A molecular genetic analysis of the communal nesting of the ostrich (Struthio camelus)
Abstract
The ostrich breeding system is complex and unique; communal clutches are laid by several females, although only one female, the major female, and the resident territorial male provide parental care. More eggs are laid in the nest than can be incubated and the major female ejects surplus eggs from the incubated central clutch. Microsatellite markers were used to analyse the parentage of communal nests in Nairobi National Park. This revealed that major females contributed a disproportionate number of fertile eggs to the central, incubated clutch and that multiple paternity and maternity within a nest were common; 68.9% of all incubated eggs on a nest were not parented by both the resident territorial male and the major female of that nest. All the males fertilized eggs on the clutches of neighbouring males. Unexpectedly, every major female with her own nest was also simultaneously a minor female with incubated eggs on neighbouring clutches. The relatedness between females laying in the same nest was not significantly different from the population average and significantly less than that between chicks hatched from the same nest.
Introduction
Communal avian breeding systems in which group members provide care to offspring that are not their own are usually characterized by only one breeding pair per group and the presence of nonbreeding helpers (Brown 1978, 1987). Plural breeding, when there are two or more breeding females, is rarer (Brown 1978; Koenig & Mumme 1987; Vehrencamp et al. 1988; Macedo 1992, 1994; Quinn et al. 1994). In some of these species the breeders are close relatives and tolerating their breeding in the nest has indirect benefits through inclusive fitness gains (Curry & Grant 1990; Craig & Jamieson 1990; Koenig & Stacey 1990). Other possible benefits include a reduction in mortality through a reduction in the time spent incubating per female (Vehrencamp 1978), or increased vigilance and group defence of the nest (Macedo 1992). Neither of these possibilities applies to the ostrich communal nesting system in which up to 18 females may lay in a nest, but only one female, the major female, together with the territory-holding male, provides parental care from incubation to the independence of the chicks (Sauer & Sauer 1966; Bertram 1979, 1992; Hurxthal 1979). The major female is also the one that lays the first egg in a particular nest. Although the other, minor, females contribute nothing but their eggs and are thus parasitizing the parental investment of the major female, she is remarkably tolerant of their coming to the nest and makes no attempt to prevent their laying. Other ratite species also have communal nesting with multiple females laying in one nest, but in those species only the male provides parental care (Handford & Mares 1985; Coddington & Cockburn 1995).
In the ostrich, the major female ceases laying and begins incubation 16 days (range 11–18 days) after she starts laying (Hurxthal 1979). This is before she has laid as many eggs as she can efficiently incubate. If she lays every other day as in captivity (Smit 1963), and does not lay in any other nests once she has started her own, she would be expected to lay 6 to 10 eggs with a mean of 7.4 eggs in her nest (Hurxthal 1979). Because ostriches can incubate up to 20–21 eggs, there is ‘spare space’ in the clutch that can be utilized by minor females. Females in captivity can lay many more than 21 eggs, so the major female may be constrained to stop laying before she has laid as many eggs as she could incubate. Hurxthal (1979) and Bertram (1992) suggested that this might be due to the risk of nest predation, but Bertram & Burger (1981) and Wilson et al. (1997) found that the hatchability of artificially incubated farm eggs declined sharply after 15 days. Thus major females may be constrained to start incubation when further delay would lower the survival of their own first laid eggs.
One to six minor females usually lay 20–40 additional eggs in the nest with some clutches containing up to 67 eggs, considerably in excess of the number that can be incubated (Sauer & Sauer 1966; Hurxthal 1979; Bertram 1992). At the start of incubation the major female arranges the eggs into a central, incubated clutch and a ring of peripheral, unincubated eggs. The unincubated eggs never develop.
The major female may not lose reproductive success provided that she ensures her eggs are among those incubated. Bertram (1979) identified one egg laid by the major female in each of five nests. He then classified the remaining eggs into those that did or did not closely resemble this one in morphological features. He found that only one putative major female egg was ejected into the peripheral ring and eggs that did not resemble the major female's egg were much more likely to end up unincubated. He concluded that the major female recognized her own eggs and included them in the central clutch and thus did not lose reproductive success by incubating eggs other than her own.
A female might also increase her fitness while incubating the eggs of the minor females provided they were related to her. This could be the case if the major female and the minors were from the same nest. However, Bertram (1992) argued that even long-term associations as juveniles would not guarantee a high degree of relatedness between females. Communal laying, multiple parentage and the subsequent creching of young would all result in a low average degree of relatedness between pairs of female companions.
Because all the females laying in a nest usually range daily over four or five male territories, and the territorial males solicit copulations from any females that come into their territory (Hurxthal 1979), the male may have fertilized any of the eggs in his nest, as well as some of those in the nests of neighbouring males. Hurxthal also observed that the major female mated more often with her resident territorial male than with neighbouring males.
Here we describe the use of seven microsatellite loci to identify the parentage of the eggs selected for incubation and to quantify the genetic relationship between the females that have eggs incubated on a nest and between the eggs that are incubated. We predicted that: (i) the major female would have laid 6 to 10 of the incubated eggs on her nest. Because we were not allowed to remove the peripheral eggs for incubation to analyse parentage, major females were expected to have laid a mean of 7.4 eggs per nest and the majority of incubated eggs would be laid by minor females. (ii) Territorial males would have fertilized the majority of the major female's eggs as he has most matings with her as well as some of the eggs of the minor females that were incubated on his nest. He would also have fertilized some of the eggs incubated on the nests of neighbouring males (iii) Relatedness between cooperating females and between males and females would not be higher than the population average.
Materials and methods
Field site and sampling
The study was carried out at Nairobi National Park during the breeding season from July to December 1998. This work was carried out under permit from, and with the active cooperation of, the Kenya Wildlife Service (KWS) who are responsible for the park. Individual identification of the territorial males was possible both by slight distinctive features and, once the breeding season started, by location, as they were virtually always on their territory and not very distant from the nest. However, identification of the females was especially difficult due to their cryptic grey brown coloration and lack of distinctive features. We were only able to identify them as at a particular nest, but frequently there were several females at a nest. Once the incubation started we were able to identify the major female as the only female incubating. The major female incubated between ≈ 07.00 and 17.00 each day and the territorial male did the remaining 60% of the incubation. Nests were found only after incubation started so we did not have nest initiations dates.
Skin samples of ≈ 0.1 g were collected by a KWS veterinary officer in Nairobi National Park using Palmer® biopsy darts fired from a rifle. Adult skin samples were collected after incubation began. The major female was sampled by waiting until she left the nest at the changeover in incubation at about 17.00 each day. The female was followed as she left the nest and a sample was taken once she was > 200 m away. The male was sampled during the day as he remained near the nest. We had intended to collect samples from the chicks by plucking feathers soon after hatching before they left the nest with the parents. Unfortunately, in the 1997/8 breeding season there were exceptionally heavy rains from late September. These continued throughout the breeding season. Much of the park was flooded with standing surface water and all the ostrich eggs in the park were drowned and the adults deserted the nests. After it became clear that the nests were deserted and the eggs dead, the KWS permitted collection of the entire clutch, both incubated and peripheral eggs at five nests. In some nests a proportion of the eggs had already been lost to predators (Table 2).
Most of the central, incubated eggs in four nests had begun to develop and yielded chorioallantoic membrane and foetal tissue (Table 2). The fifth nest was evidently only in the very early incubation phase and no embryonic tissues were recoverable from any of the eggs. None of the peripheral eggs in any nest showed any sign of development and we were unable to establish the parentage of these eggs. Because no eggs hatched, any reference to fertilization or reproductive output refers only to eggs that were incubated and started development.
In order to check for the existence of null alleles, an additional set of two families of chicks with known parents from Massai Ostrich Farm were genotyped (Kimwele 2000).
DNA extraction and microsatellite analysis
Sample tissue from the eggs was transported in a cool box and frozen at −20 °C in the laboratory. Genomic DNA was extracted from the egg membranes using a standard protocol with overnight digestion with proteinase K and subsequent phenol/chloroform extractions (Sambrook et al. 1989). Genotyping was determined for eight microsatellite loci developed for ostriches: OSM1, OSM2, OSM4, OSM5, OSM6, OSM7 (Kimwele et al. 1998) and List005 and List009 (Kumari & Kemp 1998). The cycling conditions were as per Kimwele et al. (1998) and Kumari & Kemp (1998) except that the number of cycles was reduced to 25 to minimize allele slippage and improve resolution. All polymerase chain reaction (PCR) amplification and detection procedures were run at least twice to verify the genotyping.
The PCR products were loaded with 0.5 vol. of formamide dye solution, denatured for 2 min at 95 °C and then 5 µL was loaded onto a denaturing 6% polyacrylamide gel and run at 1500 V. A 10-bp ladder (GibcoBRL) was used to size the alleles. The amplified loci were visualized by silver staining following Promega's protocol and applications guide (Promega 1996). The alleles were then typed by length across the eight loci to give a genotypic profile of the individuals.
Population genetic diversity and parentage analyses
genepop Version 3.2a (Raymond & Rousset 1995) was used to determine allele frequencies and to test linkage disequilibrium, to calculate Hardy–Weinberg expected heterozygosities and the probability of deviations from Hardy–Weinberg equilibrium using the Markov chain method.
Parentage in the communal nests is complex. On each nest the expectation was that there were incubated eggs in the central clutch that were not fertilized by the territorial male or laid by the major female. Because major females may lay eggs not only in their own nest, but also in other females’ nests (Hurxthal 1979; Bertram 1992), a complex scenario was expected for attributing parentage. In order to analyse this data we used the logarithm of the odds (LOD) scores on cervus Version 2.0 (Marshall et al. 1998). These scores are calculated from a simulation using the allele frequencies from the study population assuming that the alleles are in Hardy–Weinberg equilibrium. This is used to generate criteria that permit assignment of parentage to the most likely individual and to give a level of statistical confidence for this assignment. This approach eliminates the exclusion of parentage on the basis of a rare allelic mismatch. Although an allelic mismatch may reflect correct exclusion of a putative parent, it may also arise from erroneous laboratory typing, the presence of null alleles or mutations. These sources of error may be approximated from a known pedigree. Unfortunately, natural populations offer little opportunity for such a pedigree and none existed for our study.
Marshall et al. (1998) suggest that any locus with a null allele frequency of > 0.05 should be excluded from the analysis. cervus found a possible, but not significant, frequency greater than this for OSM7. Although we found no evidence for null alleles at this locus in the small study with a known pedigree, we performed the analysis both with and without this locus. The differences in the two analyses were minor, 10 chicks had their father and 3 chicks had their mother identified at the 80% level instead of at the 95%. We report here only the results with locus OSM7 excluded.
Because neither parent was known we followed the recommendations of cervus and ran the parent with the fewer candidates, males in this case, first and then re-ran the analysis for females using the results of this first analysis for males.
Relatedness
We used relatedness Version 5.0 (Queller & Goodnight 1989) to estimate the genetic relatedness among the incubated eggs, among the identified females laying in a nest and the identified males that fertilized incubated eggs. Relatedness was estimated separately both among the females that laid in a given nest and between the major female of a given nest and the recipient chicks within her nest. We used two estimates of relatedness: (i) average relatedness of subsets of interest within the population, i.e. groups of females laying in a given nest and the chicks from that nest; and (ii) pairwise relatedness between all pairs of individual adults. Estimation of standard errors and confidence interval was performed by jackknifing over the seven loci.
Resampling
Because we did not know the parentage of the peripheral eggs, we estimated the probability that the major females were including their own eggs in the central clutch for incubation using resampling (Version 5.0.2, Simon 1999). We assumed that each major female laid 11 eggs in the total clutch on her nest. This is a conservative estimate as both Hurxthal (1979) and Bertram (1992) found that the major female laid a maximum of 11 eggs on her own nest and usually fewer. For the resampling we assumed that loss of eggs from the central clutch to predation after desertion was random, as was infertility of eggs.
Results
A total of 25 adults and 61 incubated eggs were typed. Ten of the adults were territorial males and major females at identified nests, while 15 others were sampled at Maasai Ostrich Farm. These were hatched from eggs collected from the same population at the southern area of Nairobi National Park. From the 25 adult individuals typed, 6–25 alleles per locus were detected with an observed heterozygosity of 0.61–0.84 (Table 1). An exact Hardy–Weinberg equilibrium test found no significant deviation from expectation (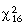 = 19.4, P = 0.25) indicating that there was no population substructure, no selection acting on any of the loci, no bias towards typing of any genotypes and no locus segregating in a sex chromosome.
= 19.4, P = 0.25) indicating that there was no population substructure, no selection acting on any of the loci, no bias towards typing of any genotypes and no locus segregating in a sex chromosome.
| Locus | No. alleles | No. individuals typed | Observed heterozygosity |
|---|---|---|---|
| OSM1 | 15 | 85 | 0.73 |
| OSM2 | 12 | 85 | 0.67 |
| OSM4 | 7 | 66 | 0.61 |
| OSM5 | 12 | 84 | 0.74 |
| OSM6 | 6 | 65 | 0.66 |
| List005 | 8 | 85 | 0.78 |
| List009 | 25 | 86 | 0.84 |
Parentage scores
The total exclusion probabilities for first and second parents were 0.995 and 0.999, respectively. Ten candidate parents including four pairs of territorial male and major female at a nest were sampled plus the major females at two nests in which we did not have samples from the territorial male. Each parent of an egg was identified at either the 95% confidence level (83 assignments), at the 80% confidence level (13) or not at all (27). We could not assign fathers to 12 and mothers to 15 eggs at either 95% or 80% confidence levels. Thus a maximum of 12 males and 15 females were unsampled. Only three eggs were unassigned to either a mother or a father.
Males
The territorial males fertilized 39–83% of the incubated eggs on their nest (Fig. 1a). All four territorial males had fertilized eggs that were incubated on other nests, and the resident male on nest 3 had as many incubated eggs on nests 6 and 4 as he did on his own. Between one and seven eggs on a nest were fertilized by other, unsampled males. Of the 31 incubated eggs fertilized by the resident males and laid on his nest, 19 (61%, Table 2) were laid by the major female on his nest and 12 (39%) by minor females on his nest.
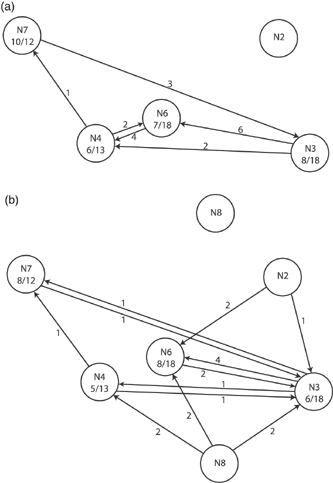
(a) Location of the incubated eggs fertilized by the territorial males. The number of incubated eggs assigned to the parent at the nest is given as the numerator and the number remaining in the central clutch the denominator. The arrows indicate parentage of eggs in nests other than their own with the number of incubated eggs fertilized in each nest. The males on nests 2 and 8 were not sampled. The distances between adjacent nests are not drawn to scale. They ranged between 2 km in the nests bordering each other (nests 4 and 6) and 16 km between the two furthest nests (nests 3 and 7). (b) Location of the incubated eggs laid by the major females at six nests.
| NEST | 3 | 4 | 6 | 7 |
|---|---|---|---|---|
| Central eggs | 26 | 19 | 27 | 24 |
| Major female's eggs | 6 (5) | 5 (2) | 8 (5) | 8 (7) |
| Minor females’ eggs | 12 (3) | 8 (4) | 10 (2) | 4 (3) |
| Central eggs lost to predation | 0 | 4 | 3 | 12* |
| Infertile central eggs (no tissue) | 8 | 2 | 6 | 0 |
| Peripheral eggs | 12 | 18 | 14 | 3 |
| Total eggs laid in nest | 36 | 33 | 36 | 27 |
- * The eggs on nest 7 were being preyed upon by baboons (Papio cynocephalus) when the nest was collected.
In every nest at least one of the major female's eggs was fertilized by a male other than the territorial male (x̄ = 2.0 ± 1.16 SD, Table 2). There was no significant difference in the number of incubated minor female eggs fertilized by the resident male (x̄ = 3.0 ± 1.86 SD) and those fertilized by other males combined (x̄ = 5.50 ± 3.70 SD) (t = 1.32, d.f. = 3, P = 0.278). There was no significant difference between the numbers of incubated eggs laid by a major female that were fertilized by the resident male at her nest (x̄ = 5.25 ± 2.56 SD) and the number fertilized by other males (x̄ = 4.25 ± 1.50 SD) (t = 0.71, d.f. = 5, P = 0.507).
Females
The major females incubated in their own nests a mean of 6.75 ± 1.5 (SD) of their own eggs (Table 2). If we assume that each female laid 11 eggs in her own nest and that selection for incubation was random, as was loss due to predation after desertion, then the mean number of her own eggs that a female would have incubated would have been 5.05. The probability of getting 6.75 or more is 0.021.
Unexpectedly every major female was also a minor female at another nest, and, in the case of nest 3, had as many incubated eggs on other nests, where she laid as a minor female, as she had on her own nest (Fig. 1b). Included in Fig. 2 are nests 2 and 8 where the major female was sampled, but we had no eggs. The number of incubated eggs laid by minor females that were not sampled ranged from 2 to 6 per nest. Because we observed at least nine different females laying at some of the nests, some females apparently were less successful than others in getting eggs incubated.
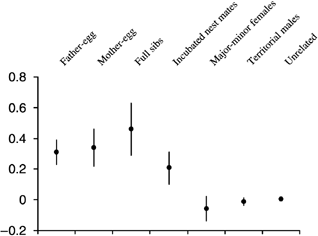
Relatedness coefficients (relate Version 5.0) of assigned fathers (cervus Version 2.0) and mothers to their fertilized eggs and among full sibs, incubated nest mates, major–minor females and territorial males with reference to the unrelated estimate.
Across the four nests including both their own and other nests the territorial males had a mean of 12.25 ± 2.99 (SD) incubated eggs while the four major females had 9.5 ± 2.08 (SD) including incubated eggs laid both as a major and as a minor female. There was no significant difference between the success rate of the males and females (t = 1.5109, d.f. = 5, P = 0.191).
Relatedness
The median pairwise population relatedness for all the adults and chicks was 0.0098 and ranged from −0.38 to 0.64 and the interquartile range was −0.09 to 0.13. We assessed the accuracy of the R-values derived from relatedness (Queller & Goodnight 1989) by estimating the relatedness between individuals identified by cervus (Marshall et al. 1998) as parents and offspring and those having the same parents. The 49 male–egg pairs had a mean pairwise relatedness of 0.31 ± 0.08, the 47 mother–egg pairs a mean of 0.034 ± 0.12, and the 29 eggs with at least one full sibling had a mean of 0.46 ± 0.17 (Fig. 2). Except for the full sibs these estimates are substantially lower than the predicted score of 0.5 for these relationships. This underestimate may be the result of the close relatedness of the samples in our population. The mean pairwise relatedness between the adults and the eggs that were not theirs (unrelated) was 0.007 ± 0.0003.
The mean pairwise relatedness between the identified major and minor females on a nest was −0.06 ± 0.08 SD, and between the territorial males it was −0.01 ± 0.03 SD. Both of these are significantly less than the mean pairwise relatedness between nest mates 0.21 ± 0.11 SD (P < 0.05, randomization test, Simon 1999).
Discussion
The communal nesting system of the ostrich is unique; the major female allows minor females to lay in her nest even though they will provide no parental care for any offspring. All incubation and posthatching care is by the major female and the territorial male. In other plural breeding avian species either none of the females provides any post laying care, as in rheas and emus (Brunning 1974; Handford & Mares 1985; Taylor et al. 2000), or there are benefits to the female from allowing others to lay in her nest. These benefits may be indirect, due to the close relatedness of the other females (Curry & Grant 1990; Koenig & Stacey 1990). Alternatively, there may be direct benefits to the female such as increased female survival due to decreased incubation time per bird (Vehrencamp et al. 1988; Koford et al. 1990) or group benefits such as nest and territory defence (Macedo 1992, 1994). In the ostrich none of these potential benefits apply; the females are not close relatives of the major female and the minor females provide no protection or other parental care. The major female may even be paying an increased cost by incubating more eggs than she has laid. In some species of birds increased clutch size requires increased energy for incubation (Moreno & Sanz 1994; Heaney & Monaghan 1996; Thomson et al. 1998; Engstrand et al. in press), but there are apparently no data on incubation costs in ostriches. The existence of a ring of ejected, peripheral eggs surrounding the nest may also attract predators to the nest, as these eggs are more visible than the eggs covered by the incubating bird (Bertram 1992). It is possible that the major female's own eggs might have a greater chance of escaping predation due to the dilution effect of risk with a larger number of eggs (Bertram 1992).
Any analysis of the costs and benefits of a complex nesting system such as that of the ostrich depends on correctly assigning parentage. In this study we have done this for the incubated eggs only, as none of the nests in Nairobi National Park survived the floods in the breeding season. As expected the parentage in the communal nesting system of the ostrich was indeed complex with multiple females laying in a nest and all females mating with multiple males. The laying of many more eggs than can be hatched in most nests has been found in different localities and in different subspecies of ostriches (Sauer & Sauer 1966; Bertram 1979, 1992; Hurxthal 1979). With the pair only able to incubate 20–21 eggs (Hurxthal 1979; Bertram 1979, 1992) the excess eggs are ejected, by the major female, to the peripheral clutch 1–2 m away where they remain unincubated and perish (Bertram 1979; Hurxthal 1979). Because the major female initiates the clutch and is thought only to lay in it once she begins it (Bertram 1979; Hurxthal 1979), she has a larger investment in it than any of the minor females. Provided that she is able to recognize her own eggs she can ensure that she does not lose any reproductive success by allowing other females to lay in her nest. Bertram suggested that the major females were using cues such as shape, size or pore pattern to distinguish between eggs. When he marked eggs using these clues from a known egg of the major female he found that eggs that closely resembled the major female's egg were not likely to be ejected to the peripheral clutch.
Our molecular findings indicated that the major females were incubating more of their own eggs than would be expected if they were selecting eggs at random to be incubated even if we assume that each major female laid 11 eggs. Of course it may be that the major females laid fewer than 11 eggs each as that was the maximum estimated for this population; the average was 7.4 (Hurxthal 1979). This calculation assumes that females laid every other day as in captivity, which may not be the case in the field. It also assumes that once a major female began a nest she laid only in that nest and this was not the case in our population. At least some of the major females must have been laying as minor females in other nests after they had started their own. Just how successful major females are in recognizing their own egg for incubation will only be answered when we have incubated and assigned parentage to all the peripheral eggs.
The ostrich mating system is primarily polygynandrous with both sexes mating with multiple partners. Hurxthal (1979) observed that females, who have a larger home range relative to the males’ territories, move through 4–7 male territories regularly and may mate with the territorial male in his territory. Ostriches copulate repeatedly and resident territorial males copulate preferentially with new females entering their territory as well as with the major female. The major female was in the territory more often than the minor females and the majority of her matings were with the resident male.
The territorial males fertilized a mean of 4.75 ± 2.06 (SD) of their major females’ eggs that were incubated in their own nest, whereas other males fertilized 2.0 ± 1.16 (SD). The resident male fertilized more of the incubated eggs of his major female than all the other males that mated with her. He also fertilized some, but not all, of the eggs of the minor females that were incubated in his nest. The number of incubated minor female eggs on the nest fertilized by the resident male ranged from two to four. Every male also fertilized some of the eggs on another nest. Indeed male 6 had as many eggs that he fertilized incubated on other nests as he had on his own.
Because the territorial male fertilized some, but not all, of the eggs of both the major and the minor females, he was in much less of a position to increase his reproductive success by selecting eggs for the central clutch than the major female was.
Every female in the group of nests that we collected was both a major and a minor female, usually on several neighbouring nests. This was completely unexpected. Bertram (1992) had reported switching status between major and a minor, but usually only after a major female lost a nest or before she gained one as a major female. He did report that a current major female laid an egg as a minor female in another nest, but he regarded it as a rare event. In our study it was the norm; some of our females must have been simultaneously major and minor females. We were also surprised at the success major females had in getting their eggs incubated on other nests. Because there were at least nine different females laying at some of the nests, the low number of incubated eggs (maximum 5) on any nest that we have not been able to assign to one of the major females suggests that the eggs of particular females who are, or will be, the major female on a nearby nest may be favoured. The existence of any such reciprocity can only be tested by incubating all the eggs ejected to the periphery and establishing their parentage as well as that of those on the nest.
Certainly laying as a minor female while also being a major female at another nest would increase the chances of reproductive success in a particular season provided that the eggs had a good chance of being incubated. This could be a successful strategy when nest predation is as high as it is among ostriches (Bertram 1992). Because nests had up to a minimum of five minor females laying up to 50% more eggs in a clutch than could be incubated, eggs of a particular female would only have a high chance of being incubated if either eggs of particular individuals were favoured by inclusion in the incubated clutch, or major females in surrounding nests laid large numbers of eggs as minor females in neighbouring nests. Bertram (1992) found one case of a major female with an extant nest laying an egg as a minor female in another nest while also continuing to lay in her nest, but he concluded that this was a very rare event. Ostriches are long lived and interact in large social groups arising from the creching behaviour. This social group breaks up only when the juveniles reach sexual maturity and begin to breed. Ostensibly, group recognition is possible and repeated interactions are likely. We know of no data for the long-term interactions of female ostriches, but investigations into the associative behaviour of known individuals in and out of the breeding season may show preferred affiliations among females.
Bertram (1992) suggested that the high first-year mortality would make it unlikely that females would have surviving nest mates to associate with. We found that major and minor females with incubated eggs in a nest were no more closely related than individuals selected at random from the population, and that they were significantly less related than nest mates would be. Because the sample size was only the six females interacting as major and minor females on four nests, the generality of this finding will only become apparent with a larger study. We also found that the major female was unrelated to the eggs, other than her own, that she incubated in her nest. A viability decrease may constrain the female to incubate 7–11 eggs (Bertram & Burger 1981; Wilson et al. 1997) leaving extra space that she may make use of by the eggs of other, nonkin females. If the costs associated with the incubation of these extra eggs are low enough, incubating extra eggs may be a strategy that counters predation by risk dilution of the offspring of the major female. Indeed, creching of the young of multiple nests is actively initiated by the escorting parents and older chicks, and Hurxthal (1979) interpreted this as circumstantial evidence for the antipredatory hypothesis. The major female then may gain increased survival prospects for her own chicks through numerical prey risk dilution and predator confusion effects. Although there is no evidence to date for an antipredator function for accepting on the nest and incubating extra eggs, it would provide a possible explanation for the unique ostrich breeding system, which combines communal nesting with monogamous parental care.
Acknowledgements
We are grateful to the Commonwealth Scholarship Commission for awarding the scholarship that enabled CK to do this work, the NERC for its funding to JG (GR9/1379), and the University of Nairobi for extended study leave for CK. We also thank D. Western, T. Manyibe, J. Wambua, S. Njumbi and S. Makalla of the Kenya Wildlife Service for administrative and field support as well as for permission to carry out the work in Nairobi National Park and J. Mugweru for technical assistance. We are also indebted to Lew Hurxthal and Brian Bertram for discussions and encouragement before this research started and to Mike Ritchie and Leon Hockham for comments on the manuscript.
References
This work is part of C.N. Kimwele's PhD thesis entitled ‘A molecular analysis of the ostrich (Struthio camelus massaicus) communal nesting system’ (St Andrews University) and is part of ongoing research on the communal nesting system of the ostrich in Kenya between the Universities of Nairobi and St Andrews. Jeff Graves works on breeding systems and reproductive success as well as population structure and phylogeny.




