Aspects of reproductive biology of Labeo horie Heckel (Pisces: Cyprinidae) in Lake Chamo, Ethiopia
Abstract
enLabeo horie Heckel is increasingly becoming commercially important in Lake Chamo but its reproductive biology in the lake is not well enough to guide its management. Sex ratio, breeding season, length at first maturity and fecundity of L. horie were studied from 1197 fish samples collected between August 1998 and October 1999 in Lake Chamo, Ethiopia. The sex ratio was significantly different (χ 2 = 12.12, P < 0.001). The peak-breeding period was during the rainy months of March to May, during which time more than 90% of both sexes had ripe gonads. The size at maturity ( L m50 ) of males was 52 cm while the L m50 of females was 62 cm. The smallest ripe male in the catch was 46.7 cm and weighed 890 g while the smallest ripe female caught was 49.5 cm and weighed 1145 g. The weight of ripe ovaries ranged from 54.3 g to 991.8 g and contained between 68,400 and 1,320,400 eggs. Relative fecundity ranged between 60 and 290 eggs per g of body weight. The relationships between fecundity and total length and between fecundity and total weight were curvilinear, while the relationship between fecundity and ovary weight was linear. L. horie conformed to the general pattern of reproduction in a tropical environment where peak-breeding activity occurred during the rainy season. In order to protect the spawning population, fishing pressure should be minimized during breeding time at the shallower littoral regions.
Résumé
frLabeo horie Heckel devient de plus en plus important au point de vue commercial dans le lac Chamo, mais la biologie de sa reproduction dans le lac n'est pas assez connue pour orienter sa gestion. On a étudié le sex-ratio, la saison de reproduction, la longueur lors du début de la maturité et de la fécondité de L. horie sur 1197 poissons récoltés entre août 1998 et octobre 1999, dans le lac Chamo, en Ethiopie. Le sex-ratio était relativement différent (χ 2 = 12,12 ; P <0,001). Le pic de la saison de reproduction se situait pendant les pluies de mars à mai ; à ce moment-là, les gonades de plus de 90% des individus des deux sexes étaient à maturité. La taille à la maturité ( L m50 ) était de 52 cm pour les mâles et de 62 cm pour les femelles. Le plus petit mâle mature collecté mesurait 46,7 cm et pesait 890 gr, tandis que la plus petite femelle mature récoltée mesurait 49,5 cm et pesait 1145 gr. Le poids des ovaires matures variait de 54,3 gr à 991,8 gr et ils contenaient de 68.400 à 1.320.400 œufs. La fécondité relative allait de 60 à 290 œufs par gramme de poids corporel. Les relations entre la fécondité et la longueur totale et entre la fécondité et le poids total étaient curvilignes alors que la relation entre la fécondité et le poids des ovaires était linéaire. L. horie se conforme au schéma général de la reproduction dans un environnement tropical, où le pic de l'activité de reproduction est atteint pendant la saison des pluies. Afin de protéger la population pendant le frai, il faudrait minimiser la pression de la pêche dans les parties les moins profondes du littoral pendant la période de reproduction.
Introduction
Labeo horie (Heckel) is a demersal freshwater fish widely distributed in the North-eastern region of Africa within the drainage basin of the Nile River, Lakes Albert, Kyoga, Turkana, Abaya and Chamo ( Reid, 1985 ). Understanding the reproductive biology of a fish population is essential because it provides basic parameters of stock assessment based on egg production methods ( Lasker, 1985 ). The rational utilization and protection of the early life stages can be planned if the time and place of spawning is known.
In tropical regions, variations in temperature and daylight length, which are two major reproductive cues in temperate regions, show little seasonal variation. Some studies conducted on the spawning periodicity of tropical fish populations indicate that breeding fish can be found at any time of the year, even though there could be a significant variation in the proportion of fish that spawn at different times (Hails & Abdullah, 1982; Tadesse, 1997; Dadebo, 2000). In many areas for which data are available, the majority of fish spawn at periods of high water level in the flood plain areas (Lowe-McConnell, 1975; Hails & Abdullah, 1982).
L. horie is a commercially important species in Ethiopia. In 1996 it contributed 1977 tonnes to the fisheries landings of Lake Chamo (Lake Fisheries Development Project (LFDP), 1996). Until the mid-1990s, L. horie was mainly used as feed for crocodiles in the crocodile farm located in the vicinity of the lake. After the Nile perch fishery collapsed due to over-fishing, the importance of L. horie as a human food resource has considerably increased. According to the report of the LFDP (1996 ), annual fish landings from Lake Chamo increased from 519 tonnes in 1994 to 3464 tonnes in 1996. Since the demand for fish has steadily increased, there is a possibility of increased fishing activity in the future.
Little work has been published on the reproductive biology of African Labeo species, even though the genus consists of some of the major food fish species in many parts of Africa. In Lake Victoria, L. victorianus commonly congregate in the shallow regions of the lake, and also in affluent rivers, where resident populations are greatly augmented by upstream migrations of breeding fishes during the rainy seasons (Whitehead, 1959; Cadwaladr, 1965). Spawning occurs in floodwater adjacent to rivers, and also in the flooded margins of small affluent streams (Fryer & Whitehead, 1959). Lowe-McConnell (1987) reported that L. mesops migrates up medium-sized rivers to spawn, but the non-migrating L. cylindricus spawns around rocks in Lake Malawi. In Lake Turkana, L. horie makes a spawning migration upstream to the Omo River and some ephemeral effluents when in spate (Lowe-McConnell, 1987). No published work is available on the reproductive biology of L. horie in Lake Chamo.
The aim of this work was to study some aspects of the reproductive biology of the fish such as sex ratio, time of spawning, size at first reproduction and fecundity, in order to provide basic knowledge for planning of the rational utilization of the species.
Materials and methods
Study site and areas
The Chamo-Abaya basin is part of a much larger drainage basin that includes Lake Turkana (Figs 1a, b). In the past, when the water level was much higher than at present, discontinuous river flows may have linked the Lakes Abaya, Chamo, Chew Bahir and Turkana to the White Nile (Grove, Street & Goudie, 1975).
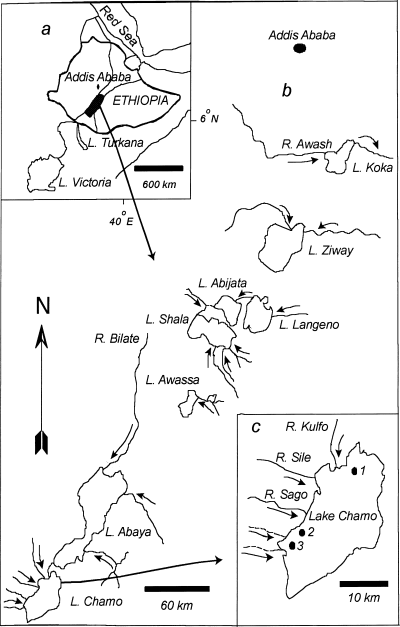
Map of eastern Africa with the relative locations of the Ethiopian rift valley lakes highlighted (a). (b) The Ethiopia rift valley lakes and their drainage pattern, and (c) Lake Chamo with the sampling stations indicated (1: Deset, 2: Bedena, 3: Bole)
Lake Chamo (5°42′−5°58′N; 37°27′−37°38′E) is the most southern rift valley lake of Ethiopia. It has an area of 551 km2 and a maximum depth of 16 m. It is located at an altitude of 1108 m and about 515 km south of the capital city Addis Ababa (Figs 1a, b). The ichthyofauna of Lake Chamo, and that of Lake Abaya, is Soudanian (Beadle, 1981). The fish species are more diverse than those in the other rift valley lakes of the country, possibly due to the free passage of the Soudanian species from the Nile system via the interconnections of Lakes Turkana and Chew Bahir (Beadle, 1981).
Kulfo River is the main affluent of the lake, which flows in at the north end. The less important feeders are the Sile and Sago Rivers from the west (Fig. 1c). There are more than 20 fish species in Lake Chamo and the inflowing rivers. The commercially important fish species are Oreochromis niloticus (L.), L. horie, Bagrus docmac (Forsskål) and Clarias gariepinus (Burchell). Capture of Lates niloticus (L.) has been banned since 1995 as a result of a sharp decline in its stock due to over-fishing.
Sampling
Monthly samples of L. horie were obtained between October 1998 and August 1999 using a graded fleet of gill nets of 100 mm, 120 mm, 140 mm and 160 mm mesh sizes. The nets were usually set during the afternoon at about 16:00 h and lifted the following morning at about 07:00 h. The total length of all fish caught was measured to the nearest millimetre, and their total weight was taken to the nearest 5 g. The sex and maturity stage of each fish was determined by a visual examination of the gonads and by use of a five-point maturity scale based on the method of Holden & Raitt (1974). According to this maturity scale, fish are categorized as: immature (I), recovering spent or developing virgin (II), ripening (III), ripe (IV) and spent (V). Ripe ovaries were weighed to the nearest 1 g and preserved in Gilson's fluid, as in Bagenal & Braum (1978).
Length at first maturity
Average length at first maturity was defined as the length at which 50% of the individuals of both sexes reach maturity (Willoughby & Tweddle, 1978). This was determined from the percentages of mature fish that were grouped in 5 cm length classes as described by the logistic function (Echeverria, 1987):
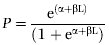 (1)
(1)where P= estimated proportion of mature fish, L= total length (centimetres), and α and β= coefficients. Equation 1 can be transformed into a logarithmic form as follows:
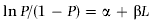 (2)
(2)The JMP program (Lehman & Sall, 1998) was used to calculate the observed mature proportion, its predicted probability and coefficients of the logistic equation. The value of Lm50 was estimated from the negative ratio (i.e. –α/β) by substituting P = 0.5 in Eqn 2.
Fecundity
Fecundity was estimated by weighing all the eggs in the ovaries and counting three subsamples of 1 g of eggs from different parts of the ovaries. Females in running condition were distinguished by slightly pressing their abdomens to check for the release of eggs. Fish found to be in running condition were excluded from the fecundity estimation. The average number of eggs per g of preserved wet weight was calculated and multiplied by the total weight of each ovary, giving the total number of eggs per ovary (Snyder, 1983). Relative fecundity was calculated by dividing the number of eggs per fish by its total body weight. The relationship between fecundity and some morphometric measurements were determined by relating total fecundity (F) data to total length (TL), total weight (TW) and ovary weight (OW) using the following formulae:
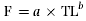 (3)
(3)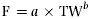 (4)
(4)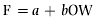 (5)
(5)where a and b are parameters of the fitted lines.
A χ2 test was used to determine if the sex ratios varied between different size classes (Frank & Althoen, 1994).
Results
Sex ratio
From a total of 1197 fish collected during the sampling period, 534 (44.6%) were males and 663 (55.4%) were females. Females were more numerous than males (χ2, P < 0.05) in the total catch as well as in all size classes above 75.0 cm TL (Table 1). Sex ratios were significantly different from the theoretical 1 : 1 in the 55.0–59.9 cm size class, and in all size classes above 70.0 cm TL. The only size class where the number of males was significantly higher (χ2, P < 0.05) was 70.0–74.9 cm TL. From the total of 112 individuals with TL > 75 cm only 24 (21.4%) were males and from 46 individuals caught with length >80 cm TL, only one was a male (Table 1). The smallest male captured was 43.5 cm in TL and 750 g in TW while the largest male was 81.3 cm in TL and 5350 g in TW. The smallest female was 41.9 cm in TL and 690 g in TW while the largest female was 87.5 cm in TL and 7900 g in TW.
| Size class (cm) | Males | Females | Sex-ratio (male : female) | χ2 |
|---|---|---|---|---|
| 40.0–44.9 | 5 | 3 | 1 : 0.60 | 0.50 |
| 45.0–49.9 | 36 | 23 | 1 : 0.64 | 2.86 |
| 50.0–54.9 | 136 | 157 | 1 : 1.15 | 1.51 |
| 55.0–59.9 | 88 | 157 | 1 : 1.78 | 19.43* |
| 60.0–64.9 | 94 | 129 | 1 : 1.37 | 2.82 |
| 65.0–69.9 | 76 | 77 | 1 : 1.01 | 0.007 |
| 70.0–74.9 | 75 | 29 | 1 : 0.39 | 20.35* |
| 75.0–79.9 | 23 | 43 | 1 : 1.87 | 6.06* |
| 80.0–84.9 | 1 | 38 | 1 : 38 | 35.10* |
| 85.0–89.9 | – | 7 | – | – |
| Total | 534 | 663 | 1 : 0.82 | 13.90* |
- * Significant difference ( P < 0.05).
Breeding season
The breeding season of L. horie was determined from the percentages of fish with ripe gonads taken monthly from August 1998 to October 1999. Although a few fish were found with ripe gonads throughout the year, the most intense breeding activity occurred during the time of peak rainfall, from February to May 1999 (Figs 2a, b). In February 1999, the fish started a spawning run 2 days after the first shower. Huge schools of fish were observed moving to the shallower areas of the lake into the dense macrophyte stands. During the rainy months, the mean monthly maximum air temperature decreased while the mean monthly minimum air temperature increased (Fig. 2a). The peak-breeding period was March to May, during which time more than 90% of both sexes had ripe gonads (Fig. 2b). The proportions of fish with ripe gonads were low during the months of August to November 1998 and June to October 1999 (Fig. 2b). No fish were found with ripe gonads in June. Spent fish were common in the catch during the months of June and July. In August and September 1998 there was an increase in breeding activity during the short rains. The same pattern of breeding activity was also observed during the same months in 1999 (Fig. 2b).
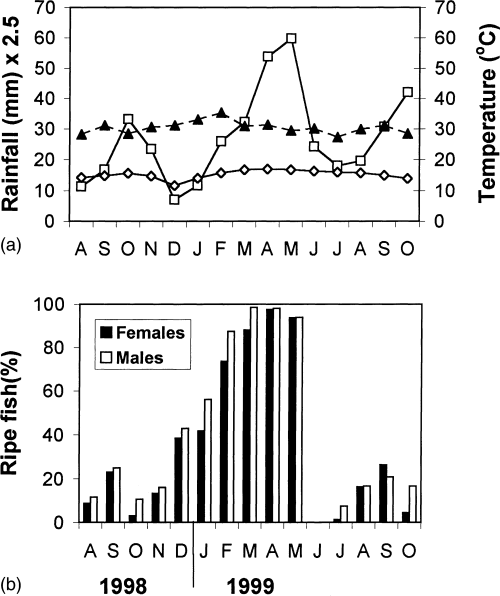
Rainfall (□), maximum (▴) and minimum (◊) air temperature of (a) Lake Chamo region, and (b) breeding season of L. horie as indicated by the percentages of fish with ripe gonads
Length at first maturity
The percentages of male and female L. horie having gonads stages three, four and five (Holden & Raitt, 1974) in different length groups, were plotted against length for each sex using data from the breeding season (February to May 1999). The average length at which 50% of the males attained maturity was 52 cm TL, while the length at which 50% of the females reached sexual maturity was 62 cm TL (Fig. 3). The smallest male found with ripe gonads was 46.7 cm and weighed 890 g while the smallest female found in breeding condition was 49.5 cm TL and weighed 1145 g.
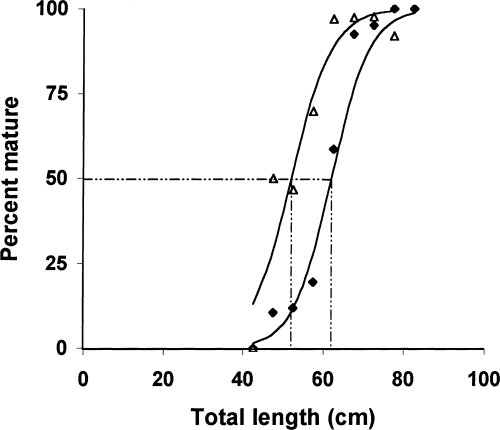
Length at first maturity of L. horie obtained by determining the average length at which 50% of the fish of both sexes reach maturity (▵, males; ◆ females)
Fecundity
Total fecundity (number of eggs per ovary) and relative fecundity (number of eggs per g of body weight) at different size classes of the fish are given in Table 2. The majority of the large fish were in running condition. From the 88 mature female L. horie (49.5–79.5 cm TL) that were used in the fecundity estimation, the mean total fecundity was 441,700 and ranged from 68,400 to 1320,400. In general, total fecundity increased with size, whereas relative fecundity seemed to be more constant above 60 cm TL (Table 2).
| Size class (cm) | Number of fish | Mean body weight (g) | Mean total fecundity | Mean relative fecundity |
|---|---|---|---|---|
| 50–54.9 | 4 | 1400 | 112,500 | 80 |
| 55–59.9 | 10 | 1985 | 228,800 | 115 |
| 60–64.9 | 22 | 2675 | 387,600 | 145 |
| 65–69.9 | 39 | 3215 | 462,200 | 144 |
| 70–74.9 | 11 | 3945 | 462,210 | 117 |
| 75–79.9 | 2 | 4175 | 707,700 | 167 |
The relationships between F and TL (Fig. 4a) and between F and TW (Fig. 4b) were curvilinear (F = 0.0003 × TL5.03, R2 = 0.71; F = 0.0001 × TW1.6, R2 = 0.79). The relationship between F and OW was linear (F = 0.121 × OW – 1.22, R2 = 0.86) (Fig. 4c). The ovaries from the smallest mature female weighed 54.3 g (4.7% of body weight), its total fecundity was 68,400 eggs and relative fecundity was 60 eggs per g of body weight. The highest total fecundity of 1320,400 eggs was in a 71.5 cm TL fish with a body weight of 4550 g. Its relative fecundity was 290 eggs per g of body weight.
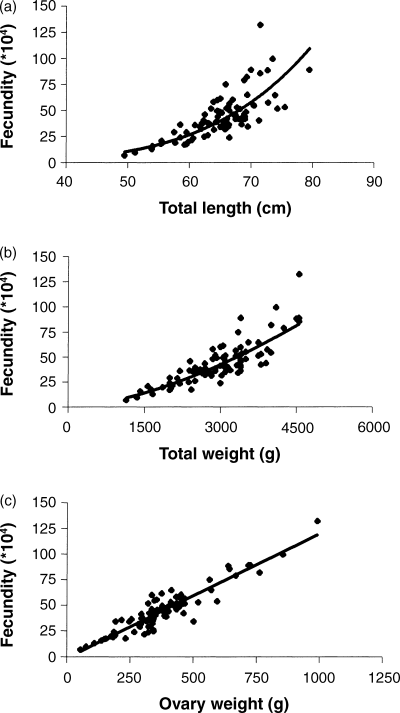
Relationship between (a) fecundity and total length, F = 0.0003 × TL 5.03 , R 2 = 0.71, n = 88, (b) fecundity and total weight, F = 0.0001 × TW 1.6 , R 2 = 0.79, n = 88, and (c) fecundity and ovary weight, F = 0.12 × OW – 1.22, R 2 = 0.86, n = 88, of L. horie from Lake Chamo
Discussion
During the breeding season L. horie exhibits a spawning run, where large numbers of fish migrate to the shallower areas of Lake Chamo. During this migration they occasionally jump out of the water in a coordinated manner making rhythmic splashes. The early rains in February probably trigger the breeding activity of this fish (Fig. 2). Kenimur (1971) observed spawning migrations of L. congoro into the inflowing streams of Lake Kariba. Hopson (1982) reported a similar spawning migration of L. horie from Lake Turkana into Omo River and some ephemeral streams. Whitehead (1959) indicated that L. victorianus enter inflowing rivers of Lake Victoria in fairly compact schools and run for 8–25 km upriver before moving laterally into flooded areas to spawn. In Lake Chamo spawning migrations of L. horie to the shallower areas of littoral regions were observed but migrations of fish upstream the inflowing rivers to any significant distance was not observed during the present study.
L. horie in Lake Chamo conforms to the pattern of breeding in tropical species ( Hails & Abdullah, 1982 ; Tadesse, 1997 ; Dadebo, 2000 ) with peak breeding occurring at the times of peak rainfall, although a few fish in breeding condition can be found at all times ( Fig. 2b ). The main pulse of breeding activity occurred during the months of February to May, coinciding with the major rainy season of the area. The second but less pronounced breeding activity occurred in August and September. It seems probable that fish which did not reproduce during the February to May breeding season, spawn during the August to September season. Since the breeding activity followed the rainfall pattern, the onset of reproductive activity may vary with rainfall pattern from season to season.
Temperature can also play an important role in governing the reproductive activity of fish (Billard & Breton, 1978). In Lake Chamo, during the rainy months, the maximum air temperature decreased but the minimum air temperature increased (Fig. 2a). Temperature regimes, i.e. cooler day temperatures and warmer night temperatures during the rainy season could possibly trigger reproductive activity.
In many areas of the tropics, rapid maturation of the gonads in many fish species starts during the early rains (Willoughby & Tweddle, 1978; Payne, 1986). This is often associated with a migratory phase in which the maturing fish enter the inflowing rivers prior to spawning in the flood plain pools. Migration is common among many non-cichlid fishes of Africa, particularly the characins and cyprinoids. It provides a mechanism for dispersion as well as for finding a favourable environment for the development of the eggs (Payne, 1986). After spawning and fertilization, the eggs are placed in some suitable flooded area with a rich food supply which the young can exploit. Migration to a stream also provides shelter from many kinds of predators for the newly hatched larvae (Jackson, 1961).
Knowledge of the size at first maturity of any fish stock is essential in order to determine the minimum size of capture by regulating the mesh size of the nets. The difference in lengths at 50% maturity (52 cm for males and 62 cm for females) of L. horie in Lake Chamo could be due to the different growth and mortality rates of the sexes. A common response to a change that increases the growth rate is a decrease at the age of maturity (Wootton, 1998). In most cases this may be accompanied by a change in the size at which the fish matures (Wootton, 1998). Differences in growth rates related to sex have been observed in a variety of fish species. In many cases, these differences are associated with sexual differences in the relative allocation of energy to the production of gametes (Weatherley & Gill, 1987). Mean gonado-somatic indexes (GSI), which are good estimators of energy allocation to the production of gametes, calculated for large numbers of ripe fish, indicate that females (GSI = 11.0%) allocate more energy than males (GSI = 3.7%) (unpublished data). In spite of this fact, females attain a larger size than the males. It is not clear whether the males expend more energy than females during courtship. Similar patterns of size difference for different sexes has been observed in L. niloticus (Hopson, 1972).
In the present study the fecundity of L. horie increased in proportion to the 5.03 power of TL and to the 1.6 power of TW (Figs 4a, b). This is high when compared with other species of Labeo in other tropical water bodies. Lowe-McConnell (1975) reported a b-value of 3.7 in the relationship between fecundity and TL of L. victorianus in Lake Victoria. In many fish species b usually is about 3 when fecundity is related to length and about 1 when related to weight (Bagenal & Braum, 1978). The high fecundity of L. horie in Lake Chamo could result from the high quality and quantity of food available there (Tadesse, Ahlgren & Boberg, 1998). L. horie is an herbivorous bottom feeding fish and its diet consists of algae, aufwuchs and detritus (Girgis, 1952; Reid, 1985). Teferra (1993), studying the food quality of an herbivorous cichlid (O. niloticus) in Lake Chamo, related the better condition of the fish to highly nutritious quality of phytoplankton that was dominated by diatoms.
In devising management strategies for this fish, protection of the spawning population should be seriously considered, because a huge amount of fish is being taken out from the shallower areas each year during their breeding period. Since spawning fish are vulnerable and easy to catch (Kenimur, 1971), fishing activity should be minimized or even banned in shallower areas of Lake Chamo during the breeding months of February to May. The greatest challenge to this management practice is the high demand for fish in the lent fasting season of the Ethiopian Coptic Orthodox Christians, which coincides with the breeding season. In order to exploit the stock on sustainable basis, protection of the breeding population is essential. Therefore fishing guidelines have to be developed concerning the time and place of fishing throughout the year. Moreover, since the females attain sexual maturity at a larger size than the males in Lake Chamo, the minimum capture size of the fish should be adjusted to that of the females. In some African water bodies, capture of Labeo species during their spawning runs has resulted in a sharp decline in stocks. de Kimpe, 1964) reported that the capture of L. altivelis populations on their spawning runs up the Luapula River from Lake Mueru (Zambia) resulted in a sharp decline of the stock.
Acknowledgements
We are grateful to Asfaw Asire, Mengistu Balcha, Tafere Gebre-Egziabher and Seblewongel Yohannes for their invaluable assistance in the field and laboratory work. We thank Gamo Gofa Zone Ministry of Agriculture Office and its staff members of the fisheries section for providing sampling equipment and for participating in sampling. The Awassa College of Agriculture (Debub University) and the Department of Limnology (Uppsala University) are acknowledged for providing laboratory facilities and the former for providing logistic support for the field trips. The study was financed by SAREC (Swedish Agency for Research Cooperation with Developing Countries).




