Streptokinase antibodies inhibit reperfusion during thrombolytic therapy with streptokinase in acute myocardial infarction
Abstract.
Objectives. To evaluate the influence of pretreatment IgG against streptokinase on the outcome of streptokinase treatment in acute myocardial infarction.
Setting. Coronary care unit.
Design. From 88 patients admitted to the coronary care unit due to chest pain, blood samples were taken for determination of the pre-existing titre of antibodies against streptokinase. The patients were treated and monitored according to standard protocols. Fifty of the patients received thrombolytic therapy with streptokinase due to acute myocardial infarction and were monitored with continuous dynamic vectorcardiography, making possible the continuous analysis of ST- and QRS-vector changes and determination of the event of reperfusion. None of these 50 patients had been given streptokinase therapy previously.
Results. According to the vectorcardiographic criteria 21(42%) patients had signs of early (within 2 h) reperfusion after streptokinase therapy. These patients had lower pre-existing antibody titres than patients without signs of reperfusion (mean values 0.20 and 0.45 arbitrary units, P = 0.01). None of the patients with a titre higher than 0.50 arbitrary units (nine patients) had signs of early reperfusion. Of the 41 patients with a titre lower than 0.50 arbitrary units 52.5% had signs of early reperfusion.
Conclusion. The present investigation indicates that pre-existing streptokinase antibodies play an important role in reperfusion failure during thrombolytic therapy with streptokinase in acute myocardial infarction. Therefore, the determination of streptokinase antibodies may differentiate between those patients who may benefit from streptokinase treatment and those who should be treated with some other regime.
Introduction
The use of thrombolytic therapy has reduced mortality and morbidity in patients with acute myocardial infarction [1–3]. For reasons not clearly understood, early reperfusion is not achieved in all patients. The TIMI-1 study showed a patency rate at 90 min of 43% [4]. In a comparison of different streptokinase studies, the patency rate after 90 min varies from 43 to 64% with a pooled mean of 51%. This is somewhat lower than with r-tPA [5]. It has been suggested that some cases of streptokinase therapy failure and the difference in results between streptokinase and r-tPA may depend on pre-existing antibodies against streptokinase.
It has been shown that infections with streptococci and treatment with streptokinase lead to development of neutralizing antibodies against streptokinase [6–9]. Clinical relevance of pretreatment streptokinase antibodies has been suggested, from angiographic studies, even though these results are conflicting [6, 10, 11].
Continuous dynamic vectorcardiography makes it possible to monitor the changes in myocardial perfusion on line [12–15], in contrast to angiographic imaging showing the macroangiographic status of the coronary arteries at one given time, usually 12 h after thrombolysis. Therefore, the reperfusion sometimes occurring at a late stage of the infarction process might be falsely interpreted by angiography as induced by thrombolytic agents, in contrast to the vectorcardiography being performed during the infusion of the thrombolytic agent.
The aim of this study was to evaluate the influence of pretreatment IgG against streptokinase on the outcome of streptokinase treatment as measured by electrophysiologic reperfusion signs, on line monitored by continuous dynamic vectorcardiography.
Materials and methods
Material
A total of 88 patients consecutively admitted to the coronary care unit due to chest pain were studied. They had ECG signs of ST-elevations and no more than 6 h of duration of symptoms at the start of thrombolytic treatment.
A blood sample was drawn from all these patients on admission to the coronary care unit, later to be analysed for the content of antistreptokinase antibodies. Fifty-five of the patients were given streptokinase (Behringwerke ) intravenously in a standardized fashion with 1.5 × 106 units in 250 mL saline during 60 min and received routine coronary care treatment. None of these patients had previously been given streptokinase. Five patients with ECG patterns that did not give reliable vector registrations or who did not develop elevations in CK-MB as signs of myocardial damage were excluded from the study, leaving 50 patients (36 males and 14 females, aged 38–85 years).
Methods
Continuous vectorcardiography
The patients were monitored with continuous dynamic vectorcardiography. On-line registrations of the X, Y and Z vectors were made, collected by Frank leads, for 12 h using an Ortivus Medical registration unit. Averaging of the continuous dynamic vectorcardiography complexes was performed for 2-min periods and for each period the resulting mean complex was compared to the reference complex collected during the start of the registration. This method is shown to give an accurate estimation of ischaemic burden and reperfusion during thrombolytic therapy in acute myocardial infarction. Reperfusion is shown by fast shift of ST-vector magnitude to a new stable and lower level simultaneously with the QRS-vector difference showing a fast change of areas [13–15]. ST-vector magnitude = square root of [STX2 + STY2 + STZ2], which is the magnitude of the ST levels measured 20 msec after the J-point. QRS-vector difference is the area that develops when the QRS complexes from different short sampling periods are superimposed on each other, reflecting the changes in the QRS complex compared to the reference complex registered at admittance, measured as the square root of [AX2 + AY2 + AZ2]. The method for obtaining these parameters has been described in detail [13]. In this work reperfusion was assumed if: (i) QRS-VD increasing at a velocity of at least 0.1 µVs (ii) QRS-VD stabilized at plateau-level within 2 hs. (iii) ST-Vm descended to base level within 2 hs.
In addition, sera were obtained from six patients between 3 weeks and 2 months after streptokinase treatment.
Elisa
ELISA was performed according to Engvall and Perlmann [16] with some modifications. The wells of flat-bottomed polystyrene microwell plates (F96 Polysorb, Nunc, Denmark) were coated overnight at 4 C with 100 µL of a streptokinase solution (1 500 000 units of streptokinase, Behring, were dissolved in tris-HCl pH 9.5 and diluted to a concentration of 2.5 µg mL–1 of streptokinase). The plates were washed three times with washing buffer (PBS, pH 7.4 containing 0.05% Tween), incubated for 1 h at room temperature with 200 µL of blocking buffer (PBS containing 1.5% ovalbumin), and washed again three times with washing buffer. A quantity of 100 µL of patient sera diluted 3200 times in blocking buffer was added, the plate was incubated for 1 h at room temperature and washed three times with washing buffer, then for 1 h at room temperature with 100 µL of peroxidase-conjugated rabbit-antihuman IgG (DAKO, Denmark, product code P214) diluted 6000-fold in blocking buffer, followed by three washes with washing buffer. 100 µL of the substrate tetramethylbenzidine (1 m mol L–1) in citrate buffer (0.1 mol L–1, pH 4.25) with 2 m mol L–1 H2O2 was added and 5 min later the enzymatic reaction was stopped by addition of 100 µL of 1 mol L–1 H2SO4. The optic density at 450 nm was determined by an ELISAreader (Multiskan Plus, Labsystems, Helsinki, Finland ). All patient sera were analysed in duplicate. The interassay variation was corrected for by a set of calibrator control sera: two dilutions (32 000 and 96 000) of sera from two patients showing high levels of streptokinase antibodies were included on each plate.
Checkerboard titration of antigen concentration versus serum dilution showed 2.5 µg mL–1 of streptokinase and 3200 times dilution of patient sera to be optimal (data not shown).
Antistreptolysine O determination was performed using an in-house agarose gel neutralization test. The toxin was prepared from a type strain of betahemolytic streptococci, and quantified using the WHO international reference serum.
The streptokinase neutralization titre was determined using a traditional in vitro clot lysis tube test [17]. In brief, a clot formed after addition of thrombin (KABI-Vitrum, final concentration 5 IU mL–1) to a mixture of plasma and a series of streptokinase (Behring) dilutions. Clot lysis occurring during a 5-min 37 C incubation period was then determined. A test tube streptokinase concentration of 500 IU mL–1 was assumed to correspond to the clinical standard dose of 1.5 million IU.
Statistics
Rank test was performed according to Snedecor and Cochran [18].
Ethics
The study was performed according to the declaration of Helsinki.
Results
Twenty-one (19 males, 2 females) of 50 patients had signs of reperfusion as determined by continuous dynamic vectorcardiography [13] within 2 hs. In this group all the patients had an antistreptokinase titre that was 0.50 arbitrary units or lower, with a mean value of 0.20 units ( Fig. 1). Amongst the 29 patients (17 males, 12 females) who did not show any signs of reperfusion, 20 had a streptokinase-antibody titre of 0.50 arbitrary units or lower. Reperfusion was not seen in any of the nine patients with a streptokinase antibody titre >0.50 arbitrary units (six males, three females). The mean streptokinase antibody titre amongst the 29 patients without signs of reperfusion was significantly higher (0.45), as compared with those patients showing signs of reperfusion (mean 0.20) (P = 0.01).
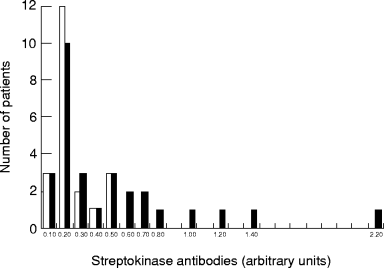
Individual patient pretreatment streptokinase antibody levels. Numbers of patients with signs of reperfusion (open bars) and with no signs of reperfusion (filled bars) are shown.
The individuals who had the highest streptokinase antibody titres also had signs of recent streptococcal infection as measured with antistreptolysin titres.
Analysis of antistreptolysine antibodies (AST-test) is a clinical routine test used for aiding in the diagnosis of poststreptococcal disease. The AST titre was determined in 85 of the 88 consecutive pretreatment patients. Amongst the 15 patients with a high level of antistreptokinase antibodies (>0.50) 5 (33.3%) showed signs of recent beta-streptococcal infection (i.e. AST was ≥200 IU), in contrast to only 2 (2.9%) in the 70 patients with AST <200 ( Table 1) (rank test P < 0.01).

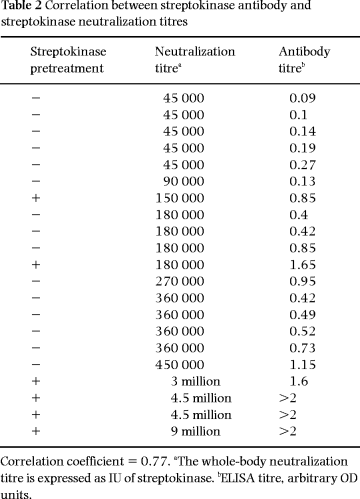
For 15 of the patients, the whole-body streptokinase neutralization titre was estimated using a clot lysis test. In addition, six patients previously treated with streptokinase, and showing high antistreptokinase antibody levels, were included ( Table 2). A positive correlation (correlation coefficient 0.77) between the neutralization titre and antibody level was seen. However, none of the patients without streptokinase pretreatment had a neutralization titre higher than the clinical standard dose of streptokinase (1.5 million IU)
Discussion
In the present study an elevated level of streptokinase antibodies is found amongst patients without signs of reperfusion (as determined by computerized continuous dynamic vectorcardiography) during thrombolytic therapy with streptokinase in acute myocardial infarction.
It has been argued that the level of streptokinase antibodies, amongst patients not previously given streptokinase, is too low for any influence on the clinical effect of streptokinase therapy to be anticipated. First, based on data on neutralization in vitro by streptokinase antibodies of the fibrinolytic capacity of streptokinase, it has been estimated that even a high antibody concentration (seen amongst the top 10th percentile of a general population) is capable of neutralizing only 10% of a standard 1 500 000-unit streptokinase dose. Secondly, the rather similar success rates observed after streptokinase treatment as after treatment with an agent evoking no immune response (e.g. r-tPA) indicate that the streptokinase antibodies are without clinical relevance. However, it can also be argued that (i) it may be that the fibrinolysis in vivo of the local coronary thrombus is affected by other concentrations of both streptokinase and streptokinase inhibitors (e.g. streptokinase antibodies), as compared with in vitro; (ii) clinical studies have shown that both plasminogen [19] and plasminogen activator inhibitor [20] levels seen in patients can be high enough to significantly and adversely affect the clinical effect of r-tPA. These findings of r-tPA inhibitors therefore indicate that the comparable clinical success rates of streptokinase and r-tPA also reflect the presence of inhibitors to streptokinase.
Recently three studies have been presented on the relation between the streptokinase antibody level and the early patency rate, giving different conclusions. Brügemann et al. [6], in a study of 21 patients given streptokinase, found no angiographic reperfusion signs in any of the patients (n = 3) showing a high streptokinase antibody titre (as measured by ELISA), whereas 16 of the 18 patients responding to therapy had low streptokinase antibody values. In contrast, in a study by Fears et al. [10] on 333 patients given streptokinase or anistreplase, no influence of the streptokinase antibody level (measured by RIA) was found on angiographic results, plasma fibrinogen or plasminogen concentrations or on hospital death rate. A third study, by Gemmill et al. [11], shows a correlation between predosing antibody titres and post-treatment coronary artery patency, indicating influence of the antibodies on the effect of the given thrombolytic therapy.
We cannot with any certainty identify any factor(s) explaining the diverging results of these four studies. In our opinion the differences in method for determination of IgG (ELISA or RIA were used) are not significant.
However, the use of different methods for assessing the myocardial blood supply or ischaemia (angiography or continuous dynamic vectorcardiography) may be important. In most patency studies the diagnosis prior to therapy is based on electrocardiographic measuring, whilst the result of therapy instead is measured by angiography. In contrast, in our study we have used the same technique before, during and after thrombolytic therapy; on-line computerized vectorcardiography, which makes it possible to directly follow the myocardial perfusion [21]. Thus, it may possibly be that some patients will turn out to have patent larger vessels at angiography, whilst a continuous dynamic vectorcardiography would have revealed persistent ischaemia due to occluded distal branches not visualized by angiography. It is known that angiographic patency after streptokinase treatment occurs later than after r-tPA treatment. It is also known that early angiography cannot predict post-thrombolytic coronary reocclusion [22]. Therefore, the results by Fears may have been affected by a relatively early time of post-treatment coronary angiography [23].
Our method for analysis of antistreptokinase IgG seems to reflect an immune response to streptococci, since the observed IgG values correlate well with the AST titres. This finding strongly indicates that our ELISA method truly measures antibodies generated during a previous exposure of the patients to streptokinase during a bacterial infection.
In agreement with previous findings of several groups [24, 25] the streptokinase in vitro neutralization titre correlates only poorly with antistreptokinase IgG. This discrepancy might reflect that the clot lysis test is not a very relevant indicator of in vivo conditions. The factors influencing intracoronary thrombolysis may indeed vary from those in the test tube, e.g. the cascade of reactions induced by formation of the plasminogen-streptokinase complex can be one such factor.
The reperfusion failure frequency from pooled data from several studies of thrombolytic therapy in AMI has been reported to be 45–52% when streptokinase was the administered drug. Our result of 58% (29/50) reperfusion seems to be in parity with the results of these studies. Interestingly, if the 18% of our patients who had high streptokinase antibody levels were excluded, the reperfusion failure would be decreased by 10.5% to 47.5% (20/41).
In conclusion, our results show that the concentration of streptokinase antibodies affects the rate of early reperfusion signs in continuous dynamic vectorcardiography during streptokinase therapy.
References
Received 25 February 1998; accepted 2 September 1998.