Intramolecular transposition of insertion sequence IS91 results in second-site simple insertions
Abstract
A series of plasmids carrying an IRL-kan-IRR transposable cassette, in which IRL and IRR are the left- and right-terminal sequences of IS91, have been constructed. These cassettes could be complemented for transposition with similar efficiency when IS91 transposase was provided either in cis or in trans. A total of 87% of IS91 transposition products were simple insertions of the element, while the remaining 13% were plasmid fusions and co-integrates. When transposase expression was induced from an upstream lac promoter, transposition frequency increased approximately 100-fold. An open reading frame (ORF) present upstream of the transposase gene, ORF121, could be involved in target selection, as mutations affecting this ORF were altered in their insertion specificity. Intramolecular rearrangements were analysed by looking at transposition events disrupting a chloramphenicol resistance gene (cat ) located outside the transposable cassette. Plasmid instability resulting from insertion of an extra copy of IRL-kan-IRR within the cat gene was observed; transposition products contained a second copy of the cassette inserted either as a direct or as an inverted repeat. No deletion or inversion of the intervening DNA was observed. These results could be explained as a consequence of intramolecular transposition of IS91 according to a model of rolling-circle transposition.
Introduction
Transposons are discrete DNA segments that insert into non-homologous DNA in reactions catalysed by transposon-encoded proteins called transposases. The mechanisms of early steps in the transposition of several well-studied transposons display remarkable similarity in a basic three-step process catalysed by transposases: end cleavage, DNA strand transfer and either replication or repair, depending on whether transposition is replicative (Tn3, replicative Mu) or conservative (Tn7, Tn10, lysogenic Mu) (Mizuuchi, 1992; Craig, 1996). Transposable elements promote a variety of DNA rearrangements in addition to simple insertions (Galas and Chandler, 1989). Intramolecular transposition results in either deletion or inversion of neighbouring DNA segments (Galas and Chandler, 1989). The capacity to generate adjacent deletions was first noted for an IS1 element located in the gal operon and, since then, has constituted a genetic hallmark of IS elements (Starlinger and Saedler, 1976; Reif and Saedler, 1975, 1977). IS10 and IS903 also generate a similar type of deletion (Kleckner et al., 1979; Weinert et al., 1983). IS10-promoted deletions are about 2% as frequent as simple transposition events (Roberts et al., 1991). IS2 (Peterson et al., 1979) and IS30 (Caspers et al., 1984) also generate deletions, although they occur at low frequencies relative to transposition frequencies. Inversion of a DNA segment has been reported for several elements, such as IS1 (Cornelis and Saedler, 1980), IS102 (Bernardi and Bernardi, 1986) and Tn10 (Ross et al., 1979).
A remarkable property of IS element transposases is that they act efficiently only in cis (McFall, 1986). Preferential cis action was demonstrated at least for IS903, IS50, IS1 and IS10 (Grindley and Joyce, 1981; Isberg and Syvanen, 1981; Machida et al., 1982; Morisato et al., 1983). Mu transposase also acts preferentially in cis (Pato and Reich, 1984). Another major group of cis-acting proteins is the replication initiator proteins of φX174 and related single-stranded DNA phages (Francke and Ray, 1972; McFall, 1986). In IS903, preferential cis action results from the instability of the transposase (Derbyshire et al., 1990). In IS10, preferential cis action depends on the abundance of transposase and on the association of its N-terminal sequence with DNA, in such a way that mutations that decrease translation initiation increase cis preference (Jain and Kleckner, 1993). In Tn5, cis preference is caused by functional inactivation of the protein resulting from non-productive transposase–transposase and transposase–inhibitor multimerization (Weinreich et al., 1994).
IS91 was originally isolated from a haemolytic plasmid of Escherichia coli and found to occur in multiple copies in a number of Hly plasmids of different incompatibility groups (Zabala et al., 1982). It contains dissimilar ends and displays a number of characteristics that are unique among IS elements, suggesting a new transposition mechanism. First, it showed an absolute transposition specificity for the target sequences CTTG or GTTC. After insertion, the right inverted repeat (IRR) lay adjacent to the 3′ end of either of these target sequences (Mendiola and de la Cruz, 1989). Secondly, no target DNA was duplicated after insertion (Díaz-Aroca et al., 1987). Thirdly, IS91 termini played different roles in transposition. The terminal 82 bp of IRR as well as the 5′-flanking sequence (CTTG or GTTC) were absolutely required for transposition, while the left inverted repeat (IRL) was dispensable. One-ended transposition of IS91 (in elements lacking IRL) resulted in tandem repetitions of the donor plasmid inserted in the recipient molecule. Any CTTG or GTTC sequence of the donor DNA could be used as a secondary end (Mendiola et al., 1994). Fourthly, sequence analysis of IS91 indicated that the amino acid sequence of IS91 transposase is related to the Rep class of proteins of rolling-circle replication plasmids (RCR plasmids) and single-stranded DNA phages (Mendiola and de la Cruz, 1992). There is also conservation between the DNA sequence around the cleavage site in IRR (5′-CTTG ↓ AT) with the origins of replication of a superfamily of RCR plasmids and phages (see Discussion ). These results taken together lead to the proposal that IS91 transposes by a rolling-circle mechanism similar to the replication of φX174 (Mendiola et al., 1994).
This study was set up to analyse some properties of IS91 transposition. For this purpose, we constructed a series of plasmids that contain an IS91 cassette in which the three ‘modules’ of the transposon, i.e. transposase gene, IRL and IRR, could be manipulated separately, taking advantage of unique flanking restriction sites. Several basic properties of IS91 transposition were manifested by the use of this series of plasmids. Besides, the results obtained are consistent with IS91 transposing by a novel pathway, as intramolecular transposition resulted in second-site simple insertions and not in inversions or deletions of adjacent DNA, as occurs for previously analysed transposons.
Results
Construction of a series of plasmids carrying a kanamycin-resistant (Kmr) transposable cassette
A series of plasmids was constructed carrying an IS91-derived transposable cassette of the type IRL-kan-IRR and an external transposase gene (tnpA) in different relative locations. The cassettes carried a kanamycin resistance gene (kan ), which allowed the detection of direct transposition events. Each of the four structural ‘modules’ of these series of plasmids, i.e. the kan and tnpA genes, IRR and IRL, was flanked by unique restriction sites, which allowed separate manipulation of each module, including exchange by modules from related ISs. Figure 1 explains the construction of plasmid pSU2620, which carried the basic IRL-kan-IRR cassette. In pSU2620, IRR is flanked by unique EcoRI and KpnI restriction sites, the kan gene by BamHI sites, and IRL by SphI and XbaI restriction sites. Transposition of IRL-kan-IRR occurred after complementation with the transposase gene, which was provided in trans by either of the two helper plasmids shown in 2Fig. 2B. In pSU2637, transposase expression could be induced by an upstream lac promoter (Plac ), whereas in plasmid pSU2638, the transposase was cloned in the opposite orientation to Plac. Alternatively to the trans configuration for the transposase, a series of plasmids was also constructed that carried the IRL-kan-IRR cassette and tnpA in cis. There were four configurations, which were represented by plasmids pSU2631 to pSU2634, as shown in Fig. 2A. In plasmids pSU2631 and pSU2632, tnpA was expressed under the control of Plac, as in pSU2637, while in plasmids pSU2633 and pSU2634, tnpA was reversed, as in pSU2638. In pSU2631 and pSU2633, IRR is proximal to tnpA, while in pSU2632 and pSU2634, IRL is proximal to tnpA.
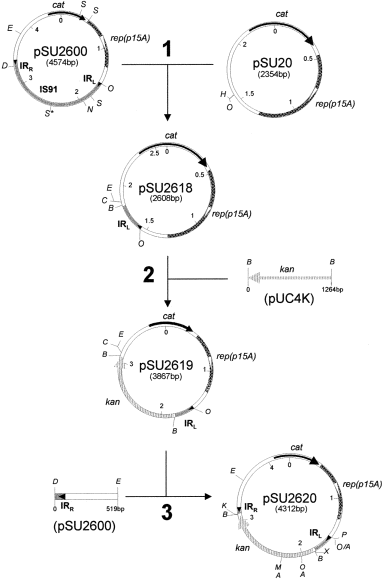
. Construction of plasmid pSU2620. pSU2620 was constructed in three steps. Step 1: the 254 bp XhoI/SspI fragment of plasmid pSU2600, which contained the IRL, was cloned in pSU20 digested with XhoI and HincII. The resulting plasmid was designated pSU2618. Step 2: the 1264 bp BamHI fragment of pUCK4 (Pharmacia), which contained kan, was cloned at a single BamHI site of pSU2618, producing the plasmid pSU2619. Step 3: pSU2600 was digested with NdeI, this site was made blunt with Klenow polymerase, and then digested with EcoRI, the 519 bp fragment that contained the IRR was cloned in pSU2619, digested with SacI, filled in with Klenow and EcoRI to obtain plasmid pSU2620. Abbreviations: cat, chloramphenicol resistance gene; kan, kanamycin resistance gene; rep(p15A), origin of replication derived from p15A; IRL, left inverted repeat; IRR, right inverted repeat. Restriction enzymes sites: A, AvaI; B, BamHI; C, SacI; D, NdeI; E, EcoRI; H, HincII; K, KpnI; M, SmaI; N, NaeI; O, XhoI; P, SphI; S, SspI; Xb, XbaI. S*, new SspI site produced by a G to A transition at nucleotide 880 of IS91 (Mendiola et al., 1992) that does not affect the transposase amino acid sequence.
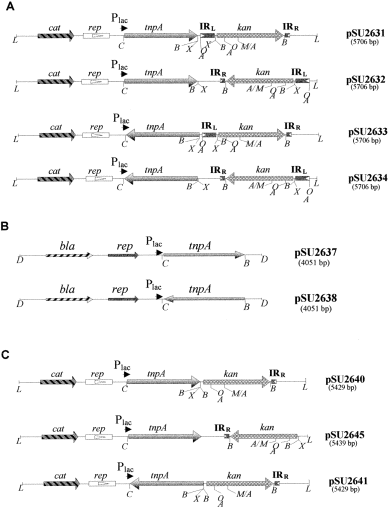
. Genetic organization of a series of plasmids carrying structural elements of IS91. A. Plasmids carrying the IRL-kan-IRR cassette and IS91 tnpA in cis. These plasmids were constructed in two steps. Step 1: plasmid pSU2600 DNA was digested with NaeI and NdeI restriction enzymes. Recessed ends of the 1363 bp fragment, containing tnpA, were filled in with Klenow polymerase, and this fragment was cloned in the SmaI site of plasmid pSU18. Plasmids pSU2629 and pSU2630 were obtained; in pSU2629, tnpA was cloned in the sense orientation with respect to Plac, whereas in pSU2630 it was cloned in the antisense orientation. Step 2: to construct plasmids pSU2631 and pSU2632, plasmid pSU2620 DNA was digested with EcoRI and SphI. The recessed ends of the 2014 bp fragment, containing IRL-kan-IRR, were filled in with Klenow polymerase, and this fragment was cloned in the HindIII and Pst I sites of pSU2629 after being made blunt with Klenow polymerase. For plasmid pSU2633 and pSU2634 construction, plasmid pSU2620 DNA was digested with EcoRI and SphI. The recessed ends of the 2014 bp fragment, containing IRL-kan-IRR, were filled in with Klenow polymerase, and this fragment was cloned in the HindIII and Pst I sites of pSU2630 after being made blunt with Klenow polymerase. B. Plasmids carrying tnpA. For the construction of plasmids pSU2637 and pSU2638, DNA from plasmids pSU2631 and pSU2633, respectively, was digested with SacI and BamHI restriction enzymes, and the resulting 1379 bp fragment was cloned in the SacI and BamHI sites of pUC18. C. Plasmids carrying one-ended derivatives of IS91. To construct plasmids pSU2640 and pSU2641, plasmid pSU2631 and pSU2633, respectively, DNAs were digested with XbaI and then religated after deletion of the 277 bp fragment that contained the IRL region. pSU2645 was constructed in several steps: the recessed ends of the two Pst I sites of pSU2620 (nucleotides 1837 and 3577) were sequentially filled in with Klenow polymerase after partial digestion of plasmid pSU2620 DNA with Pst I. Plasmid pSU2644 was obtained. Then, plasmid pSU2644 DNA was digested with EcoRI and XbaI. The recessed ends of EcoRI sites were filled in with Klenow polymerase, and the resulting 1745 bp fragment, containing kan-IRR, was cloned in the BamHI and XbaI sites of pSU2629 after the BamHI site was made blunt with Klenow polymerase. Abbreviations: bla, ampicillin resistance gene; Plac, lac promoter; rep(pMB8), origin of replication of pMB8; tnpA, transposase gene from IS91. Direction of transcription from the lac promoter is shown with an arrow. Restriction enzyme sites as in Fig. 1. L, Bgl I.
Transposase overexpression led to an increase in the transposition frequency
Transposition frequencies obtained for the series of plasmids carrying tnpA in cis are shown in Table 1. When tnpA expression was driven by Plac, transposition frequencies increased by 200-fold (pSU2631) or 15-fold (pSU2632) upon Plac induction by the addition of IPTG to the medium. Conversely, when tnpA was reversed with respect to Plac (pSU2633 and pSU2634), transposition frequencies did not increase upon Plac induction. They were very similar to the basal levels of expression of the equivalent set-ups in pSU2631 and pSU2632. Basal levels of transposase activity were higher in pSU2632 and pSU2634 than in pSU2631 and pSU2633, as judged by transposition frequencies in the absence of induction. Thus, induction seems to be stronger for pSU2631, although transposition frequencies in induced cultures were identical in both cases. We think that basal transposition levels could be dependent on a number of parameters, such as the proximity of IRR or IRL, external transcription effects or even an effect of tnpA transcription on plasmid copy number. In any case, the increase in transposition frequencies upon Plac induction indicated that the transposition frequencies of the cassettes were dependent on the level of IS91 transposase, which could be correlated with the expected activity of Plac.

IS 91 transposase is active in trans
The fact that the relative position of IRR had a minor effect on the transposition frequencies of the IRL-kan-IRR cassette suggested that positional effects may not be important for transposase function. In fact, the results obtained in Table 1 can be compared with those obtained when the transposase gene was provided in trans (Table 2). pSU2637 is equivalent to pSU2631 and pSU2632, in that tnpA is in the sense orientation with respect to Plac. The transposition frequency obtained when transposase was provided in trans was only three times lower than that obtained when transposase was provided in cis (3 × 10−4 for pSU2637 versus 9 × 10−4 for both pSU2631 and pSU2632). These results showed that IS91 transposase is also trans-acting. It has been shown previously that transcription from Plac is unregulated in the pUCs, even in the presence of a lacI q gene product, because of the high copy number of these plasmids of over 100 copies per cell (Stewart et al., 1986; Stark, 1987). Therefore, it was not surprising that the frequency of transposition of IRL-kan-IRR in plasmid pSU2637 was the same irrespective of the presence or absence of Plac induction.

The frequency obtained for pSU2638, in which tnpA was reversed with respect to Plac, was 100-fold lower than that obtained for pSU2637 (4 × 10−6 versus 3 × 10−4). In both cases, IRL-kan-IRR was complemented by the transposase encoded by a gene carried in another plasmid. Therefore, the observed difference must be caused by differences in transposase level. Basal transposase activity in plasmid pSU2638 was about the same as that of its equivalent pSU2633, which provided tnpA in cis (4 × 10−6 versus 3 × 10−6).
Transposition products of IS 91
Transposition of IRL-kan-IRR was detected by the presence of Kmr transconjugants produced by transposition of the cassette into the conjugative plasmid R388. As shown in Fig. 2, donor plasmids contain a cat gene outside the cassette, as well as the kan gene. Cmr transconjugants occurred at a frequency of roughly 10–20% of the frequency of Kmr transconjugants for all donor plasmids (Tables 1 and 2). The Cmr transconjugants were the result of co-integrate formation or multiple tandem insertions of donor plasmids into R388. DNAs from both KmrCms and KmrCmr transposition products between R388 and donor plasmids carrying tnpA both in cis and in trans were analysed with the restriction enzyme AvaI. Digestion of plasmid pSU2620 with AvaI resulted in three fragments of 274 bp, 435 bp and 3602 bp (Fig. 1). In the case of plasmids pSU2631 to pSU2634, digestion with AvaI resulted in three fragments of 274 bp, 435 bp and 4997 bp (Fig. 2A). R388 also produced 15 AvaI fragments, ranging from 0.54 kb to 6.9 kb (Avila and de la Cruz, 1988). AvaI digests of plasmid R388 DNA were compared with DNAs from R388 recombinants in which the transposable cassette had inserted. One R388 DNA band disappeared, while new fragments appeared as a result of the insertion. All the KmrCms transconjugants analysed were simple insertions of the IRL-kan-IRR cassette into R388. KmrCmr transconjugants were the sum of co-integrates between R388 and donor plasmids, and multiple tandem insertions of donor plasmids into R388 (plasmid fusions). The relative proportion between co-integrates and plasmid fusions ranged from 6:4 in the case of plasmid pSU2631 to 3:7 in plasmid pSU2632 (Table 1). In the case of plasmid pSU2631, 87% of transposition products were simple insertions of the transposable cassette resulting from direct transposition; the remaining 13% were the sum of co-integrates and plasmid fusions. We assume that co-integrates and plasmid fusions are the result of the failure in IRL recognition when the transposase encounters IRL during rolling-circle transposition (Mendiola et al., 1994).
One-ended transposition of kan-IRR occurs at similar frequencies to transposition of IRL-kan-IRR
To analyse one-ended transposition, the IRL fragment of the IRL-kan-IRR cassette was excised from plasmids that carried tnpA in cis. In this way, plasmids pSU2640, pSU2645 and pSU2641 were obtained, which represent one-ended derivative versions of pSU2631, pSU2632 and pSU2633 respectively (Fig. 2C). Transposition frequencies obtained with these constructions are shown in Table 3. One-ended transposition occurred at nearly the same frequency as transposition of the complete cassettes (2 × 10−4 for pSU2640 versus 9 × 10−4 for pSU2631). When Plac was activated by the addition of IPTG to the medium, most recombinants (65% for pSU2640 and approximately 90% for both pSU2641 and pSU2645) were multiple tandem insertions of the donor plasmid, as described previously (Mendiola et al., 1994), and they were selected as KmrCmr transconjugants; the remaining 35–10% were KmrCms transconjugants corresponding to incomplete plasmid fusions, which did not carry the cat gene of donor plasmids. On the contrary, when there was no induction, most recombinants were incomplete plasmid fusions.

Two mutants of IRL were constructed in which 2 bp or 3 bp of IRL were deleted. The DNA sequences of wild-type IRL in plasmid pSU2600 and of the mutant IRLs of plasmids pSU2635 and pSU2636 are shown in Table 4, together with the transposition frequencies obtained with these plasmids. IS91 transposition from these mutants resulted in multiple tandem insertions of the donor plasmid in the recipient, indicating that the mutant IRLs were inactive. These results imply that the tetranucleotide CGAG is essential for IRL function. Transposition frequencies obtained for these one-ended derivatives were equal to that of pSU2600 (2–3 × 10−4 versus 4 × 10−4), further indicating that the lack of IRL function does not impair IS91 transposition.

Specificity of insertion of IS 91 derivatives lacking ORF121
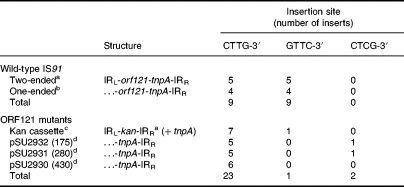
It was known that IS91 inserts specifically 3′ of the sequences CTTG or GTTC, both of them used at roughly the same frequency (Mendiola and de la Cruz, 1989; Table 5). The same specificity of insertion is maintained in one-ended transposition (Mendiola et al., 1994; Table 5). However, when we sequenced the junctions of eight recombinants produced by simple insertion of IRL-kan-IRR in R388, we found great specificity for the sequence CTTG-3′ (Table 5). Wild-type IS91 contains an additional open reading frame (ORF), named ORF121, which precedes the tnpA gene (Mendiola et al., 1992). Three Ω-interposon insertions in ORF121 were available from previous work (Mendiola et al., 1992). IS91 derivatives with Ω inserted in ORF121 were transposition proficient, so these insertional mutants were transposed to R388 to determine whether the lack of ORF121 expression was causing the change in IS91 insertion specificity. We sequenced the junctions between IRR and R388 DNA in 18 of the resulting transposition products. The results (Table 5) confirm that ORF121 mutants give very similar specificity to IRL-kan-IRR. The χ2 test was applied to ascertain if the results obtained with wild-type IS91 were significantly different from those obtained with the mutants. A χ2 value of 15.2 was obtained, corresponding to a P < 0.001 for a set of data with two degrees of freedom, implying that the two sets of experiments differ very significantly, i.e. target selection was altered in these mutants. It is of special interest that two of the mutants inserted in the sequence 5′-CTCG (or its complementary 5′-CGAG), which corresponds to the last four nucleotides of IS91 IRL. These results imply that ORF121 could be involved in target selection and specificity of insertion of IS91.
Intramolecular transposition of IS 91
Storage of derivatives of strain DH5α carrying either pSU2631 or pSU2632 in 50% glycerol at −20°C resulted in the loss of Cmr recovered bacteria. When these bacteria were cultured and plated on Km-containing plates, about 35% of individual colonies were Cms. Loss of antibiotic resistance was not detected in strains containing plasmids pSU2633 and pSU2634, which have low transposase levels, or in strains carrying pSU2620 and the helper plasmids pSU2637 or pSU2638. Furthermore, there was no loss of antibiotic resistance when plasmids pSU2631 and pSU2632 were carried in strain D1210, which carries the lacI q gene. Inactivation of cat seemed to be a useful result, as it could have been produced by deletion of sequences adjacent to the IS91 cassette resulting from intramolecular transposition, as occurs for other IS elements (see Introduction ). Nine plasmid DNAs resulting from independent cat inactivation events were analysed. All nine Cms plasmids contained an extra copy of IRL-kan-IRR inserted within cat. Insertions were mapped to three precise locations by endonuclease digestion and DNA sequencing (Fig. 3). The DNA sequence located between the original and the duplicated copies of the cassette remained intact, without having experienced any rearrangement such as deletion or inversion.
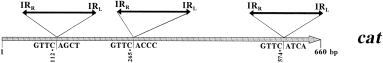
. Localization of second-site insertions of IRL-kan-IRR within the cat gene of plasmids pSU2631 and pSU2632. The DNA sequences of the cat gene at the site of insertion (/) are shown under the grey arrow, which represents the DNA sequence of cat. Also shown are the co-ordinates of the insertion points in the cat gene sequence.
Discussion
Previously reported properties of IS91 transposition made it clear that this element transposes by a peculiar mechanism, different from most transposable elements. In this work, we present some additional properties of IS91 transposition, which can be compared with other well-studied transposable elements. They emphasize further the unique nature of this insertion sequence. A series of modular cassettes was constructed to help in this and future work. Results obtained suggest the following conclusions.
Transposition frequency of IS 91 cassettes is related to transposase transcriptional level
The results shown in Tables 1 and 2 indicate that transposition of IS91 cassettes can be induced to levels of 10−3 transposition events/recipient DNA molecule. Such high levels of transposition are convenient for genetic analysis. The fact that a high transposition frequency can be achieved by simply activating a lac promoter, suggests that transposition of IS91 could be regulated mainly at the level of transposase gene expression. This is a common mechanism of transposition regulation (Kleckner, 1990).
IS 91 transposase activity does not show cis preference
Transposition mediated by cis-acting transposases (IS10, IS903 ) is extremely sensitive to the distance between the 3′ end of tnpA and the nearest transposon IR (Morisato et al., 1983; Derbyshire et al., 1990). However, IRL-kan-IRR transposition did not show any dependence on the distance between tnpA and IRR, as transposition of IS91 cassettes occurred at the same frequency in plasmids pSU2631 and pSU2632, the distance between tnpA and IRR being 1.6 kb in pSU2631 but only 0.4 kb in pSU2632. This result could also imply that IS91 transposase is trans-acting, unlike the majority of IS transposases. In fact, transposition of the IRL-kan-IRR cassette when tnpA was carried in a helper plasmid (pSU2637 or pSU2638) occurred at roughly the same frequencies as those obtained when tnpA was carried in cis. Relative transposition frequencies when cis-preferring transposases act in trans are 100-fold lower (Isberg and Syvanen, 1981; Machida et al., 1982; Morisato et al., 1983; Derbyshire et al., 1990). It is unlikely that tnpA gene expression is higher in pSU2637 than in pSU2631, as expression signals are conserved in both constructions.
IS 91 one-ended transposition
After the excision of IRL, IS91 transposable cassettes undergo one-ended transposition at frequencies similar to the transposition frequency of IRL-kan-IRR (Table 3). We can conclude that only the transposase, the 82 bp IRR region and the adjacent GTTC or CTTG sequences are involved in the interaction between a donor IS element and a target DNA sequence and, consequently, in the initiation of IS91 transposition. On the other hand, in all other transposons analysed, interaction between both IRs and the transposase is essential for transpositional cleavage and strand transfer reactions (Mizuuchi, 1992; Bainton et al., 1993; Sakai et al., 1995). One puzzling result appeared when one-ended transposition products were analysed with respect to their termination sites (Table 3). When one-ended transposition occurred at low levels (no induction), most recombinants (60–70%) were plasmid fusions in which RC transposition had finished before cat, so they do not contain an entire copy of the donor plasmid. Conversely, most fusion products were Cmr when Plac transcription was induced. It seems that RC transposition does terminate at more proximal sites when there is active transcription from Plac. The explanation cannot be that transposase levels affect site selection, as pSU2640 and pSU2641 both show the same effect, in spite of the fact that Plac induces TnpA expression in pSU2640 but not in pSU2641. One more likely explanation could be that transcription is diminishing the availability of DNA sites for termination directly. How this occurs has yet to be determined.
IS 91 specificity of insertion can be modulated by ORF121 accessory protein
IS91 uses the target sequences 5′-GTTC and 5′-CTTG indistinctly (Mendiola and de la Cruz, 1989; Table 5). However, IRL-kan-IRR, as well as the mutants in ORF121, showed a clear preference for the target sequence 5′-CTTG (Table 5). Thus, the cleavage site of these recombinants was 5′-CTTG/AT, that is the same cleavage site described for the pC194 group of rolling-circle replication plasmids and for phage φX174 (Gruss and Ehrlich, 1989; Novick, 1989). It can be inferred that ORF121 could be involved in target selection by broadening IS91 insertion specificity. As a consequence, the sequence 5′-GTTC could be used efficiently only in the presence of ORF121. Interestingly, ORF121 mutants used 5′-CTCG as a second preference site. This result can be interpreted in connection with the fact that alteration in just two or three of the IRL terminal nucleotides, that is in the sequence 5′-CTCG, abolished IRL function (Table 4). Thus, it seems that 5′-CTCG is a secondary cleavage site recognized by TnpA, while 5′-CTTG is the real primary site. This notion will explain why the termination reaction in IS91 transposition is not 100% efficient.
IS 91 intramolecular transposition results in second-site simple insertions
Intramolecular transposition of well-known transposons generates adjacent deletions. In the case of IS91, deletion formation could be analysed conveniently by looking for Cms derivatives of plasmids pSU2631 and pSU2632. These derivatives were in fact produced spontaneously upon storage of strains containing these plasmids, presumably because of high transposase intracellular levels. Their analysis showed that, in all cases, antibiotic resistance loss was caused by the insertion of a second IRL-kan-IRR element into cat. Insertion of a second cassette could be the result of intermolecular transposition, as IS91 did not show immunity to transposition (unpublished results). However, existing transposition models for other transposons do not predict this outcome as the unique result of intramolecular transposition. Simple secondary insertions occur in sequences adjacent to transposons, but always together with deletions and/or inversions (Galas and Chandler, 1989; Jilk et al., 1993; Craig, 1996). On the other hand, according to the RC transposition model that we proposed for IS91 (Mendiola et al., 1994), IS91-mediated intramolecular transposition would not result in adjacent deletions or sequence inversions. Rather, it would result in the insertion of a second element either as a direct or as an inverted repeat with respect to the pre-existing element, depending on which strand of the target sequence is nicked. The proposed mechanism is explained in Fig. 4. As we always found second-site insertions, but never deletions, when Cms derivatives were analysed, we believe that this result further strengthens the validity of our model of RC transposition. It should be noted that second-site insertions were obtained in only one orientation for the plasmids used in these experiments. All pSU2631 recombinants contained the second copy of IRL-kan-IRR inserted as an inverted repeat, whereas all pSU2632 recombinants contained the second copy inserted as a direct repeat. That is, irrespective of the orientation of IRR, insertions always occurred at the same orientation with respect to the plasmid origin of replication. A unique orientation was also produced when we analysed the transposition of a one-ended derivative of IS91 to the pBR322 plasmid derivative pSU4053 (Mendiola et al., 1994). We can offer no explanation for this orientation effect at the moment.
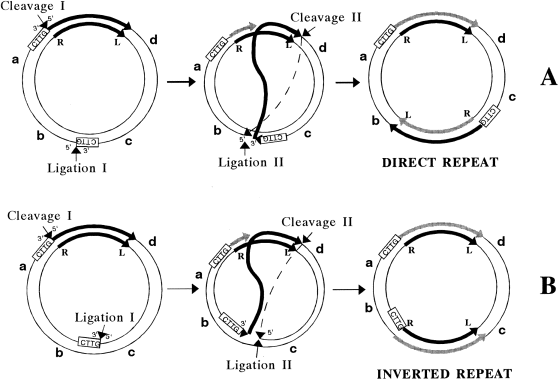
. Model of IS91 intramolecular transposition. Thick arrows represent IS91 sequences; black lines representing pre-existing IS91 sequences and grey lines representing newly synthesized IS91 sequences. R and L represent IRR and IRL respectively. a, b, c and d represent the position of four arbitrary plasmid genes. Small arrows represent transposase-mediated cleavage and ligation reactions. Cleavage I and Ligation I represent cleavage and ligation at IRR, while Cleavage II and Ligation II represent cleavage and ligation at IRL. IS91 transposase cleaves its recognition site at IRR in the sequence CTTG/AT (or GTTC/AT) of the IRR and presumably binds covalently to the resulting 5′ end. The transposase recognizes the target sequence CTTG; it cleavages one strand at the 3′ site of the target sequence and catalyses the first strand transfer event, which binds the 5′ end of the nicked IRR to the 3′ end of the nicked target DNA. Replication initiates from the newly exposed 3′ end of the nick site in the IRR as IS91 donor strand is displaced; as a result, the pre-existing IS91 copy is regenerated. Upon arrival to IRL, the transposase verifies the second strand transfer event by nicking the 3′ end of IRL and linking it to the 5′ end of the nicked target site. This product can be passively replicated by the bacterial replication machinery. As a result, the new IS91 copy would insert in two possible orientations with respect to the pre-existing copy, either as a direct repeat (A) or as an inverted repeat (B), depending on which strand is nicked in the target site.
Experimental procedures
Bacterial strains and plasmids
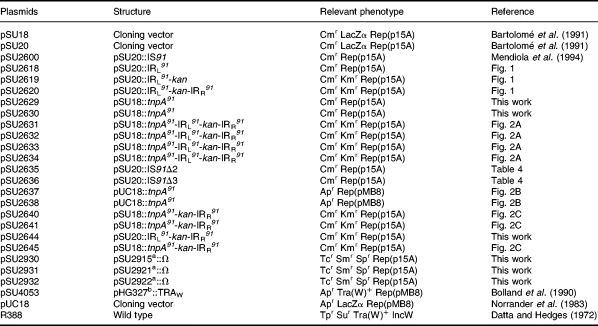
Bacterial strains used were E. coli K-12 derivatives DH5α [F−endA1 recA1 gyrA96 thi-1 hsdR17 supE44 relA1 Δ(arg F-lacZYA)U169 φ80ΔlacZΔM15; Grant et al., 1990], D1210 (F−recA lacI qrpsL hsdR hsdM ; Sadler et al., 1980), UB1637 (F−lys his trp rpsL recA56; de la Cruz and Grinsted, 1982) and UB5201 (F− pro met recA56 gyrA ; de la Cruz and Grinsted, 1982). Plasmids used are listed in Table 6.
Genetic techniques
Plasmid DNAs were introduced in bacteria either by transformation, using the method of Chung and Miller (1988), or by electroporation (Dower et al., 1988). Luria broth (LB; Sambrook et al., 1989) was used for bacterial growth in liquid. LB agar (1.5%) was used for growth on plates. Selective media included antibiotics at the following concentrations: sodium ampicillin (Ap) 100 μg ml−1; chloramphenicol (Cm) 25 μg ml−1; kanamycin sulphate (Km) 50 μg ml−1; nalidixic acid (Nx) 20 μg ml−1; streptomycin sulphate (Sm) 300 μg ml−1; and trimethoprim (Tp) 25 μg ml−1 (in Mueller–Hinton agar; Difco).
Transposition assays
These were carried out by a mating-out assay as described previously (Mendiola et al., 1992, 1994). Donor strains were derivatives of D1210 (Nxs) containing the conjugative plasmid R388 (Tpr) plus a non-mobilizable plasmid that carried the transposon. The recipient strain was DH5α. Before mating, donor strains were streaked out on LB agar plates containing antibiotics for plasmid selection and incubated at 30°C for 2 days. IPTG (Boehringer Mannheim) at a final concentration of 0.5 mM or glucose at a final concentration of 0.5% were added to the plates where indicated. Nalidixic acid was used to counterselect donors. Transposition frequency is the number of transconjugants carrying the transposon marker divided by the number of transconjugants carrying the marker of the conjugative plasmid (Tpr).
Construction and analysis of plasmids DNAs
Plasmid DNA was isolated by a modification of the alkaline lysis method of Ish-Horowicz and Burke (1981), as described previously (Martínez and de la Cruz, 1988). The method of Serghini et al. (1989) was used for small-scale preparations. Molecular cloning techniques were carried out according to Sambrook et al. (1989). Restriction endonucleases, the large fragment of DNA polymerase I (Klenow fragment; Pharmacia Biotech) and T4 DNA ligase (New England Biolabs) were used according to the manufacturers’ recommendations. Construction of plasmid pSU2620 is explained in Fig. 1. Construction of plasmids pSU2637, pSU2638, pSU2631, pSU2632, pSU2633 and pSU2634 is explained in Fig. 2. For the construction of plasmids pSU2635 and pSU2636, 5 μg of pSU2600 digested with XhoI restriction enzyme was treated with 0.8 U of S1 nuclease for 10 min at room temperature; then, plasmid DNA was treated with Klenow polymerase and religated. Construction of plasmids pSU2640, pSU2641 and pSU2645 is explained in Fig. 2. To construct plasmids pSU2930, pSU2931 and pSU2932, the Ω fragment of pHP45 (Prentki and Krisch, 1984), digested with EcoRI, was cloned at the single EcoRI site of plasmids pSU2915, pSU2921 and pSU2922 (Mendiola et al., 1992) respectively.
DNA sequencing
Sequencing was carried out by the dideoxynucleotide chain-termination method (Sanger et al., 1977) with the fmol DNA sequencing System (Promega), which is a polymerase chain reaction (PCR)-directed sequencing kit, and Sequenase version 2.0 (US Biochemical) using plasmid DNA as substrate. Primer oligonucleotides were labelled in their 5′ ends using T4 polynucleotide kinase (New England Biolabs) and [γ-32P]-ATP (3000 Ci mmol−1; Amersham). The oligonucleotides used were M13 reverse sequencing primer (5′-CAGGAAACAGCTATGAC-3′) (Boehringer Mannheim), and two oligonucleotides corresponding to positions 1761–1777 and 1790–1806 of the upper strand of IS91 respectively (Mendiola et al., 1992) (5′-ACAAGGGGTGTTGAAG-3′ and 5′-GTGGTGCTTTTTTAGTC-3′).
Footnotes
Acknowledgements
This work was supported by a grant (PB95-1269) from the DGICYT (Spain) to Fernando de la Cruz. I.B. was a recipient of an FPI fellowship from the Spanish Ministry of Education.




