Design and characterization of a DNA-encoded, voltage-sensitive fluorescent protein
Abstract
Optical imaging of electrical activity has been suggested as a promising approach to investigate the multineuronal representation of information processing in brain tissue. While considerable progress has been made in the development of instrumentation suitable for high-speed imaging, intrinsic or extrinsic dye-mediated optical signals are often of limited use due to their slow response dynamics, low effective sensitivity, toxicity or undefined cellular origin. Protein-based and DNA-encoded voltage sensors could overcome these limitations. Here we report the design and generation of a voltage-sensitive fluorescent protein (VSFP) consisting of a voltage sensing domain of a potassium channel and a pair of cyan and yellow emitting mutants of green fluorescent protein (GFP). In response to a change in transmembrane voltage, the voltage sensor alters the amount of fluorescence resonance energy transfer (FRET) between the pair of GFP mutants. The optical signals respond in the millisecond time-scale of fast electrical signalling and are large enough to allow monitoring of voltage changes at the single cell level.
Introduction
Voltage-sensitive fluorescent dyes capable of monitoring fast electrical signalling of nerve cells have been known for many years (Salzberg et al., 1973; Grinvald et al., 1982). In principle, they provide a relatively noninvasive methodology for imaging of the activities of thousands of nerve cells simultaneously. Voltage-sensitive dyes allow for staining whole tissue via incubation with dye solution, or individual cells by dye injection via microelectrodes. With both staining protocols, only a minor fraction of the applied dye associates with the membranes of interest. Additional stained membranes give rise to an uncertainty of distinguishing which population of cells contributes to the observed signals and causes a poor signal-to-noise ratio of signals from individual cells. Therefore, it is highly desirable to find a way to stain specific cell populations with a voltage-sensitive fluorescent dye. With the advent of green fluorescent protein (GFP), construction of genetically encodable and therefore targetable fluorescent probes that permit the measurement of a range of physiological quantities became feasible. Existing concepts for the generation of such probes exploit the intrinsic dependency of GFP fluorescence on pH (Kneen et al., 1998) or halide ion concentration (Jayaraman et al., 2000) or are based on the fusion of a GFP-based reporter to a second protein that transduces environmental factors into conformational changes. The latter concept requires that the GFP-based domain reports conformational changes, a property that can be achieved by fluorescence resonance energy transfer (FRET) between pairs of GFP (Romoser et al., 1997; Miyawaki et al., 1999; Pollok & Heim, 1999) or by circular permuted GFP variants that are sensitive to conformational perturbations at their new fusion sites (Baird et al., 1999). We reasoned that a voltage-sensitive fluorescent protein could result from an appropriate fusion between a GFP-based fluorescent reporter with a voltage-sensing domain of a potassium channel. A related concept was recently implemented by the generation of the fusion protein FlaSh that monitors C-type inactivation of an insect potassium channel (Siegel & Isacoff, 1997). At least in our hands, the FlaSh protein did not give detectable voltage-dependent signals when expressed in mammalian cells (unpublished observations) and in Xenopus oocytes, the response time-constant to a change in membrane voltage was only in the order of 100 ms and hence too slow to faithfully report fast electrical signalling. Other approaches exploited FRET between two dyes, one of which is membrane-bound and a second that is redistributed in the stained membrane in a voltage-dependent manner (Cacciatore et al., 1999). Probes based on this concept are also too slow to report voltage changes in the millisecond time-scale (Cacciatore et al., 1999).
The fourth transmembrane helix (S4) of voltage-gated potassium channels contains an excess of cationic residues. A change in transmembrane voltage causes S4 to rotate, which results in a net displacement of positive charge along the direction of the electric field (Cha et al., 1999; Glauner et al., 1999; Sansom, 2000). We envisaged that this movement might be transduced into a change in FRET between a pair of GFP variants (Romoser et al., 1997; Pearce et al., 2000), taking advantage of the fact that the efficiency of FRET depends on the relative orientation of the dipoles of donor and acceptor chromophores and is, therefore, sensitive to rotational movements.
Methods
Gene construction
A series of cDNAs of cyan variant of GFP (CFP, Miyawaki et al., 1999) with variable linker sequences at its 3′ end was generated by the PCR using the following primers: sense primer containing a NotI site, 5′-CAA TCT TGC GGC CGC GGA GGT ACT GGA GGT TCA GGA GGT ATG GTG AGC AAG GGC-3′ or 5′-CAG TCC TGC GGC CGC ATG GTG AGC AAG GGC GAG GAG CTG-3′; antisense primer containing a BamHI site, TCA GGA TCC GAG AGT GAT CCC GGC GGC GGT CAC GAA CTC-3′, 5′-TCA GGA TCC GGA GCC GAG AGT GAT CCC GGC GGC GGT CAC GAA CTC-′, or 5′-TCA GGA TCC GCC GCC GGA GCC GAG AGT GAT CCC GGC GGC GGT CAC GAA CTC-3′. Similarly, a cDNA of yellow GFP mutant (YFP, Miyawaki, 1999) was amplified with a sense primer containing BamHI site, 5′-ATG AGC CCG GGA TCC ATG GTG AGC AAG GGC GAG GAG CTG TTC ACC-3′, and an antisense primer containing an EcoRI site, 5′-TCA GCG GCC GCA TTA GAA TTC CTT GTA CAG CTC GTC CAT GCC GAG AGT GAT CCC GGC-3′. Engineered restriction sites are in bold. The restricted products of cDNAs encoding CFP and YFP were ligated at the BamHI site resulting in six different fused CFP-YFP constructs flanked by NotI at the 5′- and EcoRI at the 3′-ends. These fragments were then inserted into the pGEM-T easy vector (Promega, Madison, WI, USA) for subsequent screening and expression of cyan-yellow fluorescent proteins (CYFPs) in Escherichia coli. The six different CYFP/pGEM-Teasy constructs were transformed into XL1-Blue competent cells (Stratagene, La Jolla, CA, USA) and plated on solid Luria-Bertani (LB) charcoal plates containing 20 mg/mL charcoal powder, 100 µg/mL ampicillin and 200 µm isopropylthio-beta-d-galactoside (IPTG). After incubation at 37 °C for 24 h, fluorescent colonies were selected.
Insertion of CYFP mutants
A cDNA encoding rat Kv2.1 potassium channel (Shi et al., 1994) was engineered by the overlapping extension method of PCR to introduce NotI and EcoRI sites at amino acid positions 316 and 320, respectively, with flanking sites XbaI and Acc65I. The following primer set was used: a sense primer with XbaI site, 5′-CGC TGG TCT AGA ATG ACG AAG CAT GGC TCG CGC TCC ACC AGC TCG-3′, and an antisense primer with NotI–EcoRI sites, 5′-CAG TTC GTT GTA GCT TCT GCG GAA TTC GGA GCG GCC GCA GGA CTG CAG GCC AGT GGA G-3′; and sense primer with NotI–EcoRI sites, 5′-CTC CAC TGG CCT GCA GTC CTG CGG CCG CTC CGA ATT CCG CAG AAG CTA CAA CGA ACT G-3′, and an antisense primer with Acc65I site, 5′-GTG GTA GGT ACC TCA GAT ACT CTG ATC CCT AGT GCT CCC GTG TGC-3′. Engineered restriction sites are in bold. The resulting product was ligated at the XbaI–Acc65I sites of a mammalian expression vector pcDNA3.1(–) (Invitrogen, San Diego, CA, USA) to generate the host mutant Kv2.1/pcDNA3.1(–) plasmid. The coding region of CYFP was then inserted at the NotI–EcoRI sites of Kv2.1/pcDNA3.1(–), resulting in two fusion proteins comprised of mutant Kv2.1 and CYFP variants: (i) full-length Kv2.1 with CYFP4 inserted at the in the S4–S5 link, and (ii) truncated Kv2.1 (Δamino acids 316–853), which lacks the downstream regions of the S4–S5 link of Kv2.1, with CYFP4 inserted at NotI–EcoRI sites of the host plasmid. All constructs were confirmed by sequence analysis.
Protein expression, purification and spectroscopic measurements
Escherichia coli strain XL-1 blue (Stratagene) transformed with CYFPs was grown in 100 mL LB cultures at 37 °C until OD600 0.6, induced for expression of CYFPs with the addition of 0.1 mm IPTG and incubated overnight at 37 °C. The recombinant CYFPs were subsequently extracted using the Bugbuster reagent (Novagen, Madison, WI, USA). The isolated protein was then concentrated by three-phase partitioning using t-butanol and 1.5 m ammonium sulphate, and purified by hydrophobic interaction chromatography with a macro-prep methyl support (Bio-Rad, Hercules, CA, USA) using 10 mm Tris (pH 8.0) for elution. Visually inspected fractions showing intense fluorescence were filtered at 6000 g using an ultrafree centrifugal filter with a 10-kDa cutoff membrane (Millipore, Bedford, MA, USA) and reconstituted in storage buffer with the following composition (mm): K-gluconate (115), HEPES (25), NaCl (10), pH 7.4. Purified CYFPs were spectroscopically characterized using a SPEX spectrofluorometer (Jobin Yvon, Edison, NJ, USA) at 25–28 °C. For acceptor bleaching experiments, 515 nm light from an argon laser (≈ 10 mW) was passed directly onto a cuvette containing 50 µL protein solution.
Expression in mammalian cells
VSFPs were transfected into human embryonic kidney 293 (HEK) cells using Fugene 6 (Boehringer Mannheim, Palo Alto, CA, USA) according to the manufacturer's instructions. HEK cell lines stably expressing prototypes of VSFPs were established by G418 selection (Gibco). HEK cells expressing VSFPs were grown on poly l-lysine-coated coverslips at 37 °C, and were then used for functional characterization at room temperature (25–28 °C).
Electrophysiology and fluorescence measurements
Coverslips carrying HEK cells were mounted on a Zeiss Axiovert microscope equipped with a photomultiplier (Hamamatsu H5784-01, Hamamatsu Photonics K.K., Shizuoka, Japan) and coupled by fibre optics to the SPEX spectrofluorometer. In some control measurements, cells were stained for 5–10 min with the voltage-sensitive dye di-4-ANNEPS (Fluhler et al., 1985; 0.5 µm, Molecular Probes, Eugene, OR, USA). Interference filters and dichroic mirrors were obtained from Omega Optical and Chroma Technologies (Brattleboro, VT, USA). HEK cell membrane potential was controlled using the whole-cell configuration of the patch clamp method (Axopatch 200B, Axon Instruments). The bath was perfused with a solution containing (in mm): NaCl 150, KCl 4, MgCl2 1, CaCl2 2, d-glucose (5) and HEPES (5) at pH 7.4. For high K+ solution NaCl was replaced by an equimolar amount of KCl. The pipette solution contained (in mm): K-aspartate 120, NaCl 4, MgCl2 4, CaCl2 1, EGTA 10, Na2ATP 3 and HEPES 5 at pH 7.2.
Results and discussion
In previous successful designs of engineered FRET- and GFP-based sensors, an environmentally sensitive protein domain was fused between a GFP mutant acting as donor and a second GFP mutant acting as the acceptor in such a way that a change in the conformation of the sensing domain directly modulated FRET efficiency (Romoser et al., 1997; Miyawaki et al., 1999; Pearce et al., 2000). Considering the requirement for membrane targeting and steric constraints, we did not investigate the possibility of fusing an S4-containing potassium channel motif between two GFP mutants and, in contrast, explored the possibility of modifying FRET efficiency of a fusion protein consisting of cyan and yellow variants of GFP (CFP and YFP) through a rotational disturbance at its N-terminal flank (Fig. 1). This scheme requires a linker between the two GFPs that allows changes in the relative orientation and/or distance between the two chromophores. We therefore excluded long floppy linkers that might facilitate dimerization and thereby induce a rigid distance and/or orientation between the GFPs. Furthermore, we reasoned that the rotation of S4 would more likely change the relative orientation rather than the distance between the CFP and YFP. Because FRET efficiency is most sensitive to orientation changes when the chromophore dipoles are at an angle of 45°, we selected a pair of GFP mutants that exhibited about half maximal FRET efficiency. Among a series of fusion proteins between cyan and yellow GFP involving different linkers, the construct CYFP4 with a single amino acid linker satisfied these conditions. Acceptor bleaching experiments confirmed FRET between CFP and YFP in CYFP (Fig. 2A).
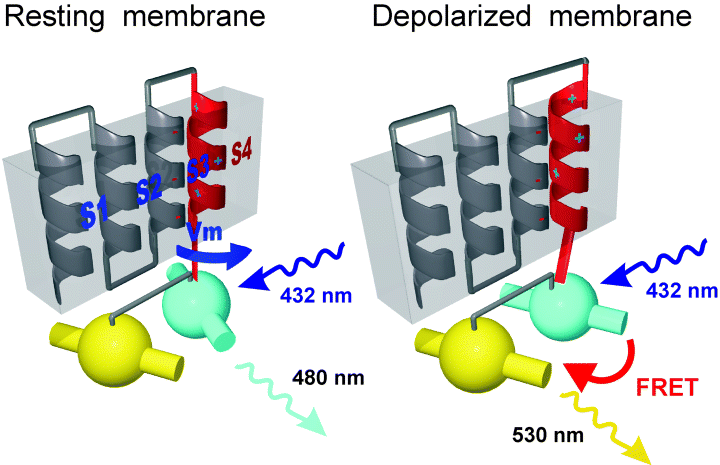
(A) Schematic representation of the conceptual design and motivation of the voltage-sensitive fluorescent protein. The four helices represent helices S1–S4 of a Kv potassium channel, and the balls with embedded cylinders represent cyan and yellow GFP variants and their optical dipole moments, respectively. Membrane depolarization results in a rotational movement of S4, which is transduced to a change in the orientation of the dipole moments. FRET occurs when the chromophore's dipole moments are parallel to each other, as indicated by an emission in the yellow spectrum at λ ≈ 530 nm upon excitation in the cyan spectrum at λ ≈ 432 nm, but not when they are perpendicular to each other.
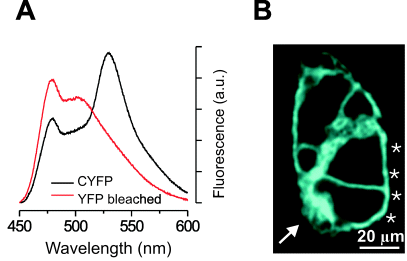
Spectroscopic characterization of CYFP4 and expression of VSFP1 in HEK cells. (A) Black trace, fluorescence emission spectra of CYFP4 with excitation at 420 nm. Note the two emission peaks at 480 and 530 nm for cyan and yellow fluorescence, respectively. Red trace, fluorescence emission spectra following photobleaching of YFP. Note increased cyan fluorescence demonstrating FRET in CYFP4. (B) Confocal image of a cluster of living HEK cells transfected with VSFP1 showing plasma membrane localization of VSFP1 fluorescence (excitation 442 nm; emission 465–495 nm). Asterisks indicate plasma membrane fluorescence and arrow indicates fluorescence associated with endoplasmic reticulum and the Golgi apparatus.
We inserted the CYFP4 coding sequence into the S4–S5 linker region of the potassium channel Kv2.1. The resulting fusion protein still exhibited fluorescence spectroscopic properties of CFP but lacked yellow fluorescence, suggesting that YFP failed to fold under the constraint imposed by the insertion (not shown). We then fused the CYFP4 construct C-terminal to the S4 domain of Kv2.1 (Fig. 1a), deleting the downstream sequences of Kv2.1. This prototypic fusion protein (voltage-sensitive fluorescent protein 1, VSFP1) was expressed and targeted to the plasma membrane in HEK cells (Fig. 2B, asterisks). Trafficking to the plasma membrane was only partial and additional fluorescence was associated with the endoplasmic reticulum and the Golgi apparatus (Fig. 2B, arrow). Spectroscopic measurements demonstrated CFP and YFP fluorescence, as well as FRET between the two chromophores (Fig. 3F).

Functional characterization of VSFP1. (A) Fluorometric and whole-cell recordings from a HEK cell stably expressing VSFP1. The cell was voltage-clamped and subjected to a command voltage time-course, as indicated by lower trace. Yellow fluorescence (upper trace, excitation 432 nm, emission > 530 nm) followed membrane voltage. (B) Fractional fluorescence response (ΔF/F) of VSFP1 as a function of imposed change in membrane voltage. Straight line represents linear regression (r = 0.99) with a slope of 1.8 ± 0.1% per 100 mV (n = 11 cells). (C and D) Optical recordings using the conventional, fast, voltage-sensitive dye di-4-ANNEPS (excitation at 530 nm, emission > 590 nm). Linear regression (r = 0.98) had a slope of −5.3 ± 0.3% per 100 mV (n = 10 cells). (E) Time-course of fluorescence change of VSFP1 (upper trace) upon step in command voltage (from −80 mV to +20 mV, lower trace) in comparison with response obtained with di-4-ANNEPS (middle trace). Red curves are exponential fits with time-constants 0.7 ms (VSFP1) and 1.2 ms (di-4-ANNEPS). (F) Emission spectra of HEK cells expressing VSFP1. Spectra were measured with excitation at 432 nm in control solution containing 5 mm K+ (blue line) and during depolarization of the cells by a solution containing 150 mm K+ (red line). The graph represents the average of five independent experiments and is scaled to the peak of the spectrum. Insets show increase of YFP emission and decrease in CFP emission during depolarization of the cells. Lower graph shows the difference between the control and high K+ spectra.
Modulation of fluorescence intensity by membrane potential was investigated in voltage-clamped HEK cells stably expressing VSFV1 (Fig. 3). Depolarizing voltage jumps resulted in an increase in the emission by YFP (> 530 nm) with excitation of CFP (432 nm), while hyperpolarization of the membrane resulted in a decrease in fluorescence output (Fig. 3A). The current-to-voltage relationship of HEK cells expressing VSFP1 did not differ from that of untransfected cells, demonstrating that VSFP1 did not form functional ion-conducting channels (not shown). The relationship between voltage change and fluorescence change was close to linear (r = 0.99) with a slope of 1.8 ± 0.1% per 100 mV (n = 11 cells, Fig. 3B). In parallel measurements using the prototypic conventional voltage-sensitive dye di-4-ANEPPS, we obtained a sensitivity of −5.3 ± 0.3% per 100 mV from clean HEK cell membranes (n = 10 cells, Fig. 3C and D).
VSFP1 responded rapidly to a change in command voltage with a response time-constant similar to that obtained with di-4-ANEPPS (Fig. 3E). The time-constants of fluorescence changes resulting from hyperpolarizing and depolarizing steps (± 100 mV) of command voltage were 0.74 ± 0.12 ms (n = 19) for VSFP1 and 0.97 ± 0.15 ms (n = 20) for di-4-ANEPPS. These response time-constants appeared to be limited by our ability to impose sudden voltage changes to these in single electrode voltage-clamped cells (Fluhler et al., 1985).
To demonstrate that the voltage-dependent modulation of YFP fluorescence (with CFP excitation) results from a change of FRET efficiency, we measured the fluorescence emission spectrum (excitation at 410 nm) as a function of membrane depolarization. In order to obtain enough fluorescence signals to be fed into a spectrometer, we sampled whole-field (60 × objective) fluorescence and depolarized the cells by switching the extracellular solution to a solution containing 150 mm K+. Based on control measurements with di-4-ANEPPS (using the above calibration of sensitivity) and application of the Nernst equation, we estimated that this high K+ solution induced a change in transmembrane voltage in the order of 100 mV. As expected for a FRET-based mechanism, membrane depolarization decreased cyan emission with an increase in yellow emission (Fig. 3F).
In HEK cells, the fractional fluorescence change of our VSFP1 is comparable with that of di-4-ANNEPS, one of the best organic voltage-sensitive dyes available, and reports similarly fast changes in transmembrane voltage. In whole tissue, such as acute brain slices or in vivo, signals obtained with di-4-ANNEPS are reduced by at least one order of magnitude (Iwasato et al., 2000). VSFPs are expected to provide signals not contaminated by fluorescence from glia cells or other electrically silent neurons. We therefore expect that in intact tissue, VSFPs targeted to specific cell populations will produce a signal-to-noise ratio superior to the signals obtained with conventional voltage-sensitive dyes.
In general, GFP-based probes of environmental factors suffer from the intrinsic dependence of GFP mutants on local pH and, in the case of some YFP variants, on halide ion concentration (Kneen et al., 1998; Jayaraman et al., 2000). Interpretation of signals obtained with fast VSFP has to carefully consider these dependencies. However, we expect that fast changes in transmembrane potential can be easily differentiated from the usually much slower changes in ion concentrations.
The relation of fractional fluorescence change of VSFP1 and change in transmembrane voltage was close to linear. This property might be unexpected considering the sigmoidal voltage dependency of native potassium channel activation. However, ion channel gating involves conformational changes of protein domains other than S4, probably involving mainly helices S5 and S6 (Perozo et al., 1999) and it is interesting to note that these rearrangements appear to determine the actual voltage dependency of channel gating. Application of the Boltzmann equation for a single charge indicates that only a small fraction (≈ 1%) of the membrane potential change is transformed into a change in FRET. Therefore, there is still much potential for improvement of the sensitivity of VSFP1. It should also be noted that our work was motivated by the principal concept illustrated in Fig. 1, however, we are aware of the fact that the actual mechanisms underlying the voltage sensitivity of VSFP1 is not as yet demonstrated. Considering the amount of work done towards understanding the detailed mechanisms underlying the function of naïve potassium channels, this task is far beyond the present work.
VSFP1 does not form channels as expected because of the lack of protein segments carboxy-terminal to S4. We cannot, however, exclude the possibility that VSFP1 interacts with endogenous potassium channel subunits. Such interactions would be undesirable but might be avoided by deletion of the tetramerization domain (Kreusch et al., 1998) and/or by fusion between several VSFPs and nonconducting subunits. In addition to such further improvements of the prototypic VSFP described here, the next step will be to place the construct under the transcriptional control of promoters defining specific populations of neurons in transgenic animals.
Acknowledgements
We thank J. Trimmer for providing cDNA encoding Kv2.1, M. Siegel for providing cDNA encoding FlaSh, A. Miyawaki for providing a cDNA encoding cyan and yellow GFP variants and Ms Ayako Takada for expert administrative and secretarial assistance.
Abbreviations
-
- CFP
-
- cyan fluorescent protein
-
- CYFP
-
- cyan-yellow fluorescent protein
-
- FRET
-
- fluorescence resonance energy transfer
-
- GFP
-
- green fluorescent protein
-
- HEK
-
- human embryonic kidney
-
- S4
-
- fourth transmembrane helix
-
- VSFP
-
- voltage-sensitive fluorescent protein
-
- YFP
-
- yellow fluorescent protein.




