Some limitations in the detection of residual aneuploid cells with dna quantification on immunophenotyped cells by flow cytometry
A recent report by Almeida et al (1999) showed data about the flow cytometric detection of residual aneuploid myeloma cells in bone marrow before and after autologous transplantation of peripheral blood stem cells (PBSCT) and the contamination of leukapheresis samples with aneuploid myeloma cells. The sensitivity of the described assay deserves further comment. The sensitivity level for detecting aneuploid cells depends on technical flow cytometric, as well as biological, parameters. These parameters are the DNA index of aneuploid cells, the coefficient of variation (CV) of the G0/G1 phase, the ratio between diploid and aneuploid cells, the antigen intensity of immunophenotyped malignant plasma cells and the S phase of diploid cells. On the one hand, we can confirm the incidence of aneuploidies in multiple myelomas ( Nowak et al, 1998 ). On the other hand, we want to direct attention to the fact that we found only small aberrations (< 10%) in the DNA content in about 20% (16 out of 75) of stage II and III myelomas, resulting in near diploid DNA indices (recent unpublished data). Benson & Braylan (1994) reported for those near diploid DNA indices and a given CV of 3·0 that the relation of diploid to aneuploid cells has to be in a range between one and five (optimum 1) for reaching a clear separation of the two populations. But in leukapheresis samples and in bone marrow after autologous PBSCT, the percentage of diploid plasma cells is often more than 10-fold higher than that of the aneuploid cells, as described in the paper discussed here and in our data ( Nowak et al, 1999 ). Furthermore, the sensitivity level is influenced by the antigen intensity of the immunophenotyped plasma cells. Even in leukapheresis samples the DNA quantification of CD138-positive cells is more suitable than that of CD38 in our experience ( Fig 1). This could be explained by a better separation of CD138-positive and CD138-negative cells compared with those of CD38. In particular, leukapheresis samples often contain a lot of CD38dim-positive monocytes and activated T and B cells, possibly covering some of the signals of the aneuploid cell population. Finally, the S phase of diploid cells has a negative influence on the detectability of residual hyperdiploid cells ( Nowak et al, 1997 ).
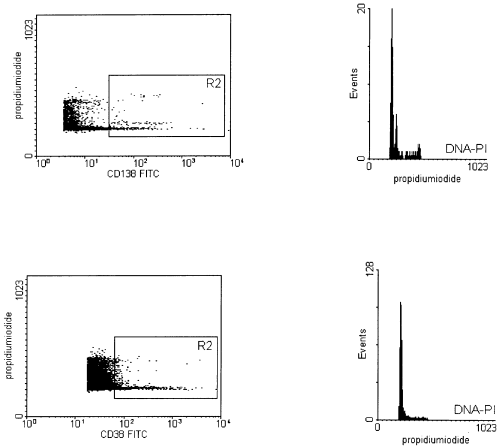
Simultaneous immunphenotyping and DNA quantification (left dot plots) in a leukapheresis sample of a patient with 0·06% aneuploid multiple myeloma cells (DNA index 1·17) with anti-CD138 (upper left) and CD38 (lower left). The CD138+ or the CD38++ cells, respectively, are gated in R2. Histograms show the DNA content of cells from gate R2. In both measurements, 100 000 cells were analysed. The CV of G0/G1 peak of all cells is 2·74% and 2·81% respectively. Histograms and dot plots are zoomed for better recognition. The axis for DNA content is depicted in linear scale.
Because of these reasons, the sensitivity for the detection of residual aneuploid cells is limited in a considerable part of multiple myelomas.
We described the sensitivity level as one aneuploid in 1 000–10 000 normal bone marrow cells ( Nowak et al, 1997 ) or in leukapheresis samples ( Nowak et al, 1998 , 1999). With these limitations taken into account, the method discussed here is suitable for the detection of residual malignant lymphatic cells in a considerable number of myeloma patients ( Nowak et al, 1998 ), as well as in leukaemia patients ( Nowak et al, 1994 ). It is a less expensive and less time-consuming method for the quantification of residual malignant cells than that of the clone-specific polymerase chain reaction, but with a two-decade lower sensitivity.




