Introgression patterns in the mosaic hybrid zone between Mytilus edulis and M. galloprovincialis
Abstract
Hybrid zones are fascinating systems to investigate the structure of genetic barriers. Marine hybrid zones deserve more investigation because of the generally high dispersion potential of planktonic larvae which allows migration on scales unrivalled by terrestrial species. Here we analyse the genetic structure of the mosaic hybrid zone between the marine mussels Mytilus edulis and M. galloprovincialis, using three length-polymorphic PCR loci as neutral and diagnostic markers on 32 samples along the Atlantic coast of Europe. Instead of a single genetic gradient from M. galloprovincialis on the Iberian Peninsula to M. edulis populations in the North Sea, three successive transitions were observed in France. From South to North, the frequency of alleles typical of M. galloprovincialis first decreases in the southern Bay of Biscay, remains low in Charente, then increases in South Brittany, remains high in most of Brittany, and finally decreases again in South Normandy. The two enclosed patches observed in the midst of the mosaic hybrid zone in Charente and Brittany, although predominantly M. edulis-like and M. galloprovincialis-like, respectively, are genetically original in two respects. First, considering only the various alleles typical of one species, the patches show differentiated frequencies compared to the reference external populations. Second, each patch is partly introgressed by alleles of the other species. When introgression is taken into account, linkage disequilibria appear close to their maximum possible values, indicating a strong genetic barrier within all transition zones. Some pre- or postzygotic isolation mechanisms (habitat specialization, spawning asynchrony, assortative fertilization and hybrid depression) have been documented in previous studies, although their relative importance remains to be evaluated. We also provided evidence for a recent migratory ‘short-cut’ connecting M. edulis-like populations of the Charente patch to an external M. edulis population in Normandy and thought to reflect artificial transfer of spat for aquaculture.
Introduction
Barriers to gene flow promote genetic divergence between populations, differential adaptation and eventually speciation. This depends on their intrinsic strength and on the dispersal potential of the organism concerned (Barton 1979; Barton & Bengtsson 1986). In hybrid zones, an equilibrium is reached between the homogenizing effect of migration and forces acting to maintain the integrity of parental genomes (e.g. selection against hybrids, Barton & Hewitt 1985). This results in stable clines of allele frequencies at loci directly involved in reproductive isolation (review in Barton & Hewitt 1989) and in genetic barriers to neutral gene flow (Barton 1979, 1986; Bengtsson 1985; Barton & Bengtsson 1986). Hybrid zones are therefore excellent systems to investigate the structure of genetic barriers (Hewitt 1988), especially if a range of organisms displaying contrasted dispersal abilities is studied. However, most empirical studies concern terrestrial organisms with low to moderate migration rates, which thus typically display narrow hybrid zones (up to tens of kilometres wide: reviewed by Barton & Hewitt 1985, 1989; Harrison 1993; Arnold 1997). In contrast, most marine organisms with planktonic larvae are characterized by extensive dispersal abilities. Unfortunately, there are only few detailed studies of marine hybrid zones (reviews in Palumbi 1994; Gardner 1997).
The hybrid zone between the blue mussels Mytilus edulis and M. galloprovincialis along the Western European coast has revealed an unusual complexity. It has considerable extension, from the Southwest of France to Scottish coasts (Skibinski et al. 1978, 1983; Coustau et al. 1991) and has a mosaic structure, in which populations of parental genotypes alternate with mixed populations (Skibinski et al. 1983; Coustau et al. 1991; Daguin et al. 2001). Mosaic structures usually are explained by differential adaptation in patchy environment (Harrison & Rand 1989). However, the maintenance of genetically contrasted patches by local adaptation requires that the scale of dispersal does not greatly overwhelm that of ecological variation (the environmental grain sensuLevins 1968). In mussels, the spatial distribution of alleles correlates with environmental factors such as salinity, wave exposure and tidal height (Gosling & Wilkins 1981; Skibinski 1983; Skibinski et al. 1983; Gardner & Skibinski 1988; Gosling & McGrath 1990; review in Gardner 1994) at a local scale. If, on one hand, increasing evidence (Hellberg et al. 2002; Swearer et al. 2002) and theoretical consideration about the evolution of dispersal (Strathmann et al. 2002) suggest that local recruitment may be much more common in marine organisms than thought previously, on the other hand it is still accurate that a substantial proportion of long-range larval dispersal could account for the massive gene flow often recorded in genetic surveys (Palumbi 1992; Strathmann et al. 2002). In mussels, for instance, a survey of the range expansion of recently introduced M. galloprovincialis in South Africa has indeed revealed that the great majority of successful recruits appeared within < 5 km of the parent population, but it has also revealed a spread of > 50 km per year in the direction of currents and > 10 km against currents (McQuaid & Phillips 2000). In addition, intraspecific genetic variation rarely shows significant differentiation over huge distances in Mytilus (e.g. the whole Mediterranean sea) and only slight differentiation is observed in trans-oceanic comparisons (McDonald & Koehn 1988; Varvio et al. 1988; McDonald et al. 1991; Sanjuan et al. 1997). However, mussels dispersal seems to overwhelm the scale of presumably important environmental variation (e.g. sheltered/exposed or estuarine/oceanic habitats) as well as the observed patchiness in genetic variation. The alternation between sheltered and exposed habitats is sometimes very rapid, and M. galloprovincialis as well as mixed adult populations are found as soon as exposed habitats begin, sometimes no more than a few hundreds of metres apart from M. edulis-recruiting sites (Bierne et al. in press). In such conditions, the maintenance of this fine-grained mosaic structure requires very strong selection (Slatkin 1973; Harrison & Rand 1989) and/or habitat choice (Rice 1987; Gosling & McGrath 1990; Kingsford et al. 2002).
Several factors may have limited progress in our comprehension of the European hybrid zone between M. edulis and M. galloprovincialis. (i) The geographical coverage of this very large zone is incomplete, because sampling has been much less intensive along the Atlantic coasts of France (but see Coustau et al. 1991; Daguin et al. 2001) than around the British Isles (Skibinski et al. 1983). (ii) Most studies of the M. edulis/M. galloprovincialis hybrid zone have used allozymes. Cases of direct selection have been described in intraspecific surveys on some of them (e.g. the leucine amino-peptidase locus in pure M. edulis populations of Long Island Sound, Koehn et al. 1980; Hilbish & Koehn 1985). Although the possibility that all the allozymes used are under selection seems remote, it is important to recall that these loci have been chosen because they were the most diagnostic between M. edulis and M. galloprovincialis. Unfortunately, the uncertainty around the neutral or selected status of allozymes makes them difficult to interpret, as different inferences can be drawn from neutral and selected clines using hybrid zone theory. In addition, the five allozymes used classically in Mytilus spp. populations genetics might belong to a single linkage group (Beaumont 1994). In particular, the two most-used loci, Est-D and Odh, are either linked or under strong epistatic interaction (Hilbish et al. 1994). To circumvent a possible redundancy, it is thus desirable to expand the sampling of nuclear loci in studies of Mytilus hybrid zones after having verified that these new loci themselves do not belong to a single linkage group. (iii) A third problem lies with the analysis of hybridization itself. Although previous surveys (Skibinski et al. 1983; Coustau et al. 1991; Daguin et al. 2001) have well documented geographical variation in allele frequencies, the analysis of Hardy–Weinberg and linkage disequilibria, two crucial parameters according to hybrid zone theory, has remained insufficient for two reasons. The first is the lack of a proper statistical technique to analyse multilocus data under the framework of hybrid zone theory. As a consequence, the term ‘hybrid population’ masks a diversity of situations: while some allegedly hybrid populations can show various degrees of disequilibrium (Skibinski et al. 1983; Coustau et al. 1991; Daguin et al. 2001), others are in Hardy–Weinberg plus linkage equilibrium (HWLE). The second reason lies with the necessary use of reference populations that represent parental gene pools. Previous studies on the M. edulis/M. galloprovincialis hybrid zone have often used two regions, one in the North Sea and the other in the Mediterranean, as reference populations for M. edulis and M. galloprovincialis, respectively (Skibinski et al. 1983; Coustau et al. 1991). However, this ignores within-species genetic differentiation. For instance, Daguin et al. (2001) have pointed out that M. galloprovincialis populations from Atlantic coasts of the Iberian Peninsula, which may serve as parental populations for hybridization in the south of France, differ from their Mediterranean conspecifics. The mosaic hybrid zone between M. galloprovincialis and M. edulis is so large that geographical differentiation within each parental species must be taken into account.
In the present study we propose to refine our knowledge on the genetic structure of the zone along the Atlantic coast of France. Three length-polymorphic DNA loci were used, one in the adhesive plaque protein gene, Glu-5′ (Rawson et al. 1996), and two introns, mac-1 (Ohresser et al. 1997; Daguin et al. 2001) and EFbis (Bierne et al. 2002). The last two loci correspond to noncoding parts of the genome and display sufficient variability to reveal possible genetic differences between patches of the same species. Genetic analysis of F2s and backcrosses did not show evidence of linkage between these three loci (N. Bierne, unpublished results). Three independent genomic regions were therefore screened here, although the possibility that one of these three loci might belong to the suspected Est–Odh linkage group cannot be ruled out. Departure from HWLE was analysed using recently developed maximum-likelihood multilocus estimates of between-genome associations (Hardy–Weinberg disequilibrium) and within-genome associations (linkage disequilibrium), a well-suited method for hybrid zone analysis (Barton 2000). To evaluate the level of associations among genes, we avoided the use of a single reference population for each species but rather identified a posteriori parent populations that are adjacent to regions where hybridization is detected.
Materials and methods
Collection of samples
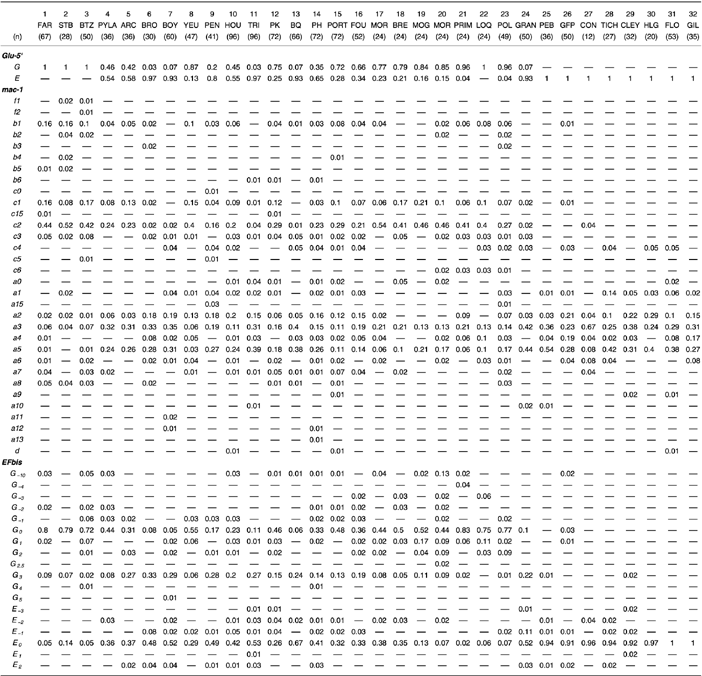
Mytilus spp. samples of adult individuals (size > 2 cm) were collected at 32 localities along the European Atlantic shore numbered according to their order along a South–North gradient (Fig. 1). Two samples were difficult to number a priori because they are located in the British Isles (samples 23 and 27). These were in fact numbered a posteriori, that is after the genetic analysis, to simplify and clarify the presentation of the complex structure of the hybrid zone (see Results). Sample 23 in Southwest Britain appeared to be very similar to populations of Brittany just on the other side of the English Channel, and sample 27 very similar to populations in the North Sea. Samples numbered 1, 2, 3, 6, 7, 8, 9, 16, 23, 26, 31 and 32 in the present study are, respectively, samples FAR, STB, BTZ, BRO, BOY, YEU, PEN, FOU, POL, GFP, FLØ and GIL of Daguin et al. (2001). We used the DNA extracts prepared by these authors. Individuals of all the other samples were all sized and preserved in alcohol (80%) immediately after collection and the extraction of their genomic DNA was performed using the Chelex protocol as described in Bierne et al. (1998). Individual sizes are also available for samples 9, 31 and 32.
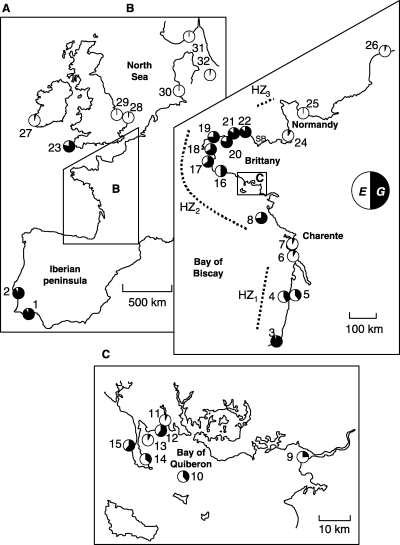
Sampling localities for Mytilus spp. in the northeastern Atlantic. (A) Western Europe. (B) Atlantic coast of France. (C) Bay of Quiberon area. Allelic frequencies, averaged over loci, of the samples after collapsing alleles characteristic of M. edulis and M. galloprovincialis into compound alleles E and G (see text and Fig. 4) are also given. Sample names and sample sizes (in brackets) are the following: 1, Faro (n = 67); 2, Setubal (n = 28); 3, Biarritz (n = 50); 4, Pyla (n = 36); 5, Arcachon (n = 36); 6, Brouage (n = 30); 7, Boyard (n = 50); 8, Ile d’Yeu (n = 47); 9, Penestin (n = 41); 10, Ile de Houat (n = 96); 11, Trinité-sur-Mer (n = 96); 12, Pointe de Kerbihan (n = 72); 13, Bay of Quiberon (n = 66); 14, Port Haliguen (n = 72); 15, Portivy (n = 72); 16, Foret-Fouesnant (n = 52); 17, Morgat (n = 24); 18, Brest (n = 24); 19, Moguériec (n = 24); 20, Morlaix (n = 24); 21, Primel (n = 24); 22, Locquémau (n = 24); 23, Polzeath (n = 49); 24, Granville (n = 50); 25, Port-en-Bessin (n = 36); 26, Grand-Fort-Philippe (n = 50); 27, Connemara (n = 12); 28, Tichwell (n = 27); 29, Cley (n = 32); 30, Helgoland (n = 20); 31, Flødevigen (n = 53); 32, Gilleleje (n = 35). The ‘SB’ symbol indicates the localization of the Saint Brieuc sample of the Coustau et al. (1991) study.
Molecular markers
DNA fragments at loci mac-1, EFbis and Glu-5′ were PCR-amplified using the following primer sequences: forward (5′-CGTCTAGCGTAGTACTTAAATTG-3′) and reverse (5′-CGAAAATTGTAGTCTAGTTTTGTG-3′) primers of Daguin & Borsa (1999) for locus mac-1; EFbis-F (5′-ACAAGATGGACAATACCGAACCACC-3′) and EFbis-R (5′-CTCAATCATGTTGTCTCCATGCC-3′) for locus EFbis (Bierne et al. 2002); Me15 (5′-CCAGTATACAAACCTGTG AAGAC-3′) (Inoue et al. 1995) and a newly designed reverse primer, Me17 (5′-CTGGTGGATAATTTGTCTTTGC-3′), for locus Glu-5′.
The nomenclature for mac-1 size-alleles was that of Daguin et al. (2001). In a previous study (Bierne et al. 2002) we have already used locus EFBis to analyse experimental crosses between M. edulis and M. galloprovincialis. Five alleles have been described in these crosses, four (G0, G1, G2 and G3) being found in M. galloprovincialis and one (now called E0) in M. edulis. G0 and E0 were sequenced previously and the sequences were deposited in GenBank under accession nos AF424742 and AF424743, respectively. All other size-alleles, including G1, G2 and G3, and all novel size alleles observed in this study were numbered consecutively by increasing size. The electrophoresis method used here (see below) does not allow precise measurement of the length of an allele and only a relative scale can be used. Consecutive alleles are thought to differ by ∼1 pb and Gi and Ei size classes are separated by approximately ∼15 pb. Locus Glu-5′ exhibited two alleles with primers Me15 and Me17, a short one of approximately 160 bp (here called G) and a long one of approximately 210 bp (called E). Genotype scoring using the new Me15/Me17primer pair was straightforward as no secondary bands due to nonspecific priming (Rawson et al. 1996) were visible.
Two kinds of labelling techniques, radioactivity and fluorescence, were used. When radioactive labelling was used, polymerase chain reactions (PCR) and electrophoresis were performed for all three loci as described in Daguin & Borsa (1999) except that annealing temperatures for the Glu-5′ and EFbis loci were set at 54 °C. Using the fluorescent dye 5′ end-labelled-primer technique, the dye 6-FAM (Sigma Genosys) was used to label the forward primers for mac-1 and Glu-5′ while primer EFbis-F was labelled with the dye TAMRA (Sigma Genosys). The concentration of both forward and reverse primers were adjusted to 400 pm in the PCR reaction mixture, other parameters remaining unchanged (see Daguin & Borsa 1999). In the particular case of fluorescent dyes, after electrophoresis gels were scanned in a FMBIO II fluorescence imaging system (Hitachi Instruments) at 505 nm and 605 nm.
Data analysis
Genetic differentiation was studied by performing correspondence analysis (CA) on the matrix of allele counts per sample using the genetix 4.03 software (Belkhir et al. 2002). CA is particularly well-suited for hybrid zone analysis as it allows extraction of the between-species differentiation on the first axis of the CA (CA1), whereas differentiation between populations of the same species emerges on secondary axes (Dod et al. 1993; Daguin et al. 2001). Guinand (1996) has shown that the eigenvalue associated with each factorial axis is analogous to a partial FST estimate. CA also offers the advantage of simultaneously expressing the genetic differences present in the data set and the respective contributions of each allele to these differences. Estimations of FST were performed using Weir & Cockerham's (1984) estimator θ, and population differentiation tested using the permutation procedure in genetix 4.03 (Belkhir et al. 2002).
Within-species diversity is not relevant to the analysis of disequilibria within the hybrid zone framework that considers only disequilibria between pairs of alleles typical of either species (Barton 2000). To solve this problem, alleles at a single locus were pooled into species-specific compound alleles according to their coordinates on CA axis 1, as described in Daguin et al. (2001). Pairwise associations between genomes (κ1,1, Hardy–Weinberg disequilibrium) and within genomes (κ0,2, linkage disequilibrium) were estimated following the method described in Barton (2000), using mathematica 3.0 (Wolfram 1996) add-ons provided by N. Barton on his website (http://helios.bto.ed.ac.uk/evolgen/). The usefulness of this method is that both linkage and Hardy–Weinberg disequilibria are estimated jointly. As recommended by Barton (2000), the likelihood of different nested models was estimated: we assumed first that the population is in HWLE, then allowed for pairwise associations between genomes (Hardy–Weinberg disequilibrium), within genomes (linkage disequilibrium) or both, and finally for higher–order associations. The same sequence of models was repeated allowing different contributions of each locus to the association. The most appropriate model was chosen using the Akaike information criterion under which model 2 is more likely than model 1 if log(L2)-2ν > log(L1), where ν is the number of additional parameters used in model 2 relative to model 1. A 95% confidence interval (CI) was estimated using a random walk algorithm with parameter T (temperature of the simulated annealing) initially set up to 1 (Barton 2000).
Results
Analysis of the total genetic variation
Thirty-two alleles differing by size were detected in the total sample at locus mac-1. These included all alleles already reported by Daguin et al. (2001), except allele f3, and two novel alleles, b6 (with an intermediate length between c0 and b5) and a13 (shorter than a12). At locus EFBis, 18 alleles were detected in the total sample and denominated according to their size by using alleles E0 and G0 of Bierne et al. (2002) as references. G0 and E0 were the most frequent alleles in Atlantic M. galloprovincialis populations and M. edulis populations, respectively. Six alleles were close in size to E0 (E−3, E−2, E−1, E0, E1 and E2) and 12 alleles were close in size to G0 (G−10, G−4, G−3, G−2, G−1, G0, G1, G2, G2.5, G3, G4 and G5). Allele frequencies are presented in Appendix I.
Figure 2A is the projection of samples on the plane defined by the first two factorial axes and Fig. 2B is their projection on the plane defined by the first and the third axes of the CA. The eigenvalue (analogous to a partial FST estimate) of the first axis was 0.49, a value that indicates strong differentiation among samples. Axis 1 reflects mainly the allele frequency gradient between the two species, from M. galloprovincialis on the left to M. edulis on the right. Differentiation was also clearly visible on the second and third axes. The second axis, which has an eigenvalue of ∼0.06, highlights differences among populations with positive first axis coordinates that share a high frequency of alleles characteristic of M. edulis. Two groups of genetically homogeneous and geographically distinct populations may be defined: a group of populations from the North Sea (generally considered as pure M. edulis) clustered on one side and a group of populations from the Bay of Biscay clustered on the other side (Fig. 2A). A slight differentiation between these two groups is also visible along CA axis 1 (Fig. 2B), revealing that populations of the Bay of Biscay group possess some alleles characteristic of M. galloprovincalis at low frequency (see below). We will refer to these two groups hereafter as ‘North Sea M. edulis’ and ‘Bay of Biscay M. edulis-like’ populations.

Correspondence analysis (CA) on the matrix of allele counts per sample. (A) Projection of samples on the plane defined by the two first factorial axes of the CA. The star is a subsample from sample 24 after M. galloprovincialis-like individuals have been removed. (B) Projections of samples on the plane defined by the first and the third axes of the CA.
The third axis, which has an eigenvalue of ∼0.05, highlights differences among populations with negative first axis coordinate that share a high frequency of alleles characteristic of M. galloprovincialis. Once again, two groups of genetically homogeneous and geographically distinct populations can be defined: M. galloprovincialis populations of the Atlantic coast of the Iberian Peninsula on one hand and populations of Brittany and SW England on the other hand (Fig. 2B). Slight differences between these two groups are also visible along CA axis 1 (Fig. 2A), revealing that populations of Brittany and SW England possess some alleles characteristic of M. edulis at a higher frequency than M. galloprovincialis populations of the Atlantic coast (see above). We will further refer to these two groups as ‘Iberian M. galloprovincialis’ and ‘Brittany M. galloprovincialis-like’ populations.
Before analysing further the geographical interspecific structure by using compound, species-specific alleles, the total variation may be used to track for heterogeneity among loci. To do this, F-statistics analysis was also performed. The three marker loci do not contribute equally to the differences between North Sea M. edulis and Bay of Biscay M. edulis-like populations, with θ = 0.02 (P < 0.001) at loci Glu-5′ and mac-1, while at locus EFbis θ = 0.16 (P < 0.0001). This is due mainly to one allele, EFbis-G3, as it can be deduced from the relative contributions of alleles to axis 2 of the CA (Table 1). EFbis-G3 has a frequency of approximately 0.30 in Bay of Biscay M. edulis-like populations while it is completely absent from North Sea M. edulis populations and is rare in Iberian M. galloprovincialis as well as Brittany M. galloprovincialis-like populations (Fig. 3B). This allele is therefore characteristic of populations from the Bay of Biscay, in the midst of the hybrid zone, a feature that had never been noticed previously in this zone with other markers. The degree of genetic differentiation was also unequal among loci between the two groups assigned as Iberian M. galloprovincialis and Brittany M. galloprovincialis-like populations in Fig. 2B. The differentiation appears to be too strong for the FST-values to remain undisturbed by the strong differences in variability among loci (from two alleles for Glu-5′ to 32 alleles for mac-1) (Hedrick 1999). The highly significant FST are, respectively θ = 0.08, θ = 0.04, θ = 0.02 at loci Glu-5′, EFbis and mac-1 using the total variation. However, using the artificially constructed bi-allelic loci (see below) FST values become θ = 0.08, θ = 0.03 and θ = 0.15, respectively. We are left with the fact that genetic differences between these two groups of populations are unequally distributed among loci as well as among alleles within loci (Table 1).
| Axis 1 | Axis 2 | Axis 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Locus | Allele | Contribution | Locus | Allele | Contribution | Locus | Allele | Contribution |
| Glu-5′ | G | 0.982 | EFbis | G 3 | 0.793 | mac-1 | c6 | 0.534 |
| Glu-5′ | E | 0.978 | mac-1 | a4 | 0.340 | mac-1 | b5 | 0.315 |
| Mac-1 | c2 | 0.895 | mac-1 | a6 | 0.265 | EFbis | G 1 | 0.289 |
| EFbis | G 0 | 0.845 | EFbis | E 2 | 0.233 | EFbis | G 2 | 0.265 |
| mac-1 | c1 | 0.783 | EFbis | E 0 | 0.185 | mac-1 | b4 | 0.252 |
| EFbis | E 0 | 0.776 | EFbis | E −1 | 0.181 | mac-1 | b1 | 0.241 |
| mac-1 | a5 | 0.762 | EFbis | E −2 | 0.106 | EFbis | G −4 | 0.215 |
| mac-1 | b1 | 0.624 | mac-1 | b6 | 0.105 | EFbis | G 2−5 | 0.202 |
| mac-1 | a3 | 0.579 | EFbis | G 5 | 0.085 | mac-1 | a8 | 0.174 |
| mac-1 | a8 | 0.345 | mac-1 | a11 | 0.085 | mac-1 | f1 | 0.174 |
| EFbis | E 2 | 0.295 | mac-1 | a9 | 0.084 | EFbis | G −10 | 0.141 |
| EFbis | G 1 | 0.271 | mac-1 | a12 | 0.080 | EFbis | G −3 | 0.136 |
| EFbis | G −2 | 0.234 | EFbis | E −3 | 0.069 | mac-1 | a7 | 0.112 |
| mac-1 | a7 | 0.230 | mac-1 | a1 | 0.047 | mac-1 | c3 | 0.082 |
| mac-1 | a2 | 0.227 | mac-1 | c0 | 0.045 | EFbis | G 0 | 0.072 |
| mac-1 | c3 | 0.223 | mac-1 | a10 | 0.044 | mac-1 | c15 | 0.070 |
| EFbis | G −1 | 0.215 | mac-1 | a5 | 0.041 | mac-1 | b2 | 0.069 |
| mac-1 | b2 | 0.200 | mac-1 | a15 | 0.041 | mac-1 | a4 | 0.051 |
| mac-1 | c4 | 0.164 | mac-1 | b5 | 0.035 | mac-1 | d | 0.033 |
| mac-1 | c6 | 0.154 | mac-1 | d | 0.033 | mac-1 | b3 | 0.030 |

Allele frequency in the 32 Mytilus spp. samples ranked by numerical order (see legend to Fig. 1). (A) Frequency of the G compound allele at each of the three nuclear loci. Samples with both significant Hardy–Weinberg and linkage disequilibrium are indicated by two stars and samples with significant linkage disequilibrium only, by one star. (B) Frequency of the G3 allele at locus EFbis.
Analysis with species-specific compound alleles
Alleles were pooled to form bi-allelic loci at both mac-1 and EFbis according to the outcome of the CA (Daguin et al. 2001; Bierne et al. in press). Sixteen size-alleles at locus mac-1 (a0, a1, a15, a2, a3, a4, a5, a6, a9, a10, a11, a12, a13, d, b6, c4) and seven size-alleles at locus EFbis (E−3, E−2, E−1, E0, E1, E2, G3) were pooled to form a compound E allele characteristic of M. edulis. The remaining 16 size-alleles at locus mac-1 (f1, f2, b1, b2, b3, b4, b5, c0, c1, c15, c2, c3, c5, c6, a7, a8) and 11 size-alleles at locus EFbis (G−10, G−4, G−3, G−2, G−1, G0, G1, G2, G2.5, G4, G5) were similarly pooled to form a compound G allele characteristic of M. galloprovincialis. Allele size generally, but not always, was characteristic of a species. The average frequency, over the three loci, of the compound G allele was presented as black sectors in the pie diagrams of Fig. 1. Clear mosaic structure was thus revealed. The frequencies of the compound G allele at each locus in each sample are presented in Fig. 3A. From South to North roughly three transitions were observed, delimiting two enclosed patches: (i) a first transition from Iberian M. galloprovincialis populations to Bay of Biscay M. edulis-like populations; (ii) a second, irregular transition with strong variation in allele frequencies over short distances, toward Brittany M. galloprovincialis-like populations; (iii) and a third transition toward North Sea M. edulis populations. This representation confirms that Bay of Biscay M. edulis-like populations exhibit M. galloprovincialis alleles at low frequency (0.05 < G < 0.1). This also illustrates the discrepancy among loci in the rate of introgression in the Brittany M. galloprovincialis-like populations. The latter harbour M. edulis alleles at high frequency at the mac-1 locus (E∼0.4), whereas they possess only few M. edulis alleles at locus Glu-5′ (E∼0.05) and resemble Iberian M. galloprovincialis populations at locus EFbis. Genetically intermediate populations were present in geographically intermediate locations, thus delineating three possible distinct hybrid zones (hereafter coined HZ1, HZ2 and HZ3 according to their position from south to north; see 1, 3).
Analysis of disequilibria
Pairwise associations of alleles at a locus between genomes, κ1,1 (Hardy–Weinberg disequilibrium) and across loci within a genome, κ0,2 (linkage disequilibrium) are presented as a function of synthetic G allele mean frequency in Fig. 4. The estimations of κ1,1 and κ0,2 were not possible in samples from the North Sea because M. edulis alleles were fixed for these populations. The theoretical values for these parameters were thus used for Fig. 4 (κ1,1 = 0; κ0,2 = 0). No disequilibrium was observed among M. edulis alleles (data not shown). Six samples showed significant Hardy–Weinberg, κ1,1 and linkage, κ0,2, disequilibrium (nos 4, 5, 9, 10, 14 and 24, indicated by two stars in Fig. 3A) and three samples a significant linkage disequilibrium, κ0,2, only (nos 16, 18 and 19, indicated by one star in Fig. 3A). Allowing for higher–order associations did not increase the likelihood value enough to match the Akaike criterion, except for samples 4 and 5.
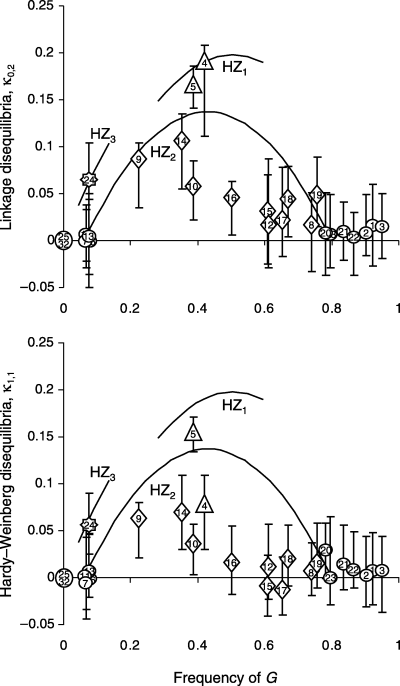
Pairwise associations within genomes, κ0,2 (corresponding to an average pairwise linkage disequilibrium) and between genomes, κ1,1 (corresponding to an average Hardy–Weinberg disequilibrium) estimated following Barton's method (Barton 2000) organized as a function of G allele frequencies. Samples within hybrid segment HZ1 are represented by triangles, samples within hybrid segment HZ2 are represented by diamonds, sample within hybrid segment HZ3 is represented by a cross, and other samples are represented by circles. Theoretical maximum values of κ1,1 and κ0,2 under different models (see text) are represented by curves. For clarity, only a part of the curve is shown for HZ1 and HZ3. Vertical bars represent 95% confidence intervals.
To evaluate Hardy–Weinberg and linkage disequilibria it is useful to examine their maximum values. However, maximum values depend on the level of differentiation between parental populations that are the source of hybridization (Barton 2000). For example, if both taxa are fixed for alternative alleles (M. edulis: E= 1, G= 0; M. galloprovincialis: E= 0, G= 1), the maximum value is κ0,2 = 0.25 for an allele frequency of 0.5, but if both taxa are differentiated incompletely (M. edulis: E= 0.8, G= 0.2; M. galloprovincialis: E= 0.2, G= 0.8), the maximum value decreases to κ0,2 = 0.09. Moreover, if introgression is asymmetrical the maximum value is not expected at an allele frequency of 0.5. If partly introgressed populations serve locally as parent populations for hybridization, and less introgressed populations external of the zone are taken as references, the maximum value is overestimated and consequently the level of hybridization is overestimated. This question does not usually arise in hybrid zones that consist of a single cline but is clearly relevant in the case of mosaic hybrid zones, especially here where differently introgressed patches are observed. Theoretical maximum values of κ1,1 and κ0,2 were provided under different null models, taking into account that the three possibly distinct hybrid segments (HZ1 to HZ3) involve differentially introgressed parental populations: Iberian M. galloprovincialis (G∼0.95) and Bay of Biscay M. edulis-like (G∼0.05) populations for HZ1, Bay of Biscay M. edulis-like (G∼0.05) and Brittany M. galloprovincialis-like (G∼0.8) populations for HZ2 and Brittany M. galloprovincialis-like (G∼0.8) and North Sea M. edulis (G∼0) populations for HZ3. Linkage disequilibria (κ0,2) were generally near their maximum values [except for the most M. galloprovincialis-like populations of HZ2 (Fig. 4)]. Hardy–Weinberg disequilibria (κ1,1) followed the same trend but were smaller. A more classical representation of genotypic frequencies within populations, the distribution of the hybrid index (number of G alleles per individual), is also given for representative populations located in and around HZ1 (Fig. 5).
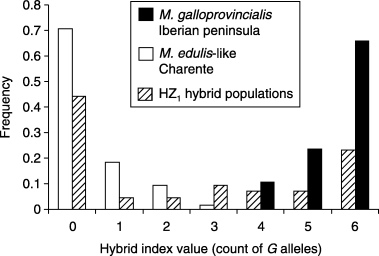
Distribution of individual hybrid index values (count of G alleles over the three loci in an individual) in M. galloprovincialis populations of the Iberian Peninsula (samples 1–3), in Charente, Bay of Biscay M. edulis-like populations (samples 6, 7) and in intermediate populations of segment HZ1 (samples 4, 5).
The case of sample 24 (Granville, in the Bay of Mont Saint-Michel, Fig. 1B) was an exception. This sample presented a strong association with M. edulis from the Bay of Biscay in the CA (Fig. 2) and was characterized by the large frequency (0.22) of allele G3 at locus EFbis. However, contrary to the Bay of Biscay M. edulis-like samples, alleles characteristic of M. galloprovincialis were not distributed randomly among individuals in sample 24. They were rather associated within the same individuals. The disequilibrium was so large that one could easily identify these M. galloprovincialis-like individuals (five of 50) and remove them. The first coordinate of the reduced sample, represented by a star in Fig. 2A, was significantly different from that of the complete sample. Thus sample 24 was a mixture of a few M. galloprovincialis-like individuals from the North of Brittany and many nonintrogressed M. edulis displaying, however, a high frequency of the G3 allele, typical of Bay of Biscay M. edulis-like populations.
Discussion
Genetic differences were detected among the populations analysed, belonging either to M. galloprovincialis and M. edulis external parent populations outside the contact zone, or to patches of M. edulis-like and M. galloprovincialis-like populations within the zone. Coupled with a multilocus analysis of Hardy–Weinberg and linkage disequilibrium, the present results shed light on patterns of hybridization and introgression in the French part of this complex mosaic hybrid zone.
The analysis of a hybrid zone depends on the status of the genetic variation screened with regard to reproductive isolation. Genes directly involved in reproductive isolation, for example those causing hybrid breakdown, form stable allele frequency clines (Barton & Hewitt 1981a, 1989). In contrast, neutral markers, such as the loci we used, are only slowed down by the genetic barrier created by isolation genes (Barton 1979, 1986; Bengtsson 1985; Barton & Bengtsson 1986). Neutral clines are transient unless the barrier is complete. Allele frequencies at neutral markers must ultimately reach homogeneity over the hybrid zone, although this may take a very long time. At a given time, the degree of introgression may vary among neutral loci depending on the amount of linkage to isolation genes, as observed in the grasshopper Podisma pedestris (Barton & Hewitt 1981b; Halliday et al. 1983) or in sunflower species (Rieseberg et al. 1999). Introgression (the progressive spread of neutral or favourable alleles from one species into the other) can be directly observed using allele frequencies at neutral markers. However, as mentioned above, only isolation genes are stable components in a hybrid zone, and hybridization (i.e. the temporary combination of two differentiated genomes within the same individual even if unfit) should ideally be detected using isolation genes rather than neutral markers. Neutral allele frequencies give little information in this respect. Fortunately, Hardy–Weinberg and linkage disequilibria at neutral loci do depend on hybridization and on isolation mechanisms because they reflect the extent to which isolation genes prevent the mixing of different gene pools within the same individuals. Briefly, when a population shows strong Hardy–Weinberg and linkage disequilibria at neutral markers, it means that alleles of the two species must be segregating at isolation genes. When Hardy–Weinberg and linkage equilibrium is observed, isolation genes are probably fixed for one particular species type, and the population is called ‘introgressed’ as soon as neutral alleles of the other species are observed. Hardy–Weinberg and linkage disequilibria at neutral loci therefore yield indirect estimation of the hybridization provided the current state of neutral introgression in the populations analysed is taken into account.
In the following sections, we discuss first the geographical distribution of allele frequencies and introgression, then the strength of isolation mechanisms attested by HWLE analysis within populations, and finally the possible impacts of mussel culture.
Several hybrid segments
Classical models of hybrid zones describe two pure populations separated by parallel, relatively narrow clines of allele frequencies resulting from the balance between migration and selection. Clines are often sigmoidal, nicely fitted by theoretical expectations (Barton & Hewitt 1989), but sometimes they are more irregular (Harrison & Rand 1989; Bridle et al. 2001). Narrow clines have been observed in many terrestrial species (Barton & Hewitt 1985, 1989; Harrison 1993; Arnold 1997). One could expect that marine species, whose main characteristic is a high dispersion potential, would show an elongated version of this model. However, this is not the image we observed for the M. edulis/M. galloprovincialis hybrid zone in France. Instead, 1, 3 indicate a noticeably discontinuous transition between M. galloprovincialis populations in the Iberian Peninsula and M. edulis populations in the North Sea. This discontinuity is manifested by the presence of a patch of M. edulis-like populations in the Bay of Biscay and a patch of M. galloprovincialis-like populations in NW Brittany and SW England. Both patches are separated from their external ‘conspecific’ populations (respectively, those of the North Sea and of the Atlantic side of the Iberian Peninsula) by the patch of the other species. Such a mosaic structure confirms and extends the patterns observed around the British Isles by Skibinski (1983) using allozymes, and in France by Coustau et al. (1991) and Daguin et al. (2001) using allozymes and the mac-1 markers, respectively. Mosaic hybrid zones have also been observed in terrestrial organisms (e.g. Harrison & Rand 1989; Howard & Waring 1991). They have been interpreted as a succession of clines (Harrison & Rand 1989; Cain et al. 1999) assuming a coarse-grained environment — i.e. dispersal is smaller than the grain of genetic and/or environmental variation. In the present case, the same sort of situation may occur and transitions correspond to steep (HZ1, HZ3 on 1, 3) or very irregular (HZ2) clines. The irregular structure observed within HZ2 would correspond to a fine-grained mosaic structure, the conditions of maintenance of which are still not clearly known (Slatkin 1973; Bridle et al. 2001).
Genetic originality of the internal patches
In both patches (Bay of Biscay and Brittany), HWLE within populations was not rejected. Therefore, there seems to be no restriction to gene exchange among individuals within these populations, meaning that the genes potentially involved in isolation between the two mussel taxa are in a fixed state (for M. edulis alleles in Bay of Biscay and M. galloprovincialis alleles in Brittany). We can therefore assume that each patch is a homogeneous, monospecific entity. However, the presence of alleles typical of M. galloprovincialis (in the Bay of Biscay) and M. edulis (in Brittany) in low to moderate frequencies demonstrate introgression. It was predictable that introgression be higher in internal patches than in external populations given the comparatively small geographical size of the internal patches and their being surrounded by genetically different populations on both sides.
Apart from introgression, internal patches also exhibit genetic originalities with respect to their intraspecific allelic composition, corroborating further their isolation from their conspecific external populations. To appreciate this, one needs to know whether alleles are in similar frequencies in the Bay of Biscay and in the North Sea external populations (and symmetrically for the Brittany patch and Spanish populations) letting aside the introgression by E or G alleles, respectively. The gradient of introgression being represented on axis 1 of CA, this question can be addressed by restricting the geographical analysis of allele frequencies to CA axes 2 and 3. Although the details of this analysis were complex, significant differentiation along these axes appeared between internal patches (Bay of Biscay, Brittany) and their respective conspecific external populations (respectively North Sea, Iberian Peninsula). This differentiation is not due to changes in the overall frequency of the M. edulis and M. galloprovincialis allele classes, but rather to changes in frequency of the different alleles within each class. Importantly, the Bay of Biscay M. edulis-like populations are characterized by a high frequency (∼0.3) of the G3 allele at locus EFbis that is absent or rare in the two reference populations (Fig. 3B). Such levels of differentiation, which cannot be related to the level of introgression, were not noticed previously. They suggest that, despite considerable potential for the dispersal of mussels through their planktotrophic larval stage, actual dispersal is insufficient for genes to ‘jump over’ patches of the other species, nor can they diffuse progressively through them. The patterns of geographical differentiation uncovered in the present study may have been masked previously by the lack of intraspecific variation at the classical diagnostic enzyme loci (e.g. Est-D and Mpi, Beaumont et al. 1989) and by the focus on interspecific differences.
Another surprising feature of the genetic differences between internal patches and external populations (be they related or not to introgression) is their unequal distribution among loci. Differences seem to concentrate on a single locus, EFbis for the Bay of Biscay M. edulis-like patch and mac-1 for the M. galloprovincialis-like Brittany patch. This echoes the previous finding that allozyme loci are frequently differentially introgressed within populations of the Northwestern Atlantic area (Skibinski et al. 1983; Coustau et al. 1991). Such locus-specific structures might suggest the action of positive selection; however, EFbis and mac-1 are noncoding molecular markers and direct selection is therefore unlikely. The indirect effect of directional selection at linked loci (genetic hitch-hiking) is another hypothesis that cannot be ruled out. More investigation is needed to evaluate neutral and selective historical scenarios in explaining the distinctive genetic identity of the Bay of Biscay and Brittany patches in Mytilus spp.
Barrier strength and hybridization
As explained at the beginning of this section, testing for Hardy–Weinberg and linkage equilibria provides evidence for the existence of genetic isolation and/or hybridization. Isolation mechanisms should be apparent in populations situated within transitions in the form of Hardy–Weinberg and linkage disequilibria. Hybridization can be detected when these parameters do not reach their maximum possible values (values expected assuming a mixing of parental populations without interbreeding).
The HZ1 and HZ3 zones are both located between an external population and an introgressed patch related to the other species (1, 3). They exhibit maximal linkage disequilibrium (Fig. 4). Of course, our sampling is scarce in these two zones but we had already some indication, from allozymes (Coustau et al. 1991), that the two genomes mix very little in HZ3. A sample from Saint-Brieuc [numbered 3 in Fig. 1 of Coustau et al. (1991), see the ‘SB’ symbol in Fig. 1B for localization] departed significantly from Hardy–Weinberg equilibrium and even if linkage disequilibria were not provided in Coustau et al.′s (1991) study, the high variance of their hybrid index value was indicative of a large disequilibrium (Barton & Gale 1993; Kruuk et al. 1999). The two best-studied populations of the hybrid zone, Croyde and Whitsand (see review in Gardner 1994) are located in SW England, just across the English Channel. In these populations, the strong linkage disequilibria or bimodal hybrid index distributions often recorded attest effective reproductive isolation (Skibinski et al. 1983; Gardner & Skibinski 1991; Gardner et al. 1993; Gardner 1994; Hilbish et al. 1994). Regarding HZ1, Lubet (1959) found a very low proportion of intermediate morphotypes in Arcachon (our sample 5) and a strong correlation between morphotype composition and salinity. Comesaña & Sanjuan (1997) also observed a strong microgeographical differentiation at allozyme loci in Cap Breton in the south of HZ1.
The large linkage disequilibria observed raises the question of whether hybridization occurs at all. The answer is that it does. Indeed, Hardy–Weinberg disequilibria on average reach only half of their maximum possible values (Fig. 4), indicating substantial proportions of EG heterozygotes. Moreover, the distributions of the hybrid index (example of HZ1 in Fig. 5) show that it is not possible to classify hybrid individuals unambiguously into various classes of cross (Arnold 1997) such as parental or F1 genotypes (Barton 2000) with our three loci. This suggests that more than one generation of hybridization occurs. The apparent discrepancy between near-to-maximal linkage disequilibria and relatively modest HW disequilibria was expected, because hybridization removes the latter more efficiently. For example, one generation of random mating would suffice to restore Hardy–Weinberg equilibrium while it would take several generations to restore linkage equilibirum (even if loci are physically unlinked). To summarize, hybridization does occur in HZ1 and HZ3, but isolation mechanisms (either pre- or postzygotic) are strong and prevent complete mixing of the M. edulis and M. gallorprovincialis gene pools.
Previous studies have provided insight into the isolation mechanisms at play. Post-settlement selection is probably important in this respect. Within mixed populations, alleles specific to M. galloprovincialis increase in frequency with size and age, due to differential viabilities between the juvenile and adult stages (Skibinski 1983; Gardner & Skibinski 1988; Skibinski & Roderick 1991; Gardner et al. 1993; Wilhelm & Hilbish 1998). Differential susceptibility to wave erosion in exposed shores has been proposed as an explanation (Gardner & Skibinski 1991; Willis & Skibinski 1992). One case of symmetric advantage to M. edulis-like genotypes during the settled phase has been documented in southwest Britain (Gilg & Hilbish 2000). These authors reported a decrease in M. galloprovincialis allele frequency at locus Glu-5′ during the first few weeks after settlement. On the other hand, Gosling & McGrath (1990) have observed that the strong segregation of M. edulis-like and M. galloprovincialis-like spat according to tidal level, disappeared in adults, following a uniformly large increase in G allele frequencies in all sites. These authors suggested that tide level-related structure relied on presettlement or very early postsettlement processes. All in all, what is known of selection in the adult stage rather suggests a systematic advantage for M. galloprovincialis genotypes than a disadvantage of hybrids. On the contrary, hybrid genotypes seem to be relatively vigorous at the adult stage (Gardner 1994). Increasing evidence points to isolation mechanisms acting early in the life cycle. Unlike those on postsettlement processes (see above), the following studies do not suggest directional selection and may therefore provide explanations of why genetic barriers are observed in the hybrid zone. Spawning asynchrony (Seed 1971; Gardner & Skibinski 1990; Secor et al. 2001) and assortative fertilization (Bierne et al. 2002) are documented premating isolation mechanisms that can effectively lower the production of hybrid mussels. Early hybrid depression in the larval stage has also been observed (Beaumont et al. 1993; Bierne et al. 2002).
Because selection may act throughout the life cycle, HWLE analysis may be hard to interpret if individuals are sampled at different ages in different localities. However, all mussels collected in this study were > 2 cm, a stage at which the selection documented by previous studies has already occurred and reproduction starts. In the samples where E and G alleles coexist and individual sizes are known (4, 5, 10–15, 24), no significant correlation was found between average shell-length and allele frequency or hybrid index (data not shown), suggesting that the sampling has been performed in sufficiently old individuals, after selection had operated. Note also that Hardy–Weinberg and linkage disequilibria should not be affected equally by the effect of age at sampling. Hardy–Weinberg disequilibria are broken down in one generation of hybridization only, and are therefore sensitive to the age at sampling (i.e. before or after selection). On the other hand, linkage disequilibria are only partly broken down by recombination and therefore register what has happened during previous generations. They are expected to be less sensitive to the age effect. Finally, there is little reason for a sampling size- or age bias to be correlated with geography as allele frequencies are.
The case of HZ2, the zone that separates the central patches of M. edulis-like and M. galloprovincialis-like populations, is more complex than HZ1 or HZ3. At first glance, some populations from this part of Brittany have intermediate allele frequencies (∼50%E) and do not show large departure from HWLE (e.g. populations 12, 15 and 17). The same observation was made by Coustau et al. (1991), who concluded that considerable interbreeding occurs. However, we also observed three samples (9, 10 and 14) with approximately 1/4–1/3 E alleles, that exhibited very high departure from HWLE. Although Coustau et al. (1991) did not observe any sample with allele frequencies near 1/4–1/3, Viard et al. (1994) actually did so in south Brittany (sample F90 in their Fig. 2). In agreement with our results, their sample exhibited significant departure from Hardy–Weinberg equilibrium and a high variance in the hybrid index. We conclude that hybridization is not extensive in this zone, and that barriers to hybridization are present at least locally. How can we explain that such barriers are expressed mainly when the E allele frequencies approach 1/4–1/3 rather than the usual 1/2? Our interpretation is that local barriers occur between already partly and asymmetrically introgressed genomes (in our case, M. edulis-like mussels have 6%G alleles, and M. galloprovincialis-like mussels have ∼20%E alleles or perhaps even more locally). Taking this into account, some disequilibria appear to be close to their maximum possible values (samples 9 and 14). Had Iberian M. galloprovincialis and North sea M. edulis been used as reference populations (as usual) instead of local introgressed parental populations, the strength of isolation mechanisms, as attested by departures from HWLE, would have been underestimated.
Our results suggest that genetic barriers are present in all three hybrid segments. Do these barriers add up to isolate M. edulis and M. galloprovincialis at the scale of western Europe? We have seen that dispersal is probably insufficient to effectively connect internal patches to conspecific external populations. Therefore, to introgress the M. galloprovincialis genome of the Iberian Peninsula, a M. edulis neutral allele from the North Sea has to go through a first barrier to integrate the M. galloprovincialis genomic background in Brittany then to go through a second barrier to integrate the Bay of Biscay M. edulis genomic background and at the end to extract itself from the third barrier. This may be a long way to go and it can be predicted that both species will not completely blend or will do so very slowly. The successive hybrid zones (and associated barriers) may constitute sieves through which few genes manage to pass. Important sampling effects can therefore occur in the process, leading to genetic differentiation of introgressed alleles. This could explain the previous observations that there exist slight differences between the mitochondrial sequences of introgressed M. edulis haplotypes in the Iberian M. galloprovincialis background and haplotypes of M. edulis from the North Sea (Rawson & Hilbish 1998).
The recent impact of mussel culture
Finally, the case of sample 24 in the Mont Saint-Michel bay deserves particular comments. Hardy–Weinberg and linkage disequilibria were large in sample 24 (see Fig. 4). This sample can be split into a few M. galloprovincialis-like genotypes and a majority of M. edulis-like genotypes. The latter are not genetically identical to nearby M. edulis populations from Normandy (sample 25, grouped with North Sea populations). Rather, they group with Bay of Biscay M. edulis in the CA (Fig. 2), mainly because they display a relatively high frequency of the EFbis-G3 allele, typical of the populations from the Bay of Biscay. A probable explanation is the large unilateral spat transfer from cultivated stocks of mussels located in the Bay of Biscay (samples 6 and 7) to the Mont Saint-Michel bay culture area. Humans have modified natural migration by importing spat and could have recently created a migration ‘short-cut’ between the Bay of Biscay and the North Brittany M. edulis populations. Note that, because these genotypes differ from genotypes of North Sea M. edulis at locus EFbis only, we cannot exclude the hypothesis that the allele G3 at locus EFbis has hitch-hiked with a favourable allele into the M. edulis both in the Bay of Biscay and in the Granville population (sample 24). In any case, we expect that no genetic barrier can now prevent the G3 allele from diffusing toward the north either by neutral or selective diffusion.
The previous observation raises the question of a more pronounced impact of mussel industry or other human activities (such as accidental transfer by boats). For instance, the Bay of Biscay M. edulis-like populations seems to coincide with one of the most important European mussel culture areas in Charente-Vendée. However, several arguments oppose this view. (i) Spat transfer occurs mostly from Charente-Vendée to other culture areas, not the reverse. Indeed, mussel cultivators in Charente-Vendée are not keen on importing spat because they take pride in their native small, edulis-shaped mussel. (ii) Linnaeus’M. edulis type collection, which was constituted before 1758, before the emergence of extensive mussel culture in Europe, included individuals of different mussel species among which the one that best corresponded to today's definition of M. edulis was from Baie de l’Aiguillon in Vendée (Bucquoy et al. 1898). This suggests that typical M. edulis morphologies have not appeared recently in this region. (iii) The mosaic structure described first around the British Isles (Skibinski et al. 1983) does not correlate obviously with what is known of the mussel culture industry in the UK. (iv) The genetic composition of Bay of Biscay M. edulis-like populations is unique as they share a private allele (G3) at locus EFbis. This feature is hardly compatible with a recent introduction by man. (v) Finally, the long-term persistence of, for instance, a small cultured M. edulis stock introduced in the midst of M. galloprovincialis populations, is very improbable. Indeed, the barrier to gene flow between the two species acts as frequency-dependent selection and creates an asymmetrical disadvantage to the species with smaller population sizes (Barton 1986). For all these reasons, we believe that the large-scale structure of the mussels hybrid zone should be considered as largely independent from human action.
Acknowledgements
We are very much indebted to L. Dupont, M. Szulkin and to the students preparing the 2000–2001 DEA diploma (Diplôme d’Etudes Approfondies) d’Océanologie Biologique of Paris VI University, for participating in laboratory analyses; to A. Couty for arranging in the collection of the Cley and Tichwell samples and to F. Cornette for the Granville and Port-en-Bessin samples. We also thank four anonymous referees for insightful comments on the manuscript. This research was funded in part by IFREMER URM 16.
References
Nicolas Bierne, currently postdoc at the Centre for the Study of Evolution (University of Sussex, Brighton, UK) was a PhD student in Ecology and Evolution at Université Montpellier II (UMII, France). This study was completed as part of his thesis. His supervisors were François Bonhomme [Director of Laboratoire Génome, Populations, Interactions (LGPI) and Station Méditerranéenne de l’Environnement Littoral] and Patrice David (Maître de Conférence at UMII). Philippe Borsa is population geneticist with interest in marine species. Claire Daguin was PhD student at LGPI. Both have studied population genetics and biogeography of mussels. Didier Jollivet and Frédérique Viard were collaborators from Station Biologique de Roscoff. This study was part of an experimental and theoretical project aimed at examining the structure of barriers to gene flow in mosaic hybrid zones.
Appendix I1
Allelic frequencies at the Glu-5’mac-1 and EFbis loci. -: allele absent in sample; (n): sample size.