
Structuring of the cyanobacterial community by pelagic fish in subtropical reservoirs: experimental evidence from Australia
Summary
1. Subtropical reservoirs of Australia are commonly subject to summer blooms of cyanobacteria. The potential for food web manipulation to control cyanobacterial blooms was investigated in Lake Maroon, south east Queensland using enclosures in which the density of the Australian gudgeon Hypseleotris spp. was manipulated.
2. Zooplankton biomass and community structure were strongly affected by fish density. A size dependent predation effect of Hypseleotris on zooplankton was observed at ambient fish densities, and the community shifted towards a dominance of copepod juveniles and nauplii. Substantial increases in the populations of Ceriodaphnia and calanoid copepods were observed at low fish densities and in the absence of fish.
3. At ambient fish densities total phytoplankton and the proportion of cyanobacteria were maintained at levels similar to those prevailing at day 0. Total phytoplankton and the proportion of cyanobacteria decreased substantially at low fish densities and in the absence of fish. Chlorophytes became dominant in the ‘no fish’ treatment and the grazing-resistant species Oocystis and Dictyosphaerium were significantly higher than at ambient fish densities.
4. The experiment demonstrated a strong positive relationship between Hypseleotris density and cyanobacteria, and the results suggest that subtropical reservoirs may be suited to food web manipulation as a means of controlling summer cyanobacterial blooms.
Introduction
The occurrence of toxic cyanobacterial blooms in major water systems in Australia has stimulated research into the causes and management strategies of algal blooms on this continent (Jones, 1994; Davis, 1997). The use of food web manipulation as a method for regulating algal blooms has been extensively studied in the Northern Hemisphere (Gulati et al., 1990; Reynolds, 1994) and its role in the reduction or prevention of noxious algal blooms is an important issue for the restoration and management of lakes (Elser, 1999). The potential use of food web manipulation for controlling cyanobacteria in Australia has been debated (Boon et al., 1994; Gehrke & Harris, 1994; Matveev, Matveeva & Jones, 1994), and reviewed (Matveev, 1998, 2003) but hitherto not tested.
The ability of zooplankton grazing to control cyanobacterial blooms has been questioned due to the uningestible size, grazing-resistant coverings and the toxicity associated with certain species of cyanobacteria (Haney, 1987; Bernardi & Giussani, 1990). The effectiveness of food web manipulation to control cyanobacterial blooms in Australia has been argued against mainly on this basis (Boon et al., 1994). However, whilst direct consumer control of cyanobacterial blooms may be limited, food web manipulation may act to decrease the competitive advantage of cyanobacteria by altering the consumer-driven nutrient recycling regime (Elser, 1999).
The results from food web manipulation studies suggest both grazing and nutrient recycling can affect cyanobacteria. Substantial declines of cyanobacteria biomass have been observed in response to reductions or removal of planktivorous fish, and this has been attributed to increased zooplankton grazing (Sanni & Wævågen, 1990; Christoffersen et al., 1993; Borics et al., 2000), different rates of nutrient regeneration (Reinertsen, Langeland & Olsen, 1986; Vanni et al., 1990) or both (Lyche, Faafeng & Brabrand, 1990; Søndergaard et al., 1990). However, the positive correlation of cyanobacteria with planktivorous fish does not always hold. Reduction or removal of planktivorous fish has resulted in increased cyanobacteria biomass (Lyche, 1989), and has shifted the dominance of phytoplankton to a different species of cyanobacteria (Hrbacek, 1964; Carpenter et al., 1995). Hence the complex interactions occurring in lake food webs result in unpredictability in the response of a system to manipulation.
The present study was conducted in Lake Maroon, a subtropical reservoir in Queensland, Australia. In this region reservoirs typically demonstrate a seasonal dominance of cyanobacteria, including species known to be toxic [e.g. Anabaena circinalis Raben, which was the predominant toxic species in the 1991 Darling River algal bloom outbreak (Jones, 1994)]. During the summer months cyanobacteria frequently attain concentrations which represent a potential health risk in many of these reservoirs (QDNR, 1999). Native pelagic piscivores do not occur in the reservoirs unless they are stocked, as they require either migration to coastal waters or natural flooding events to stimulate spawning (McDowall, 1996). Consequently, large populations of planktivorous fish species can develop in the absence of a viable piscivore population, making food web manipulation a feasible management strategy to control cyanobacterial blooms.
The subtropical reservoirs of Australia present novel environments for food web manipulation in terms of their biota, climate, physical limnology, age and nutrient fluxes (Banens & Davis, 1998; Matveev, 2003). The aim of the experiment was to evaluate the short-term response of phytoplankton and zooplankton to complete removal, a reduced density, and an ambient density of the Australian gudgeon (Hypseleotris spp.) during a summer period when the phytoplankton community of Lake Maroon was dominated by the cyanobacterium Anabaena circinalis. Hydroacoustic surveys of planktivorous fish in the pelagic zone of Lake Maroon were used to establish the ambient fish densities to be used in the enclosure studies.
Methods
Study site
Lake Maroon (south-east Queensland, Australia) is the result of a dam constructed during the 1960s. It has a capacity of 38 400 ML, covering an area of 326 ha with a maximum depth of 34 m (Chudek et al., 1998). The water body is monomictic and stratified from September to May, with a summer mean surface temperature of 25 °C. It is a mesotrophic lake with mean annual total nitrogen (TN), total phosphorous (TP) and chlorophyll a concentrations of 590, 27 and 8 μg L−1, respectively (V. Matveev, unpublished data).
As a consequence of the impoundment, the native piscivorous fish, which require natural flooding or downstream migration to breed (McDowall, 1996), cannot successfully reproduce. Native piscivores, predominantly Australian bass (Macquaria novemaculeata Steindachner), are stocked as fingerlings at low densities (15–200 ha−1 year) for recreational fishing (Queensland Department of Primary Industries, 1999, personal communication). A number of planktivorous fish species inhabit the pelagic zone, including the Australian gudgeon (Hypseleotris spp.) and the Australian smelt (Retropina semoni Weber).
The crustacean zooplankton community is dominated by copepods including the calanoid genera Boeckella and Calamoecia, and the cyclopoid genus Mesocyclops. Phytoplankton succession during a 2-year period (1997–99) demonstrated a summer peak of chlorophytes followed by a considerably higher biovolume of cyanobacteria. (V. Matveev & L. Matveeva, unpublished data).
Design, construction and preparation of enclosures
The enclosures were constructed from tubes of polythene film (0.5 mm thick), 1.5 m in diameter with a depth of 3 m and sealed at the bottom to give a volume of 5000 L. After conditioning in lake water for 5 days, enclosures were suspended from offshore pontoons and had clear polycarbonate lids (1 mm thick). Enclosures were filled on 22 and 23 February 1999 with filtered lake water (85 μm) pumped from a depth of approximately 2.5 m using a 5 hp centrifugal impeller driven pump.
Experimental Design
The experimental design aimed to establish initial conditions in the enclosures similar to those in the lake. Consequently, the three treatments were applied as soon as possible after enclosures were filled:
- 1
AF (ambient fish): fish stocked at the ambient density of pelagic planktivorous fish in Lake Maroon during summer (n = 3);
- 2
LF (low fish): fish stocked at one-third of the AF density (n = 2); and
- 3
NF (no fish): no fish stocked (n = 3).
Ambient densities of fish were determined from the time-weighted mean of planktivorous fish abundance in the pelagic zone of Lake Maroon during the summer of 1998–99. The density of pelagic planktivorous fish inhabiting the epilimnion of Lake Maroon was measured with a Simrad EY 500 scientific echo-sounder (Simrad-Kongsberg Co., Horten, Norway) using a 7° transducer operating at 200 KHz and a pulse rate of 0.5 m s−1. During the period from 2 December 1998 to 24 February 1999 the density of fish ranged between six and 17 fish m−3, resulting in a time-weighted mean (calculated according to Vanni & Findlay, 1990) of 11.8 ± 1.57 fish m−3 (±SE, n = 5).
Zooplankton were added to the enclosures on day 0 (24 February 1999) with four pooled 7 m vertical hauls taken with a 64 μm (50 cm diameter). A second zooplankton addition was made because zooplankton samples taken on day 0 revealed a lower biomass in the enclosures than in the lake (for the method see section on sampling below). On day 7 (3 March 1999) two pooled 15 m vertical hauls taken with a 500 μm net (50 cm diameter) were added to each enclosure.
Australian gudgeon (Hypseleotris spp.) from Lake Maroon averaging 28 mm in length and 0.3 g wet mass were transferred to enclosures on day 1. Stocking rates were 12 fish m−3 (3.6 g m−3), and 4 fish m−3 (1.2 g m−3) in the AF and LF treatments, respectively. Fish health was visually assessed on at least every sample date. On day 23 one fish death was noted in an AF enclosure and was removed. No other fish deaths were observed either during or at the termination of the experiment.
Enclosure sampling
Sampling of zooplankton, phytoplankton and chlorophyll a began on day 0 (25 February 1999), 1 day before the addition of fish, and conducted every 9 days until day 27 (24 March 1999). Temperature was recorded every third day from day 0 and Secchi depth was measured every third day after day 15. Measurements of physico-chemical conditions in the enclosures (temperature, pH and dissolved oxygen) were taken 1 week after the last sampling date using a Hydrolab multiprobe (Hydrolab-Hach Co., Loveland, CO, USA) to determine depth profiles.
Zooplankton were sampled with a 14 cm diameter Wisconsin style net with 85 μm mesh. Two hauls were taken between 2.5 and 2.7 m depth from each enclosure, pooled and preserved with ethanol and stained with Lugols solution. Samples were stored at 4 °C. Zooplankton were counted and separated into categories according to taxa and to major size classes within a taxon using a dissecting microscope. A minimum of 90 individuals was enumerated for each category. The biomass of the crustacean zooplankton was calculated from measurements of at least 20 individuals from each category using length–weight regressions from Bottrell et al. (1976) for species or genera that most closely matched the taxa. Rotifer biovolume was calculated from the mean length and width measurements of each genus using the formulae of Bottrell et al. (1976). Biovolume conversion to biomass assumed a specific gravity of 1 so that 106 μm3 = 1 μg, and dry mass was calculated as 5% of wet weight for Asplanchna and as 10% for all other genera (Bottrell et al., 1976). The biomass of each category was calculated from the product of population density and the individual mean dry mass. Community biomass was calculated by summing the biomass of zooplankton categories.
Integrated water samples for chlorophyll a and phytoplankton were taken with a 45 mm diameter flexible plastic hose, weighted at one end to ensure vertical sampling, and withdrawn in a U-shape by means of a cord attached to the weight (Lyche et al., 1996). Samples were taken between 2.5 and 3 m depth, and care was taken to prevent contact with the sides or bottom of the enclosures. Chlorophyll a samples passed through CF/C filters were immediately chilled and frozen at −20 °C within 6 h. Chlorophyll was extracted from GF/C filters in 90% acetone. Absorbency was measured with a Shimadzu spectrophotometer (Shimadzu Corp., Kyoto, Japan) at 750 and 665 nm against a blank, and chlorophyll a concentration was calculated according to the equation of Lorenzen (1967).
Phytoplankton samples were preserved with acid Lugols solution in polythene bottles for phytoplankton analysis. Phytoplankton samples were concentrated by sedimentation (APHA, 1992), and identified and counted using a compound microscope. A minimum of 100 individuals (unicellular, colonial and filamentous forms) of the most abundant taxa were counted. Biovolume was calculated from linear dimensions of at least 20 individuals from each phytoplankton taxa using formulae of appropriate geometric shapes (Wetzel & Likens, 1991). Biovolume was calculated for each phytoplankton taxa by multiplying the mean volume by the population density, and community biovolumes were calculated by summing population biovolumes of phytoplankton taxa.
Lake Maroon sampling
Temperature, pH and dissolved oxygen were measured using a Hydrolab multiprobe. Chlorophyll a, dissolved inorganic nitrogen (DIN), filterable reactive phosphorus (FRP), TN and TP were taken from a single 10-m integrated water sample, using a flexible 25-mm plastic hose. All analyses were conducted at GCL Laboratories, Queensland. Chlorophyll a was analysed using the APHA (1992) method, after filtering lake water through a 0.45-μm acetate filter. The filtered water was retained for analysis of the filterable nutrients. TP and TN were digested using the simultaneous analysis described by Hosomi & Sudo (1986). DIN and FRP were analysed using the cadmium reduction and ascorbic acid methods, respectively, according to APHA (1992). Zooplankton and phytoplankton were sampled and processed as described above.
Statistical analyses
The postexperiment oxygen, temperature and pH profiles of enclosures were analysed by a two-way mixed model anova, with fish treatment and depth as factors. To provide a balanced design for this analysis two replicates from the AF and NF treatments were randomly selected and analysed with the two replicates from the LF treatment. anovas were performed using the GLM procedure in the SAS software package (SAS, 1990).
Chlorophyll a, phytoplankton biovolume and zooplankton biomass were log10 transformed to stabilise variances. The effect of the NF, LF and AF treatments on zooplankton biomass, chlorophyll a and phytoplankton biovolume were analysed from the time averaged means of all sample dates for each enclosure after day 0 by one-way anova. Post hoc analysis to determine differences between pairs of treatments was conducted using Tukeys multiple comparison procedure. The analysis did not include day 0 sample dates because the treatments had not been applied and no treatment effects would have occurred. Because of the low number of replicates in the experimental design the power of statistical analysis is low (Sokal & Rohlf, 1995). As in studies that have employed similar experimental designs (Threlkeld, 1987; Matveev, Matveeva & Jones, 2000) this problem was addressed by setting the significance level at 10% (α = 0.1), although all P values are presented. Statistical analyses were performed using Systat software (Wilkinson, 1990).
Results
Chemical and physical factors
Mean water temperature in enclosures was 25.9 ± 0.3 °C (±SE, n = 8) for the duration of the experiment and did not differ among the treatments. Temperature and dissolved oxygen decreased with depth (P < 0.001 and P = 0.05, respectively) and pH increased with fish density (P = 0.001) in enclosures. Daytime dissolved oxygen concentration, pH and temperature in the enclosures were similar to those recorded in the lake during the summer period (Table 1).
| Time weighted mean (±SE) | |
|---|---|
| Chlorophyll a (μg L−1) | 13.1 (±2.9) |
| pH* | 10.1 (±0.1) |
| Temperature (°C) | 26.7 (±0.3) |
| DO (mg L−1) | 8.2 (±0.3) |
| DIN (μg L−1) | 31 (±5) |
| FRP (μg L−1) | 9 (±1) |
| TN (μg L−1) | 520 (±18) |
| TP (μg L−1) | 40 (±3) |
| DIN/FRP | 3.5 |
| TN/TP | 12.8 |
Zooplankton
Zooplankton biomass and community structure were strongly affected by the density of Hypseleotris. Significant treatment effects occurred on total zooplankton and all taxonomic groups, with the exception of cyclopoids (Table 2). Treatment comparisons (Table 2) revealed that LF and NF treatments differed only in the response of nauplii, whilst the AF treatment had significantly different effects on all zooplankton categories except for cyclopoids.
| Source of variation | P | Treatment comparisons |
|---|---|---|
| Total zooplankton | 0.002 | (NF LF) AF |
| Rotifers | <0.001 | AF (LF NF) |
| Nauplii | 0.002 | AF LF NF |
| Cyclopoids | 0.659 | (NF LF AF) |
| Calanoids | 0.004 | (NF LF) AF |
| Ceriodaphnia | 0.003 | (NF LF) AF |
| Daphnia | 0.011 | (NF LF) AF |
The temporal pattern of variation in zooplankton community structure was similarly affected by the NF and LF treatments (Fig. 1). In both treatments zooplankton biomass increased several times by day 9, shifting from communities predominantly composed of copepods to communities dominated by Ceriodaphnia. The copepod biomass was observed to increase over time in both the NF and LF treatments. In the NF treatment the copepod community was predominantly adult calanoids, whilst in the LF treatment the proportion of nauplii was higher. In the AF treatment larger zooplankton were suppressed and copepod nauplii were predominant. The rotifer community was comprised of fluctuating populations of Brachionus, Trichocerca, Filinia and Asplanchna (Fig. 2) and a noticeably high biomass of Asplanchna was observed on day 9.
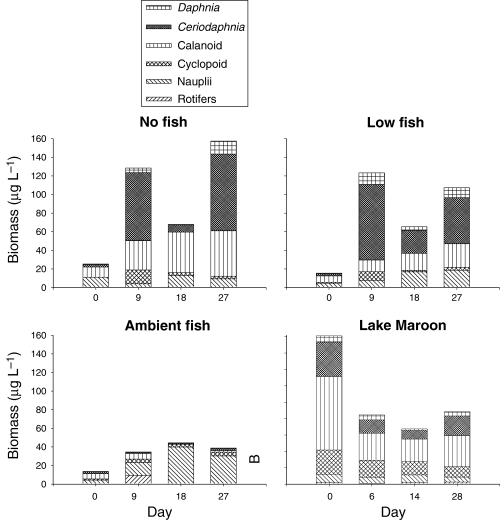
Temporal variation of biomass zooplankton for major taxa in Lake Maroon experimental enclosures and corresponding period in Lake Maroon (February–March 1999). NF, no fish; LF, low fish; AF, ambient fish.
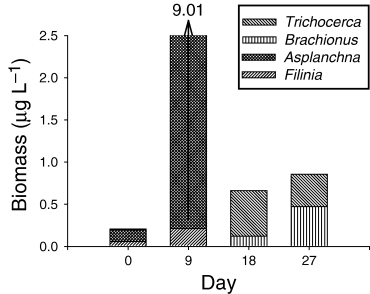
Temporal variation of rotifer biomass for major taxa in Ambient Fish enclosures in Lake Maroon (February–March 1999). NF, no fish; LF, low fish; AF, ambient fish. The day 9 column exceeded the scale of the graph and the biomass value is given above the arrow.
In all treatments zooplankton biomass increased from the levels at day 0, although the extent of the increase in the AF treatment was much reduced compared with the NF and LF treatments. The community structure of zooplankton after day 0 was strongly modified in all treatments. Size structure of the zooplankton communities showed a shift towards smaller forms in the AF treatment, and an increase in intermediate and large zooplankton forms in the NF and LF treatments (Fig. 3).
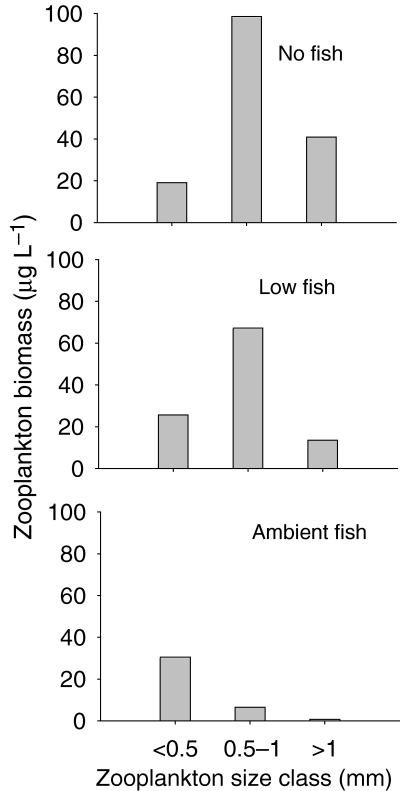
Size distribution of crustacean zooplankton in Lake Maroon experimental enclosures on day 27. Size classes based on body length measurements.
In Lake Maroon on day 0 of the experiment the zooplankton biomass was considerably higher in the enclosures, but subsequently decreased and tended to remain lower in the NF and LF treatments and higher in the AF treatment. Calanoids were the dominant taxa in Lake Maroon during the period of the experiment (Fig. 1).
Phytoplankton
Chlorophyll a concentration increased in response to ambient fish densities and declined at low fish densities and in the absence of fish, and accordingly transparency was negatively correlated with fish density (Fig. 4). The response of total phytoplankton biovolume contrasted to that of chlorophyll a, in that phytoplankton biovolume decreased in all treatments by day 9, but in the AF treatment phytoplankton biovolume subsequently increased to a level similar to that at day 0 (Fig. 4). Cyanobacteria were the dominant taxa in the phytoplankton community on day 0, comprising 75% of total phytoplankton biovolume in all treatments. Cyanobacteria were strongly affected by the density of Hypseleotris (Fig. 5); by day 27 cyanobacteria biovolume was 5, 10 and 88% of the levels on day 0 for the NF, LF and AF treatments, respectively. The effect of fish density on chlorophyll a, total phytoplankton and cyanobacteria was significant (anova, P < 0.1), and treatment comparisons revealed all three were significantly higher in the AF treatment than the LF and NF treatments (Table 3).
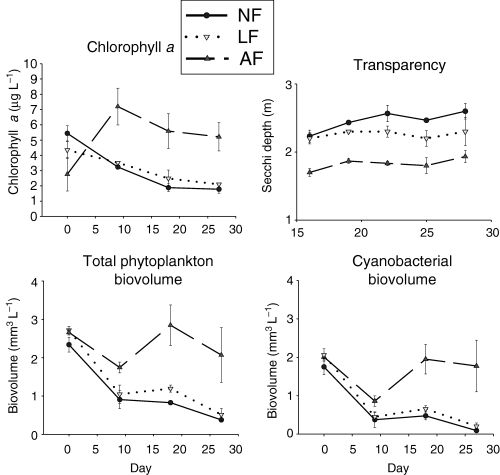
Temporal variation of chlorophyll a, transparency, total phytoplankton biovolume and cyanobacterial biovolume in the Lake Maroon experimental enclosures (February–March 1999). Transparency measurements are given for day 16 and onwards. Error bars show ±SE. NF, no fish; LF, low fish; AF, ambient fish.
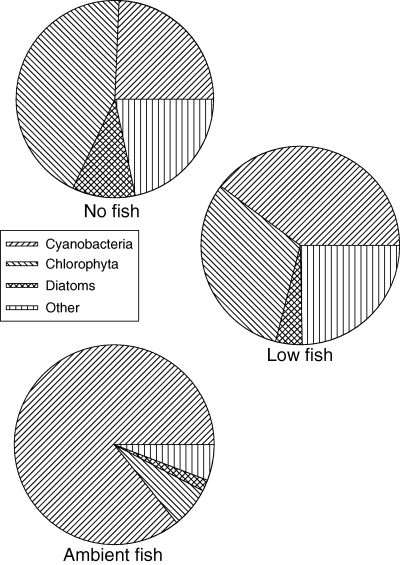
Proportion of total phytoplankton biovolume attributed to dominant phytoplankton divisions in Lake Maroon experimental enclosures on the final sampling day (day 27; March 1999).
| Source of variation | P | Treatment comparison |
|---|---|---|
| Total phytoplankton | 0.033 | AF (LF NF) |
| Cyanobacteria | 0.041 | AF (LF NF) |
| Chlorophyll a | 0.008 | AF (LF NF) |
Chlorophyll a concentrations were substantially lower in enclosures on day 0 compared with the average summer concentration in Lake Maroon (Table 1). Phytoplankton in Lake Maroon on day 0 was dominated by A. circinalis. However, succession from cyanobacteria to dinoflagellates and chlorophytes occurred in the lake by the end of the experiment.
Eight of the 16 phytoplankton taxa demonstrated significant responses to fish manipulations (Table 4, Fig. 6). The biovolume of A. circinalis which dominated the phytoplankton community in all enclosures on day 0 rapidly declined in all treatments. The declines in the enclosures coincided with a decline in Lake Maroon, but biovolumes remained significantly higher in the AF treatment compared with the NF treatment. Both Aphanizomenon issatschenkoi Ussach. and Planktolyngbya showed particularly striking responses to fish manipulations. Aphanizomenon issatschenkoi biovolume increased at ambient fish densities, remained relatively constant at low fish densities, and declined substantially in the absence of fish (Fig. 6). The biovolume of A. issatschenkoi was higher (P < 0.1) in the AF treatment than in the NF treatment and on the last day of the experiment differed by an order of magnitude between all three treatments. Planktolyngbya biovolume increased in response to low fish densities and an absence of fish, but at ambient fish densities biovolume was higher (P < 0.1). The biovolume of Cryptomonas declined in all treatments after day 0, but remained high (P < 0.1) in the AF treatment. The AF treatment exerted a positive effect (P < 0.1) on the biovolume of Fragilaria, whilst the biovolume of Ceratium was high (P < 0.1) in the LF treatment following a substantial decline after day 0 in all treatments.
| Source of variation | P | Treatment comparison | GALD (μm) |
|---|---|---|---|
| Cyanobacteria | |||
| Anabaena circinalis | 0.110 | (NF LF) (LF AF) | 53, 74, 104 |
| Aphanizomenon | 0.030 | (NF LF) AF | 72, 185 |
| Microcystis incerta | 0.262 | (AF LF NF) | 4, 25 |
| Oscillatoria | 0.651 | (AF LF NF) | 460 |
| Planktolyngbya | 0.002 | (NF LF) (LF AF) | 129 |
| Chlorophytes | |||
| Ankistrodesmus | 0.919 | (AF LF NF) | 68 |
| Closterium | 0.548 | (AF NF LF) | 500 |
| Dictyosphaerium | 0.047 | AF (LF NF) | 11 |
| Mougeotia | 0.804 | (NF LF AF) | 1870 |
| Oocystis | 0.011 | (AF LF) (LF NF) | 20 |
| Diatoms | |||
| Aulacoseira | 0.116 | (NF LF AF) | 14 |
| Fragilaria | 0.009 | (NF LF) AF | 42, 120 |
| Pinnularia | 0.197 | (LF NF AF) | 102 |
| Chrysophytes | |||
| Cryptomonas | 0.007 | (LF NF) AF | 13 |
| Rhodomonas | 0.276 | (LF NF AF) | 7 |
| Dinoflagellates | |||
| Ceratium | 0.041 | (NF AF) LF | 140 |
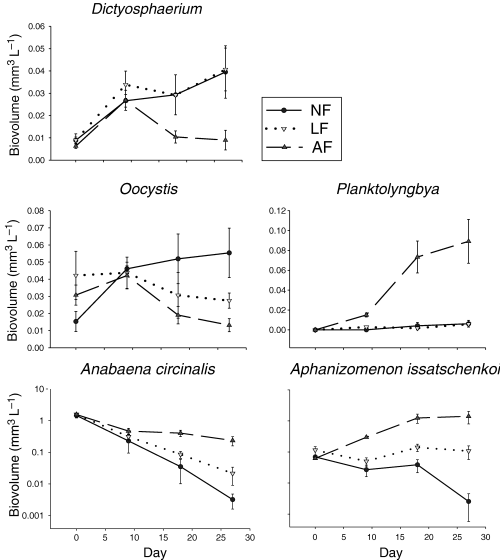
Temporal variation of biovolume for selected phytoplankton taxa that were significantly affected by fish density in Lake Maroon experimental enclosures (February–March 1999). The lower graphs for Anabaena circinalis and A. issatschenkoi display the results with a log10 scale on the Y-axis, and reveal more clearly the differences in biovolume between NF and LF treatments. Error bars show ±SE. NF, no fish; LF, low fish; AF, ambient fish.
The AF treatment had a negative effect on two phytoplankton taxa. Oocystis biovolume was significantly lower at ambient fish density compared with the absence of fish, and Dictyosphaerium biovolume was significantly lower in the AF treatment compared with both the NF and LF treatments. There were no significant effects of Hypseleotris on the biovolume of the remaining eight phytoplankton taxa (i.e. Ankistrodesmus, Aulacoseira, Closterium, Microcystis, Mougeotia, Oscillatoria, Pinnularia and Rhodomonas).
The effect of fish density on the size structure of the phytoplankton community corresponded with changes in taxon composition. Taxa with greatest axial linear dimension >100 μm were maintained in the AF treatment but declined in the NF and LF treatments.
Planktivorous fish density in Lake Maroon
The time-averaged density of pelagic planktivorous fish from three sample dates taken during the enclosure experiment (from 24 February 1999 to 24 March 1999) was 6.12 (±0.78) fish per m3. This represented a decline of 50% of the time-averaged density of pelagic planktivorous fish in Lake Maroon during the summer (from December 1998 to February 1999).
Discussion
The results of this experiment revealed that the density of Hypseleotris can have strong effects on the biomass and community structure of phytoplankton, and this was particularly evident in the response of cyanobacteria. The substantial increase in biomass of two nitrogen-fixing species of cyanobacteria (A. issatschenkoi and Planktolyngbya sp.) at ambient fish densities maintained the dominance of cyanobacteria after the initial dominance and subsequent decline of A. circinalis. This contrasted with a substantial and sustained decline of cyanobacteria and total phytoplankton at low fish densities and in the absence of fish. A positive relationship of Hypseleotris density with phytoplankton biomass and the proportion of cyanobacteria became apparent during the experiment. Phytoplankton were most strongly altered from initial conditions in the enclosures which represented a partial (LF) and complete (NF) removal of planktivorous fish, whilst at ambient fish densities the prevailing conditions in enclosures favoured the continued dominance of nitrogen-fixing cyanobacteria.
Hypseleotris exerted a typical size dependent predation effect on the zooplankton community at ambient densities. The high zooplankton biomass and large bodied zooplankton in the NF and LF treatments, predominantly Ceriodaphnia and calanoid copepods, presumably exerted a greater grazing pressure on phytoplankton than the nauplii dominated community at ambient fish densities. The decline of A. circinalis was not related to differences in grazing as it coincided in all treatments and in Lake Maroon. It is possible that grazing pressure by zooplankton, particularly Ceriodaphnia, which can have a high grazing capacity (Hambright, 1994), may have maintained the significantly lower phytoplankton biovolume in the absence of fish and at low fish densities, and suppressed the growth of phytoplankton that was observed at ambient fish densities. However, the ability of Ceriodaphnia to negatively affect phytoplankton growth under the nutrient limited conditions of late summer in Lake Maroon appears to be minimal due to the counteractive effects of nutrient regeneration (R. J. Hunt, V. Matveev, G. J. Jones & K. Warburton, unpublished data).
The relative distribution of grazer resistant phytoplankton species within and among treatments suggests that factors other than grazing were important in determining the different treatment effects on phytoplankton community structure. Phytoplankton defined as non-grazable possess the physical attributes of size and morphology which prevent or greatly impede their ingestion by zooplankton (Reynolds, 1994), such as filamentous cyanobacteria, whilst grazing-resistant forms include phytoplankton which can be ingested but can withstand passage through the gut (Porter, 1975). This group includes thick-walled greens such as Oocystis, and species protected by a mucilaginous sheath which is a trait of many cyanobacteria, including Aphanizomenon, and some greens (Vanni, 1987; Kerfoot, Levitan & DeMott, 1988), for example Sphaerocystis and Dictyosphaerium. In the NF treatment the biovolume of Oocystis and Dictyosphaerium increased to an order of magnitude higher than Aphanizomenon by day 27. Resistance of Aphanizomenon to digestion has been demonstrated for Daphnia (Holm, Ganf & Shapiro, 1983) and the calanoid Acanthodiaptomus denticornis Wierzejski (Goarant, Prensier & Lair, 1994). If the grazing resistant qualities of Aphanizomenon are comparable with or greater than those of Oocystis and Dictyosphaerium such a distinction in biovolume would indicate a competitive advantage for the chlorophytes unrelated to grazing-resistance. The biovolume of Oocystis and Dictyosphaerium were significantly lower at ambient fish densities compared with the absence of fish, despite the positive effect of ambient fish densities on total phytoplankton. By day 27 the treatment effect on both species was quite apparent; the response of Dictyosphaerium was similar for the LF and NF treatments, but Oocystis biovolume was lower at low fish densities compared with the absence of fish, and both species were lowest at ambient densities. The biovolume of A. issatschenkoi in the three treatments was separated by at least an order of magnitude by day 28 (NF < LF < AF) and this pattern was similar for A. circinalis. This response of grazer-resistant phytoplankton species provides insight into the effects exerted on phytoplankton community structure which are predominantly unrelated to grazing. In addition, the differences in phytoplankton community structure between NF and LF treatments developed whilst exposed to a similar zooplankton biomass and community structure, and therefore similar grazing pressure.
These results support the view that direct effects of nutrient excretion by Hypseleotris, and possibly zooplankton, can shift the competitive advantage towards nitrogen fixing cyanobacteria. One explanation for this could be because of different ratios of nutrient recycling in the different treatments. Current hypotheses regard the ratios at which nutrients are recycled as important in determining phytoplankton biomass and community structure under conditions of nutrient limitation (Elser, 1999), and there exists a tendency for cyanobacteria dominance to be favoured at low N : P ratios (Smith, 1983). Lakes that have a TN to TP ratio of between 10 and 17 by weight, such as Lake Maroon (mean summer TN : TP = 12), tend to have a close balance between nitrogen and phosphorus limitation (Carroll & Pelletier, 1991) and are likely to be more susceptible to transitions between nitrogen and phosphorus limitation caused by changes in food web structure which affect nutrient supply rates. Nutrient recycling by fish tends to alleviate phosphorus limitation because of low N : P supply ratios (Schindler & Eby, 1997) whilst daphnids demonstrate a tendency to sequester nitrogen and phosphorus at low N : P ratios which can increase phosphorus limitation (Sterner, Elser & Hessen, 1992; Lyche et al., 1996). A shift from nitrogen limitation to phosphorus limitation in the NF treatment due to the absence of fish and a larger zooplankton biomass dominated by Ceriodaphnia is possible. For example, low phosphorus release by fish and greater assimilation of nutrients by the zooplankton community, augmented by the tendency for lower N : P ratios sequestered in cladocerans, could potentially reduce the competitive advantage of nitrogen fixing cyanobacteria such as Anabaena, Aphanizomenon and Planktolyngbya, and increase that of grazer-resistant greens. Further experiments that manipulate N : P ratios and food web structure are required to investigate this more conclusively.
The dominance of Aphanizomenon and Planktolyngbya at ambient fish densities could be explained by adaptations of these species that result in a competitive advantage such as tolerance to low CO2 and high pH, buoyancy and nitrogen fixation. The high daytime pH values (of approximately 11) in all enclosures is indicative of low CO2 concentrations, and cyanobacteria generally show high productivity under these conditions (Shapiro, 1990). The buoyancy demonstrated by Aphanizomenon allows movement to the surface which is enriched with CO2 and this provides a distinct ecological advantage over non-buoyant species when CO2 concentration is low (Pearl & Ustach, 1982). Although nutrient limitation was not examined at the time of this experiment, nitrogen has been shown to limit the growth of phytoplankton taxa in Lake Maroon during summer period (Hunt et al., unpublished data) and these conditions would confer a competitive advantage to A. issatschenkoi and Planktolyngbya, as both can assimilate atmospheric nitrogen.
The hypothesis that an abundance of grazers promotes grazer-resistant phytoplankton forms, by selecting and removing smaller phytoplankton which compete more efficiently for nutrients (Reynolds, 1984; Haney, 1987; Kerfoot et al., 1988), represents a potential impediment to the success of food web manipulation. This conjecture was not supported by this study, although the duration of the experiment may have been too short for this to occur.
The clear distinction between the zooplankton biomass and community structure in the AF enclosures and in Lake Maroon during the experiment indicates that zooplankton were exposed to higher predation pressure by fish in the enclosures than in the lake. This may be attributable to several reasons. First, the shallow depth of the enclosures prevented zooplankton from avoiding predation by diel vertical migration into aphotic regions. Secondly, the density of planktivorous fish in Lake Maroon at the commencement and during the experiment was 50% of that in the ambient fish treatment, which was based on the mean summer densities. Thirdly, the fish stocked in the enclosures could have overestimated the biomass in the pelagic zone as fish were obtained from the littoral regions of Lake Maroon and recent evidence suggests Hypseleotris may migrate from the pelagic to littoral zones with the onset of maturity as observed in another Gobiidae species (McDowall, 1990).
The results of this experiment provide positive support for the applicability of food web manipulation in subtropical Australian reservoirs as a means of regulating summer blooms of cyanobacteria. The possibility that the ratios of nutrient excretion could explain the relative abundance of grazer-resistant nitrogen fixing cyanobacteria and chlorophytes at different densities of Hypseleotris implies that nutrient excretion is an important mechanism in determining phytoplankton community structure by affecting the relative limitation between nitrogen and phosphorus. The need to incorporate excretion rates into lake food web models has been experimentally validated (e.g. Polis & Winemiller, 1996) and it is proposed here that nutrient regeneration becomes increasingly important in determining phytoplankton response to food web manipulation as the degree or extent of nutrient limitation within a systems increases. The response of subtropical lakes to food web manipulations has been shown to deviate from expectations based on temperate lakes (Crisman & Beaver, 1990; Havens, East & Beaver, 1996). One of the ‘deviations’ of subtropical and tropical freshwater environments in Australia is an observed tendency for nitrogen to exceed phosphorus as the predominant limiting nutrient (Hunt et al., unpublished data; Udy, Mosisch & Bunn, 1999) and consequently such systems may not fit with models developed within the ‘phosphorus paradigm’ from studies predominately conducted in temperate environments.
Acknowledgments
This study was funded in part by CSIRO Land and Water, Australia. The authors whish to thank Andrew Palmer, Cheryl Orr and Lilian Matveeva of CSIRO Land and Water, and Lennie Newlove of DNR & M for their technical assistance.




