitch-Moshkovitch et al., 1998) and grown in the presence of 150 μg ml−1 G418. Hamsters (at least four in each group) were sacrificed 1 week after intrahepatic inoculation, and the formation of lesions was evaluated.
Acknowledgements
Serge Ankri was supported by a Feinberg Graduate School postdoctoral fellowship (Weizmann Institute). This work was supported in part by the Leo and Julia Forchheimer Center for Molecular Genetics at the Weizmann Institute of Science and the Center for the Study of Emerging Diseases. We wish to thank Dr Barbara Mann (Virginia, USA), Dr S. L. Stanley Jr (St Louis, MO, USA) and Dr M. Leippe (Hamburg, Germany) for the gifts of the anti-35 kDa subunit, the anti-Gal–lectin 170 kDa subunit and anti-amoebapore A and B antibodies and Dr Egbert Tannich (Hamburg, Germany) for the gift of the plasmid gEh-35/1.




