Mass mortality associated with a Sphaerospora-like myxosporidean infestation in juvenile cobia, Rachycentron canadum (L.), marine cage cultured in Taiwan
Abstract
Cultured cobia, Rachycentron canadum, of 45–80 g exhibited anaemia and ascites, and a mottled red and grey, extremely enlarged kidney with cream-coloured patches or spherical nodules. Cumulative mortality was about 90% within 1 month. Extrasporogonic or sporogonic stages of a myxosporean appeared in the blood, glomerulus, renal tubules and renal interstitium. The renal tubules were the main target tissue of the parasite and were completely occluded by sporogonic pseudoplasmodia at various degrees of maturity. Many sporogonic stages were attached to the brush border of the epithelium of the renal tubules. Mature spores were seen in the lumen of the tubules. They were elongated or spherical with numerous refractile granules in the cytoplasm. The polar filament formed 3–5 coils. No bacteria or viruses were isolated from the diseased fish. Based on the results of microbiological, histopathological and electron microscopical examinations, the cobia disease was believed to be caused by a Sphaerospora-like myxosporean. This is the first report of a myxosporean in cobia in aquaculture.
Introduction
Forty-three species of the myxosporean genus Sphaerospora have been described, mainly from freshwater fish (El-Matbouli & Hoffmann 1992). Most Sphaerospora spp. do not cause significant pathology, but some species are pathogenic, as documented in the common carp, Cyprinus carpio L. (Csaba, Kovacs-Gayer, Bekesi, Bucsek, Szakolczai & Molnar 1984) and brown trout, Salmo trutta L. (Fischer-Scherl, El-Matbouli & Hoffmann 1986). Thirty-six species of Sphaerospora were described from the lumen of kidney tubules and urinary bladders of freshwater fish by Arthur & Lom (1985). Subsequently, seven other renal species have been described (Li & Desser 1985; Fischer-Scherl et al. 1986; Lom, Desser & Dykova 1989; Baska 1990; Hedrick, McDowell & Groff 1990; Supamattaya, Fischer-Scherl, Hoffmann & Boonyaratpalin 1991).
Currently, about 1500 cage-cultures are being operated in the coastal and offshore waters of Taiwan, representing about 11% of total marine aquaculture production. The juvenile fish stocked in these cages are all hatchery-produced. One of the main cultured species is cobia, Rachycentron canadum (L.). Cobia juveniles usually grow from 30–50 g to 6 kg within 1 year and are regarded as having the highest potential for cage aquaculture in Taiwan (Su, Chien & Liao 1999). Recently, a severe mortality of cage-cultured cobia was recorded which was associated with a Sphaerospora-like myxosporean infection. This paper describes the epizootiology, clinical pathology, histopathology and microbiology of this condition.
Materials and methods
Fish
A fish farm in Penghu, Taiwan introduced 55 000 (30–40 g) cobia into marine cages on 16 August 1999. These fish developed poor appetite and anorexia on 22 September 1999 when they had reached 45–80 g in weight. Chloramphenicol was added to the fish diet (60 g per 20 kg) for 8 days but approximately 1600–1800 fish died each day from 4 to 28 October. The accumulated mortality was 90% (49 500/55 000) within 30 days. Twenty fish were sampled for this study from 16 to 24 October. After anaesthesia by MS 222 (0.008% solution), blood was taken by cardiac puncture with heparinized plastic syringes. Blood smears were stained according to the Diff–Quick method.
Bacteriology
Swabs were taken from the kidney, spleen and liver and streaked onto tryptic soy agar (TSA), TSA with 5% goat blood [blood agar (BA)], brain heart infusion agar, Sabouraud’s dextrose agar and Lowenstein–Jensen medium. Plates were then incubated at 25 °C for 21 days.
Cell culture
Kidney and tissue were homogenized (1:100 in saline) and inoculated to CHSE-214, FHM, BF-2, TO2 cell cultures grown in antibiotic-free culture with supernatants for 3 weeks at 22 °C.
Pathology
The gills, kidney, heart, liver, spleen and other internal organs with lesions were fixed in 10% buffered formalin and processed for paraffin sectioning. The sections were stained with haematoxylin and eosin (H & E).
Electron microscopy
The kidney, liver and spleen were fixed with 2.5% glutaraldehyde in 0.2 M sodium cacodylate for transmission electron microscopy and post-fixed in 1% osmium tetroxide in 0.2 M sodium cacodylate. Sections were stained in uranyl acetate and lead citrate before examination.
Results
Clinical signs and examination of blood
The fish exhibited pale livers, ascites, petechiation and gill pallor. The trunk kidney was enlarged and displayed a mottled red and grey appearance with cream-coloured patches or spherical nodules. In general, the kidney was approximately 3–25 times its normal size (Figs 1 & 2). Extrasporogonic stages of myxosporeans appeared in kidney and blood impression smears of the fish sampled (Figs 3 & 4). They were elongated or irregular in shape and measured 7–12 μm in size. These included stages containing a single primary and single secondary cell and those having 5–7 secondary cells in a single primary cell.

Dilation of the abdominal cavity and enlargement of the kidney of infected cobia. The kidney is approximately three times its normal size (arrow).
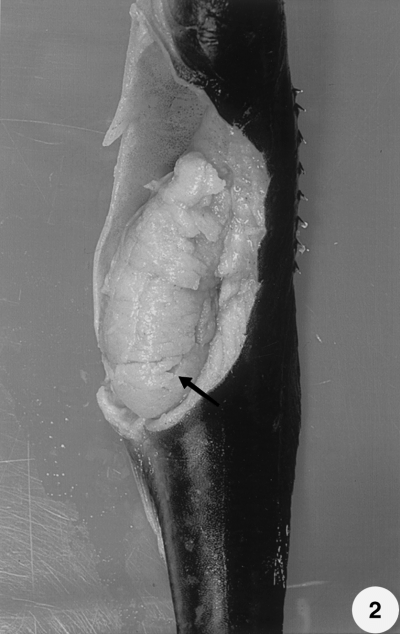
Dilation of the abdominal cavity and extreme enlargement of the kidney in cobia. The kidney is approximately 25 times its normal size (arrow). Post-fixed in 10% buffer neutral formalin solution.

Diff–Quick-stained kidney smear of cobia with Sphaerospora-like myxosporidean. Two primary (P) cells with nucleus (n) and three or five secondary cells (arrows) (× 1000).

Diff–Quick-stained blood smear of cobia with Sphaerospora-like myxosporidean. (a) Primary cell with nucleus (arrowhead) and six secondary cells (arrow). (b) Primary cell containing two spindle-shaped doubled cells (arrow) (× 1000).
Bacteriology
No bacteria were isolated using any of the media after incubation at 25 °C for 21 days.
Cell culture
No cytopathic effects were detected in any cell lines after culture for 3 weeks at 22 °C.
Histopathology
Various stages of development of a myxosporean occurred in the glomerulus, renal tubules and interstitium. Parasites were seen in the glomerular capillary loops and Bowman’s space. In some cases the glomerulus was markedly dilated while others were virtually absent. The renal tubules were the main target tissue of the parasite and in many cases were completely occluded by sporogonic stages (Fig. 5). The renal tubules showed significant dilation, hypertrophy and hyperplasia. Many sporogonic stages were attached to the brush border of the epithelium of the renal tubules by cytoplasmic projections. They were elongated or spherical, with numerous refractile granules in the cytoplasm. Mature spores were found in the lumen of the renal tubule (Fig. 6). Some presporogonic stages appeared in the renal tubular epithelium (Figs 6 & 7). The necrosis of sporogonic stages, with brown pigment and mineralization or multifocal granulomas, was also seen in the lumen of the renal tubules and in the interstitium. Renal tubular epithelium showed vacuolation, necrosis (Fig. 8) and granuloma in some cases. No significant lesions were found in other internal organs.

Renal tubule (R) completely occluded by sporogonic stage parasites (P) in various degrees of maturity (H & E, × 200).

Spores with typical two spherical polar capsules (arrows), equal in size, located in the lumen of the renal tubule. Two presporogonic stages (arrowheads) of Sphaerospora-like myxosporidean in renal tubule epithelium (× 1000).

Extrasporogonic stages penetrating renal tubule epithelium and rounding up to form trophozoites (arrows) (H & E, × 1000).

Sporogonic stages completely congesting the renal tubules and damaging the epithelium (arrow) (H & E, × 400).
Electron microscopy
Many sporogonic stages were firmly attached to the inner wall of the renal tubules by the projections of the pseudoplasmodial cytoplasm which penetrated between the lining microvilli of the epithelium (Fig. 9). Stages towards the centre of the lumen were invariably the most advanced, often containing fully formed spores (Fig. 10). The polar filament formed 3–5 coils and two unincleated sporoplasm cells were seen (Figs 11 & 12). Renal tubular epithelial cells showed a degree of vacuolation. Sporogonic stages showed large numbers of mitochondria, some Golgi and cisternae of rough endoplasmic reticulum.

Sporogonic stages attached to the brush border of the renal tubule (R) by projections of the pseudoplasmodial cytoplasm (arrow) (× 3500).

Sporogonic stages in the centre of the renal tubules showing one or two spherical polar capsules (arrow) (× 3500).

Higher magnification of Fig. 12. Maturing spore of Sphaerospora-like myxosporidean with one polar capsule within a pseudoplasmodium (P) (× 15 000).

Maturing spores of Sphaerospora-like myxosporidean within a pseudoplasmodium (P). Both polar capsules are spherical. The polar filament has 3–5 coils (arrow) (× 10 000).
Discussion
The majority of Sphaerospora species have been reported from cyprinid fish in Europe (e.g. Lom, Pavlaskova & Dykova 1985; Baska & Molnar 1988) and in North American fish (e.g. Meyers & McPherson 1985; Hedrick et al. 1990). However, more recently, Sphaerospora spp. were found in South-East Asia (Supamattaya, Fischer-Scherl, Hoffmann & Boonyaratpalin 1990; Supamattaya et al. 1991). Dykova & Lom (1982) reported that in S. renicola, both sporogonic and extrasporogonic stages have serious pathogenic potential. Large masses of sporogonic stages in the renal tubules induce tubular dilation, atrophy and result in necrosis of the epithelium. Similarly, Hermanns & Korting (1985) reported that S. tincae is a serious pathogen of European tench, Tinca tinca L., affecting the pronephros and producing severe epizootics in tench yearlings in France and Germany. Masses of parasites replace the parenchyma to provoke hypertrophy of the kidney. This appears externally as a conspicuous distension of the abdomen. In this study, the diseased cobia also showed extreme dilation of the abdomen and severe enlargement of kidney with 90% mortalities. No pathogenic virus or bacteria were isolated from the fish and it seems probable that the Sphaerospora-like myxosporidean was the causative agent of the disease.
Sphaerospora-like extrasporogonic forms observed in the blood of cobia would seem to be similar to those described by other authors (Molnar 1980; Csaba et al. 1984; Lom et al. 1985) and are presumed to be precursors of the sporogonic forms found in the kidney. Apparently identical extrasporogonic stages were seen in kidney blood vessels and glomerular capillary loops of cobia and it seems likely that these stages are transported to the kidney via the circulation passing through the glomerular capillary loops.
Severe glomerular destruction associated with Sphaerospora spp. has been noted in a number of fish species (Molnar 1980; Meyers & McPherson 1985; Supamattaya et al. 1990), as also seen in this study. Intracellular trophozoites in the renal tubules elicited a proliferation of the epithelial cells and severe effects on the kidney renal tubules were apparent during the sporogonic stages of development. Renal tubules were eventually completely occluded by developing sporogonic stages.
The occurrence of intracellular stages within the tubule epithelium in the parasite from cobia is interesting because such stages are not reported from Sphaerospora infections. Such stages are known in some genera of Myxosporea. Molnar, Fischer-Scherl, Baska & Hoffmann (1989) reported an intracellular phase in the development of Hoferellus carassii in the tubular epithelium of kidney. In this phase, the earliest developmental stages are represented by unicellular trophozoites. More developed trophozoites consist of two or three cells formed by internal cleavage of the primary cells. In the Sphaerospora-like infection of cobia, epithelial cells were infected by a single, or occasionally two, parasites. In this respect, the Sphaerospora-like myxosporidean is more similar to Myxobilatus and H. carassii (Molnar 1988; Molnar et al. 1989) than to Sphaerospora (Lom, Dykova & Lhotakova 1982).
The ultrastructure of the parasite from cobia shows similarities to previously described members of the genus Sphaerospora (Lom et al. 1982; El-Matbouli & Hoffmann 1992; McGeorge, Sommerville & Wootten 1994). The spores had two spherical polar capsules of equal size. The polar filament had 3–5 coils almost perpendicular to the longitudinal axis of the capsule. Pseudoplasmodia were disporic. Lom, Dykova & Pavlaskova (1983) and Lom et al. (1985) redefined the genus Sphaerospora and stated that the pseudoplasmodia were generally mono- or disporous according to the species. However, Supamattaya et al. (1991) described S. epinepheli from the kidney tubules of a grouper from marine and brackish waters, which developed both mono- and disporic pseudoplasmodia. El-Matbouli & Hoffmann (1992) also described both mono- and disporic pseudoplasmodia in S. scardinii and distinguished them from S. minima, which have only disporic pseudoplasmodia.
This is the first report of sphaerosporosis in marine cage culture of fish in East Asia and the disease may become significant in cobia culture.
Acknowledgment
This study was supported by the Council of Agriculture, Taiwan [grants COA 87-14-06 (10-12)].