Feeding suppression by fibroblast growth factor-1 is accompanied by selective induction of heat shock protein 27 in hypothalamic astrocytes
Abstract
It has been suggested that fibroblast growth factor (FGF)-1 serves as a physiological satiety factor in the hypothalamus, although the molecular mechanism underlying such a function is poorly understood. To gain additional insight into this issue, we used a Sendai virus (SeV) gene expression system in rats to explore genes differentially expressed subsequent to expression of FGF-1. Using cDNA arrays, we determined that infusion of FGF-1/SeV into one lateral ventricle induced selective expression of heat shock protein (HSP) 27 in the hypothalamus. Whereas FGF-1 expression was restricted to the ependymal cell layer of the cerebral ventricles, HSP27 was more widely expressed in astrocytes residing in the surrounding periventricular region. Similarly, infusion of FGF-1 polypeptide into a lateral ventricle induced dose-dependent HSP27 expression in periventricular astrocytes surrounding the third ventricle, with maximum mRNA levels being attained 6 h after infusion. This induction of HSP27 was accompanied by a significant suppression of feeding behaviour. Interestingly, suppression of feeding caused by intracerebro ventricular infusion of ciliary neurotrophic factor was also accompanied by induction of HSP27 in periventricular astrocytes, but suppression of feeding caused by infusion of leptin was not. It therefore appears that suppression of feeding by FGF-1 is accompanied by selective induction of HSP27 expression in hypothalamic astrocytes surrounding the third ventricle, and that this response may be a key component of the mechanism by which appetite is regulated by FGF-1.
Introduction
Fibroblast growth factors (FGFs) control the survival, differentiation and maintenance of neurons in the peripheral and central nervous systems (Walicke, 1988), and are thus widely distributed from early in development and throughout life (Ozawa et al., 1996). They are capable of inducing proliferation and migration of neural precursor cells, promoting neurite extension and promoting neuronal cell survival. Recent studies also suggest FGF-1 modulates neural function in the brain; for instance, it enhances long-term potentiation in the hippocampus and facilitates learning and memory (Terlau & Seifelt, 1990; Oomura et al., 1992; Sasaki et al., 1994b; Li et al., 1998a). In addition, it has been suggested that FGF-1 may act as an endogenous satiety factor: it is produced by ependymal cells located in the third ventricle (3V; Oomura et al., 1992); is released into the cerebrospinal fluid (CSF) after feeding or after intraperitoneal infusion of glucose (Hanai et al., 1989); and intracerebroventricular (ICV) infusion of FGF-1 dose-dependently inhibits food intake (De Saint Hilaire & Nicolaïdis, 1992; Oomura et al., 1992; Sasaki et al., 1994a). While such suppression of feeding by FGF-1 is apparently due to inhibition of the activity of glucose-sensitive neurons in the lateral hypothalamic area (Hanai et al., 1989), the underlying molecular mechanism for the feeding suppression is not well understood.
A novel viral vector has recently been developed based on Sendai virus (SeV), a member of the Paramyxoviridae family of viruses that has a strict cytoplasmic life cycle in mammalian cells. That the genomic RNA of SeV does not interact with the chromosomes of the host cells has enabled a variety of foreign genes with additional transcription units to be inserted at the 3′ end of the viral genome and to be expressed at extremely high levels. As such, SeV is able to effectively introduce genes into the ependymal cell layer of the ventricles via ICV infusion (Li et al., 2000). When SeV harbouring the mouse FGF-1 gene (FGF-1/SeV) was infused into the lateral ventricle (LV) of rodents, dramatic reductions in both feeding behaviour and bodyweight were observed, which reached a maximum 7 days after infusion (Hou et al., 2000).
With this as background, we attempted to identify genes involved in the suppression of feeding by FGF-1. Analysed were changes in gene expression in the hypothalamus of rats following ICV infusion of FGF-1/SeV.
Materials and methods
Animals
All animals received humane care in accordance with AIST guidelines; all animal protocols were approved by the AIST animal experimentation committee (Tsukuba, Japan). Male Wistar rats (Clea Co., Japan) weighing 250–300 g were maintained in individual cages and experienced a 12 h : 12 h light-dark cycle (lights on at 07.00 h) at a room temperature of 24–25 °C.
Sendai virus vector construction and surgical procedures
Recombinant SeV harbouring the mouse FGF-1 gene (FGF-1/SeV) or the green fluorescent protein (GFP) gene (GFP/SeV) were constructed and propagated, as described previously (Kato et al., 1996). Viral titres were determined using a standard chicken red blood cell haemagglutination assay. To administer the vector, the animals were anaesthetized by intraperitoneal (i.p.) injection of pentobarbital sodium (50 mg/kg), positioned in a stereotaxic frame, and unilaterally injected with 5 × 106 pfu of FGF-1/SeV or GFP/SeV into the right LV using a 33-gauge 10-µL Hamilton syringe. The injection coordinates were −1.0 mm posterior to bregma, 1.5 mm lateral to the midsagittal sinus and 3.5 mm ventral to the skull surface (Paxinos & Watson, 1986).
Profiling mRNA expression
Three days after SeV infusion, total hypothalamic RNA were prepared using Isogen solution (Nippon Gene, Tokyo, Japan) according to the manufacturer's protocol. Gene expression in the GFP/SeV- and FGF-1/SeV-infused hypothalami was then analysed using the Atlas Rat cDNA expression array (Clontech, Palo Alto, CA, USA) with materials provided in the kit according to the manufacturer's instructions. Briefly, 1 µg samples of mRNA purified using a PolyA tract mRNA isolation system IV (Promega, Madison, WI, USA) was converted into 32P-labelled first-strand cDNA using M-MLV reverse transcriptase. Unincorporated 32P-labelled nucleotides were removed by Chroma spin-200 (Clontech) column chromatography. The cDNA fractions with the highest activity were then pooled and hybridized to an Atlas membrane. After prehybridization for 30 min at 68 °C in Express Hyb (Clontech) supplemented with 200 µg/mL herring sperm DNA (Sigma, Deisenhofen, Germany), the heat-denatured probe was added. Hybridization was carried out overnight at 68 °C, after which the membranes were washed for 20 min in 2 × SSC containing 1% SDS at 68 °C, followed by two washes in 0.1 × SSC containing 0.5% SDS. The membranes were then exposed to X-ray film for 72 h at −70 °C.
Quantification of heat shock protein (HSP) 27 expression by Northern-blot analysis cRNA probe
Fragments of rat HSP27 were amplified by polymerase chain reaction (PCR) using the following primer set: sense, 5′-GTTAAGACCAAG GAAGGCGTGG-3′; antisense, 5′- CTACTTGGCTCCAGACTGT TCC-3′. The amplified fragments were inserted into PCR-Trap vector (GenHunter, Nashiville, TN, USA) and amplified by PCR using a pair of riboprobe primers (SP6 and T7) fused to the DNA sequences flanking the cloning site of the vector. The resultant product contained the SP6 and T7 promoters, which were then transcribed in vitro using appropriate RNA polymerase (Life Technologies, Rockville, MD, USA) in the presence of digoxygenin-UTP (Roche Diagnostics, Mannheim, Germany) and stored in 50% ethanol. Mouse FGF-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) riboprobes were prepared from the constructs based on pBluescript II SK+, which was previously constructed in our laboratory (Ozawa et al., 1996).
Northern hybridization analysis
Samples of total RNA (1 or 5 µg) were electrophoresed in a formaldehyde-agarose gel and blotted onto a nylon membrane (Hybond-N+, Amersham Pharmacia Biotech, Amersham, UK) with 20 × SSC, and then hybridized with digoxygenin-labelled cRNA probes using Easy hyb buffer (Roche Diagnostics) according to the manufacturer's instructions. The membranes were then washed twice with 2 × SSC containing 0.1% SDS at room temperature and twice with 0.1 × SSC containing 0.1% SDS at 68 °C. Bound riboprobe was detected with an antidigoxygenin-AP Fab fragment and CDP-Star (Roche Diagnostics). Thereafter, semiquantitative analysis of HSP27 was performed and corrected using GAPDH (a housekeeping gene normally present in constant amounts within samples) as an internal standard. HSP27/GAPDH mRNA ratios were calculated using image processing software running on a Macintosh computer (NIH Image, Dr Wayne Rasband, NIH, Bethesda, MD, USA).
Histochemistry
Under sodium pentobarbital anaesthesia (50 mg/kg, i.p.), blocks of brain were removed, fixed for 12–16 h at 4 °C in 4% paraformaldehyde in phosphate-buffered saline (PBS), and then treated in a graded sucrose series for 24 h. After embedding the fixed specimens in OCT compound, 16-µm coronal sections were cut, coated onto slides, dried (50 °C for 1 h), and stored at −40 °C until use.
For the purposes of in situ hybridization, slides were first soaked in 0.2 m HCl for 20 min at room temperature to inactivate endogenous alkaline-phosphatases, then treated with proteinase K (5 µg/mL) for 5 min at 37 °C, and dehydrated in an ethanol series. An excess of digoxygenin-labelled FGF-1 riboprobe (in 60 µL of hybridization buffer consisting of 300 mm NaCl, 30 mm sodium citrate at pH 7.0, 50% v/v formamide, 10% w/v dextran sulphate, and 1 µg/µL Escherichia coli tRNA) was applied to each slide, and hybridization was allowed to proceed for 20 h at 50 °C. After then washing the slides (in 300 mm NaCl, 30 mm sodium citrate and 50% formamide at 55 °C for 1 h), bound riboprobe was detected with an antidigoxygenin antibody conjugated with alkaline-phosphatase (1 : 1000, Roche). A colourimetric reaction was carried out with nitro-blue tetrazolium and 5-bromo-4-chloro-3-indolyl-1-phosphate (Promega) for 3–5 h at room temperature.
For immunohistochemistry, sections were first incubated for 30 min in PBS containing 0.3% hydrogen peroxide to eliminate endogenous peroxidase activity. The sections were then rinsed in PBS, incubated for 30 min in PBS containing 2% normal goat serum, and then rinsed for an additional 30 min in PBS containing rabbit polyclonal antibody against HSP27 (1 : 1000) (StressGen, Victoria, BC, Canada) plus 2% normal goat serum. The primary antibody was localized with the aid of an avidin-biotin system (Vector Laboratories, Burlingame, CA, USA). Bound peroxide was visualized by incubating 0.05% 3,3′-diaminobenzidine tetrahydrochloride with 0.005% hydrogen peroxide, resulting in a brown reaction product. For double immunostaining experiments, sections were blocked with 5% BSA in PBS and then incubated for 1 h with polyclonal antibody against HSP27 and monoclonal antibody against glial fibrillary acidic protein (GFAP) or microtubule-associated protein 2 (MAP2) (Neo Markers), followed by coincubation in a mixed solution of 1 : 500 Alex488-conjugated goat antimouse IgG (Molecular Probes, Eugene, OR, USA) and 1 : 200 Texas red-conjugated goat antirabbit IgG (Amersham).
Intracerebroventricular infusion of recombinant polypeptides
Under sodium pentobarbital anaesthesia (50 mg/kg, i.p.), a 23-gauge stainless-steel guide cannula (15 mm long) was inserted into the left LV (−0.6 mm posterior to bregma, 1.4 mm lateral to the midsagittal sinus and 3.8 mm ventral to the skull surface; Paxinos & Watson, 1986) and fixed to the skull with dental cement; 3 days later, cannula placement was functionally confirmed by demonstration of increased thirst after administration of angiotensin II (30 ng/rat, ICV).
Measurement of food intake was begun following a 1-week recovery from the implantation using procedures similar to those described by Li et al. (1998b). The rats were fed ad libitum except between 18.00 and 19.00 h daily, when food was removed, measured and then replaced. Food intake over selected time-spans was measured at 21.00, 01.00, 09.00 and 18.00 h (beginning 4 days before the ICV infusion), which were, respectively, 0–2, 3–6, 7–14 and 15–23 h after infusion. During dark phase measurements, a red incandescent bulb was used to provide dim illumination.
Recombinant human FGF-1 (R&D Systems, Minneapolis, MN, USA) dissolved in 0.15 m NaCl containing 200 µg/mL heparin was infused into each rat (5 µL/rat, ICV) over a 5-min period using a microsyringe. Some animals received 0.15 m NaCl containing 200 µg/mL heparin as a vehicle control. Recombinant rat leptin (1 µg/rat; Genzyme, Cambridge, MA, USA) or rat ciliary neurotrophic factor (CNTF; 250 ng/rat; R&D) in saline was infused, as described earlier. Saline served as vehicle control for leptin and CNTF. Each rat received a single infusion.
Statistics
All results are presented as means ± SEM. The data were analysed using one-way analysis of variance (anova), followed by multiple comparisons between individual groups using a post hoc Fisher's protected least significant difference test (PLSD). For experiments with small animal numbers (n = 3–5 per group), significant differences were determined using Mann–Whitney U-test. Values of P < 0.05 were considered significant.
Results
Gene expression profiling reveals selective induction of HSP27 in the hypothalamus by FGF-1/SeV
To analyse the mechanism by which FGF-1 suppresses feeding, FGF-1/SeV or GFP/SeV was infused into the LV of rats and the changes in hypothalamic gene expression were examined.
Initially determined were the regions transfected by these vectors. Using in situ hybridization with samples harvested on day 3 after the infusion of FGF-1/SeV, we found FGF-1 mRNA to be specifically expressed in the ependymal cell layers surrounding the 3V (Fig. 1A), contralateral LV (Fig. 1A), and ipsilateral LV (not shown). Control rats infused with GFP/SeV exhibited a similar distribution of GFP protein, as shown by its fluorescence (Fig. 1B). FGF-1 and GFP signals were not observed in other regions in the brain sections (coronal section from bregma −1.4 mm to −3.8 mm), including hypothalamus, hippocampus or cerebral cortex.
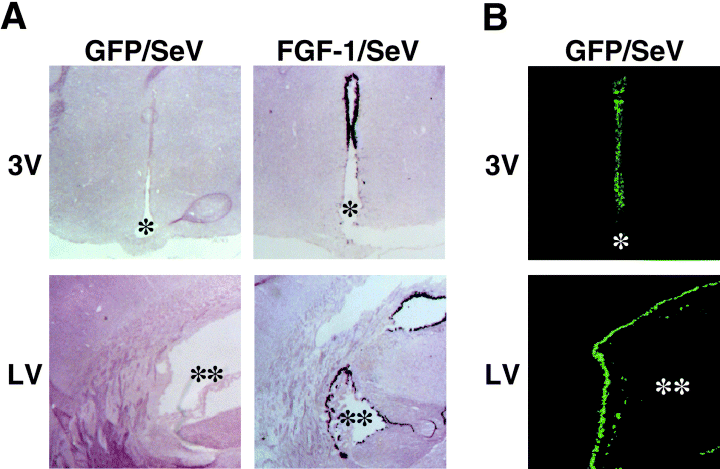
Distribution of transfected cells after FGF-1/SeV or GFP/SeV infusion. (A) Representative images of in situ hybridization to FGF-1 mRNA in GFP/SeV-infused (left) and FGF-1/SeV-infused (right) rats. (B) Representative fluorescence images in GFP/SeV-infused rats. Notably, the ependymal cell layer surrounding the third ventricle (3V) and contralateral lateral ventricle (LV) were positive. Sections were taken on day 3 after an infusion of the vector into one LV. * and ** indicate 3V and LV, respectively. Scale bar, 500 µm (A), and 250 µm (B).
The strong expression of FGF-1 in ependymal cells of FGF-1/SeV-infused rats was accompanied by marked suppression of feeding and loss of bodyweight, which is consistent with our earlier observation (Hou et al., 2000), and suggests that exogenous FGF-1 expressed in transfected ependymal cells acts as a satiety factor, perhaps utilizing the same mechanism employed by endogenously expressed FGF-1.
To examine the hypothalamic gene expression profiles in more detail, on day 3 after infusion of either FGF-1/SeV or GFP/SeV into the LV, total hypothalamic mRNA was extracted, reverse transcribed, and hybridized to cDNA array membranes. As shown in Fig. 2, the most prominent change was an increase in the expression of HSP27 mRNA in the hypothalami of FGF-1/SeV-infused rats (Fig. 2A, brackets 1). The induced expression of HSP27 mRNA was confirmed by Northern blot analysis, which showed that FGF-1/SeV infusion drastically increased HSP27 expression, as compared with GFP/SeV, and that the level of HSP27 expression paralleled the enhanced expression of FGF-1 (Fig. 2B). Expression of other HSP family members, including HSP60 and HSP70, was not detected on the DNA arrays (Fig. 2A, brackets 2 and 3, respectively). In addition, experiments using RT-PCR showed that expression of HSP60, HSP70 and HSP90 was not affected by the FGF-1/SeV infusion (results not shown). The results indicate that the observed change in HSP27 expression was not a response to the stress of infusion. Expression of the housekeeping enzyme GAPDH was also unaffected by the infusion (Fig. 2A, brackets 4).
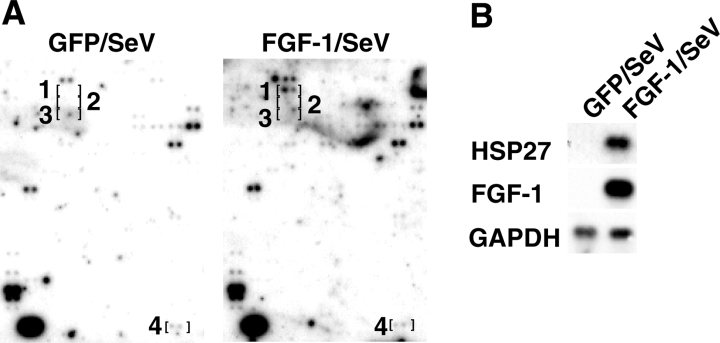
Induction of HSP27 mRNA in the hypothalami of FGF-1/SeV-infused rats. (A) Expression profiles of mRNAs from the hypothalami of rats infused with FGF-1/SeV or GFP/SeV. Corresponding regions of the two membranes are shown with brackets indicating the positions of pairs of spots representing individual genes. Brackets 1, 2, 3 and 4 denote HSP27, HSP60, HSP70 and GAPDH (a housekeeping gene), respectively. (B) Northern blot analysis of HSP27, mouse FGF-1 and GAPDH expression in the hypothalami in rats infused with FGF-1/SeV or GFP/SeV. One microgram of total RNA was applied to each lane.
HSP27 immunoreactivity was distributed around the 3V in FGF-1/SeV-infused rats
We next analysed the localization of HSP27 protein in coronal brain sections of rats infused with FGF-1/SeV or GFP/SeV. When control rats were infused with GFP/SeV, faint HSP27 immunoreactivity was observed in a thin zone surrounding the 3V (Fig. 3A). Rats infused with FGF-1/SeV, by contrast, exhibited strong HSP27 immunoreactivity, widely distributed in the parenchyma surrounding the ependymal cell layer of the 3V (Fig. 3A). Indeed, HSP27 signals were observed in all tested coronal sections from FGF-1/SeV-infused brains, encompassing a region from bregma −1.4 mm to −3.8 mm. More dispersed and weak HSP27 signals were also observed around the contralateral (Fig. 3A) and ipsilateral (not shown) LVs, but in this case, there was no difference in their intensity among GFP/SeV- and FGF-1/SeV-infused brains. No HSP27 signals were detected from other brain regions.
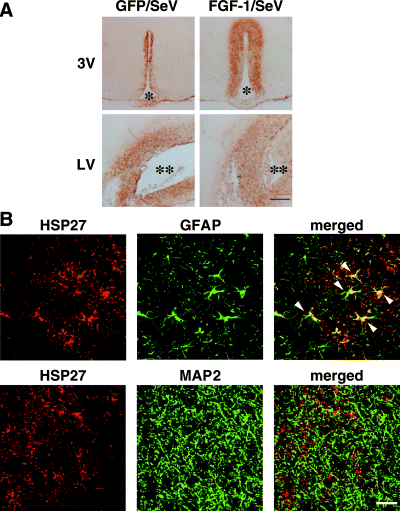
Induction of HSP27 protein within GFAP-positive cells in the hypothalami of rats infused with FGF-1/SeV. (A) Immunohistochemical staining of HSP27 surrounding the ventricles of rats infused with GFP/SeV or FGF-1/SeV. Increased expression of HSP27 protein was observed in the periventricular region of rats infused with FGF-1/SeV. (B) Double immunostaining of HSP27 (red) with GFAP (green) or MAP2 (green) in the periventricular region of FGF-1/SeV-infused rats. The merged images show that HSP27 immunoreactivity colocalizes with GFAP (arrowheads) but not with MAP2. * and ** denote the third ventricle (3V) and lateral ventricle (LV), respectively. Scale bar, 500 µm (A) and 25 µm (B).
In some experiments, brain sections were double immunostained for HSP27 and GFAP or MAP2, i.e. astrocyte and neuron markers, respectively. In large part, the HSP27 signals were localized to GFAP-positive cells that also showed hypertrophy and a stellate shape (Fig. 3B). Thus, the expression of HSP27 induced by the ependymal cell-derived FGF-1 appears to occur in astrocytes surrounding the 3V.
ICV infusion of recombinant FGF-1 polypeptide induces transient HSP27 expression
To confirm that FGF-1 in the CSF was responsible for the observed increases in HSP27 expression, we tested the capacity of recombinant FGF-1 polypeptide, infused into the LV, to induce HSP27 expression. Consistent with our previous study (Li et al., 1998b), significant feeding suppression was observed in the FGF-1-infused group (14.1 ± 1.4 g) compared with the saline-infused group (21.8 ± 0.8 g) on the infusion day (Fig. 4A), suggesting that the reduction of feeding was specific to ICV infusion of FGF-1 polypeptide, but not to infusion stress. Northern blot analysis revealed that infusion of FGF-1 elicited a clear, time-dependent increase in hypothalamic HSP27 expression: the expression was increased 6 and 14 h after FGF-1 infusion, and then gradually declined until, by 48 h, it was no longer apparent (Fig. 4B). The level of HSP27 expression 6 h after FGF-1 infusion was dose-dependent, with maximal responses being attained upon infusion of 200 ng of the protein (Fig. 5).
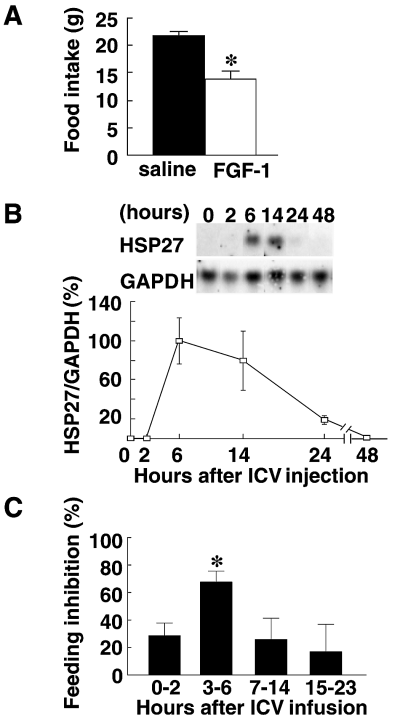
Transient induction of HSP27 mRNA expression and suppression of feeding behaviour by FGF-1 polypeptide. (A) The food intake during 24 h after intracerebroventricular (ICV) infusion of saline or 200 ng FGF-1. *P < 0.01 (vs. saline group); Mann–Whitney U-test; n = 5 for each group. (B) Time-course of HSP27/GAPDH mRNA expression after ICV infusion of FGF-1. Hypothalami were harvested at the indicated times after FGF-1 infusion (200 ng/rat, n = 3 at each time). Samples of total hypothalamic RNA (5 µg) were then assayed by Northern blotting. Typical blots of HSP27 and GAPDH, and HSP27/GAPDH mRNA expression ratios (calculated as percentages of the ratio obtained 6 h after infusion) are shown in the upper and lower panels, respectively. (C) Inhibition of feeding elicited by ICV infusion of 200 ng of FGF-1. Percentage changes were calculated based on the amounts eaten during the corresponding time-periods on the day before infusion. *P < 0.05 (vs. the other groups); one-way anova followed by post hoc Fisher's PLSD; n = 4–14 rats in each group.
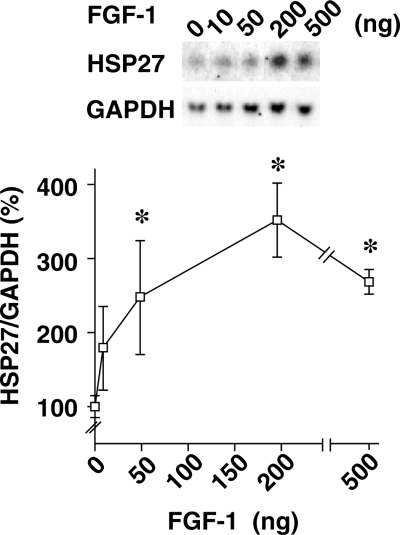
Dose-dependent induction of hypothalamic HSP27 mRNA expression by FGF-1. Hypothalami were harvested 6 h after intracerebroventricular infusion of the indicated amounts of FGF-1 (n = 3 rats for each dose). Samples of total hypothalamic RNA (5 µg) were then assayed by Northern blotting. Typical blots of HSP27 and GAPDH, and changes in the HSP27/GAPDH mRNA ratios (calculated as a percentage of the vehicle response) are shown in the upper and lower panels, respectively. *P < 0.05 (vs. saline group); Mann–Whitney U-test; n = 3 for each group.
Temporally correlated with FGF-1-induced induction of HSP27 was a decline in feeding behaviour (Fig. 4B and C). When feeding during selected periods after infusion of FGF-1 (200 ng/rat) was compared with that during the same period on the pre-infusion day, feeding behaviour was found to be inhibited 0–2, 3–6, 7–14 and 15–23 h after ICV FGF-1 infusion by 28.5% (n = 14), 67.7% (n = 11), 25.9% (n = 8) and 16.8% (n = 4), respectively, with the most significant decline occurring during the 3–6-h period (P < 0.02, Fig. 4C). Thus, induction of HSP27 expression and suppression of feeding behaviour showed similar temporal patterns.
Immunohistochemical analysis of the hypothalamus showed that HSP27 immunoreactivity in FGF-1-infused brains was distributed around the 3V (Fig. 6A). HSP27-positive regions extended 221 ± 38 µm from the wall of the ventricle in FGF-1-infused rats, which was significantly greater than that seen in rats receiving either saline or heparin (Table 1). Note that HSP27 expression associated with heparin infusion was not significantly different from that seen with saline infusion (P = 0.1; Table 1).
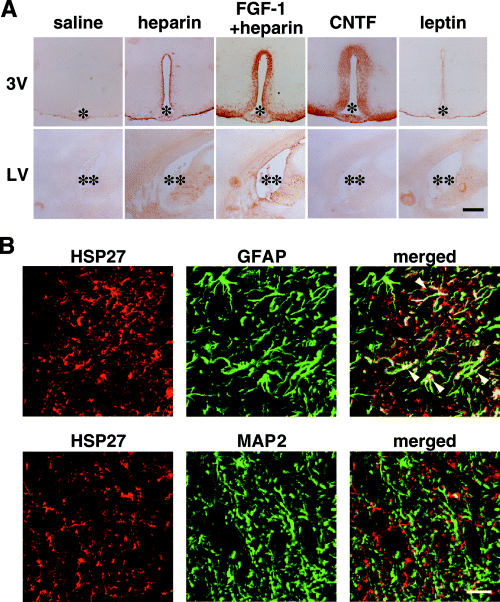
Distributions of HSP27 immunoreactivity in FGF-1-, ciliary neurotrophic factor (CNTF) -, or leptin-infused rats. (A) Representative HSP27 immunostaining of brain sections prepared 24 h after intracerebroventricular infusion of recombinant FGF-1 (200 ng/rat), CNTF (250 ng/rat), or leptin (1 µg/rat) into the lateral ventricle (LV). FGF-1 and CNTF induced strong HSP27 immunoreactivity in the periventricular area surrounding the third ventricle (3V), but not in the contralateral LV. (B) Double immunostaining of HSP27 (red) and GFAP (green) or MAP2 (green) within the periventricular region of 3V in FGF-1-infused rats. The merged images show that HSP27 immunoreactivity colocalized with GFAP (arrowheads) but not with MAP2. * and ** denote the 3V and LV, respectively. Scale bar, 500 µm (A) and 25 µm (B).
| Treatment | Third ventricle (µm) | Lateral ventricle (µm) |
|---|---|---|
| Saline | 0 | 0 |
| Heparin | 40 ± 9 | 0 |
| FGF-1 (200 ng) | 221 ± 38* † | 0 |
| CNTF (250 ng) | 293 ± 13* | 0 |
| Leptin (1 µg) | 9 ± 1 | 0 |
- Extension of HSP27 immunoreactivity from the wall of the third ventricle. Data represent means ± SEM. *P < 0.0001 (vs. saline), one-wayanova followed by post hoc Fisher's PLSD; †P < 0.0001 (vs. heparin), one-wayanova followed by post hoc Fisher's PLSD; n = 9 sections from three rats in each group.
No induction of HSP27 was seen in the area surrounding the contralateral LV in any experimental group (Fig. 6A). Double immunostaining following FGF-1 infusion confirmed the colocalization of HSP27 and GFAP signals (Fig. 6B) seen previously in FGF-1/SeV-infused rats (Fig. 3B). It is also noteworthy that HSP27 signals in both FGF-1 polypeptide- and FGF-1/SeV-infused rats were distributed not only within GFAP-positive cell bodies of astrocytes, but also in areas surrounding the MAP2-positive neurons 3, 6.
ICV infusion of recombinant CNTF, but not leptin, induces HSP27 expression
The results thus far suggest that suppression of feeding behaviour caused by FGF-1 is accompanied by the induction of HSP27 expression in periventricular astrocytes. To determine whether increased expression of HSP27 also accompanies responses to other known suppressers, we examined the effects of CNTF (Fantuzzi et al., 1995) and leptin (Zhang et al., 1994; Halaas et al., 1995). As reported previously, it was found that 24 h after ICV infusion of 200 ng of CNTF, the gain in bodyweight in CNTF-infused rats was significantly less than that in saline-infused, control rats (data not shown). Northern blot analysis of hypothalamus samples obtained 6 h after infusion showed that the level of HSP27 mRNA was augmented by infusion of CNTF (357 ± 104% compared with saline control; n = 3 in each case; P < 0.05; Fig. 7).
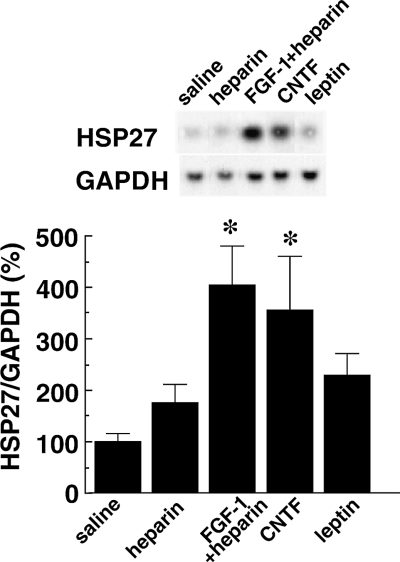
Induction of HSP27 mRNA expression by FGF-1 and ciliary neurotrophic factor (CNTF) polypeptides, but not by leptin. Hypothalami were harvested 6 h after intracerebroventricular infusion of FGF-1 (200 ng/rat), CNTF (250 ng/rat), or leptin (1 µg/rat), respectively (n = 3 for each group). Samples of total hypothalamic RNA (5 µg) were then assayed by Northern blotting. Typical blots of HSP27 and GAPDH and changes in the HSP27/GAPDH mRNA ratios (calculated as a percentage of the value in the saline group) are shown in the upper and lower panels, respectively. *P < 0.05 (vs. saline group); Mann–Whitney U-test; n = 3 for each group.
As with CNTF, rats infused with 1 µg of recombinant leptin gained significantly less bodyweight than rats infused with saline (data not shown). However, there was no significant increase in expression of HSP27 mRNA (230 ± 44% for leptin, 100 ± 15% for saline, P = 0.5; Fig. 7).
The HSP27-positive region extended 293 ± 13 µm from the wall of 3V in CNTF-infused rats, which was significantly greater than in either saline- or leptin-infused animals (Fig. 6A, Table 1). There was no significant difference between saline- and leptin-infused animals (P = 0.7).
Discussion
Previous studies have shown that FGF-1 usually resides in the ependymal cells facing the 3V; that it is released into the CSF after a meal or glucose infusion; and that it then diffuses through the ependymal cell layer into the parenchyma (Hanai et al., 1989; Oomura et al., 1992). In the present study, we have shown that infusion of FGF-1-expressing viral vector or FGF-1 polypeptide into the LV of rats causes selective induction of HSP27 expression in periventricular astrocytes surrounding the 3V. The induced expression of HSP27 was transient and coincided with suppression of feeding behaviour. Suppression of feeding and induction of HSP27 in periventricular astrocytes was also elicited by CNTF, another feeding suppresser polypeptide, strongly suggesting a common mechanism involving periventricular astrocytes and their expression of HSP27. To our knowledge, this is the first report suggesting the involvement of glial cells in polypeptide-mediated regulation of feeding behaviour.
The observed induction of HSP27 expression following infusion of either FGF-1/SeV or FGF-1 polypeptide likely mimics the response elicited by endogenous FGF-1. An earlier study showed that after eating or glucose infusion, endogenous FGF-1, expressed and stored in ependymal cells lining the 3V, was released into the CSF and diffused into the parenchyma of the ventricle (Hanai et al., 1989; Oomura et al., 1992). The physiological concentration of FGF-1 in the CSF after feeding was reported to be 7.5 nmol/mL, while that in starved rats was 0.7 pmol/mL (Hanai et al., 1989). In our study, an ICV infusion of 200 ng (12 pmol) of FGF-1 polypeptide yielded only 40 pmol/mL of FGF-1 in the CSF, assuming the total CSF volume in a 300-g rat was 300 µL. Thus, our experimental condition was within the physiological range, making the resultant induction of HSP27 a physiological likelihood.
HSP27 acts as a molecular chaperone (Jakob et al., 1993), modulating actin dynamics and therefore cell motility (Lavoie et al., 1993b; Benndorf et al., 1994; Lavoie et al., 1995; Mairesse et al., 1996). Along the same lines, HSP27 associates with GFAP and vimentin in an astrocytoma cell line, thereby regulating the intermediate filament network (Perng et al., 1999). In addition, it promotes cell survival by attenuating oxidative stress (Mehlen et al., 1996), resisting heat shock (Lavoie et al., 1993a), and resisting the toxic effects of cytokines (Mehlen et al., 1996). Induction of HSP27 expression in astrocytes has been reported during ischaemia, trauma and neural disease (Kato et al., 1995). However, the observed induction of HSP27 in periventricular astrocytes has not been described previously, although focal cerebral ischaemia induced similar selective increases in HSP27 expression in microglia and astrocytes (Kato et al., 1995). Expression of other members of the HSP family, i.e. HSP60, HSP70 and HSP90, were unaffected by infusion of FGF-1/SeV, indicating that the induction of HSP27 expression by FGF-1 and CNTF was a specific response to these polypeptides. In contrast, increased HSP27 expression was not observed upon ICV infusion of leptin, another well-characterized feeding suppresser. Leptin acts at several hypothalamic nuclei, including the arcuate and paraventricular nuclei, and regulates the expression and release of such neuropeptides as neuropeptide Y, corticotrophin-releasing hormone and alpha-melanocyte-stimulating hormone, which in turn suppress feeding behaviour. It, thus, appears that some feeding suppressers (e.g. FGF-1 and CNTF) regulate astrocyte function to suppress feeding behaviour, while others (e.g. leptin) do not utilize this mechanism.
It is unclear at present how HSP27 expressed in periventricular astrocytes modulates feeding behaviour, although we would expect it to act by modifying the activity of target neurons. Within the periventricular region of the 3V and its surroundings, many neurons secrete neuropeptides that regulate feeding behaviour (Kupfermann, 1991; Inui, 1999), and as with elsewhere in the CNS, HSP27 may be transferred from astrocytes to nearby neuronal axons (Tytell et al., 1986; Hightower & Guidon, 1989). For example, in response to hyperthermia, HSP27 is strongly induced in Bergmann glial cells and transported into radial fibres projecting to the synapse-enriched molecular layer of the cerebellum (Bechtold & Brown, 2000). One recent study also showed that exogenous HSP27 has the novel ability to induce IL-10 in human monocytes (Asit et al., 2000), making it possible that HSP27 released from the periventricular astrocytes acts directly on neighbouring neuropeptide-producing neurons to inhibit feeding. Our finding that HSP27 immunoreactivity surrounds MAP2-positive neurons in rat brains infused with FGF-1/SeV (Fig. 3) or FGF-1 polypeptide (Fig. 7) supports this possibility, as does the finding that FGF-1 inhibits the activity of glucose-sensitive neurons in the lateral hypothalamic area (Oomura et al., 1992).
Alternatively or in addition, astrocytes may directly suppress feeding by taking up neurotransmitters. Catecholaminergic nerve terminals have been shown to be present in the periventricular region, and the extracellular concentrations of norepinephrine, dopamine and serotonin are all known to increase in the hypothalamus during feeding and to contribute to the regulation of the behaviour (Hoebel et al., 1989). In that regard, an increasing body of evidence suggests that astrocytes regulate neural functions by removing neurotransmitters such as dopamine and norepinephrine from the synaptic cleft (Kimelberg et al., 1983; Semenoff & Kimelberg, 1985; Hirst et al., 1998; Inazu et al., 1999a). Indeed, FGF-2, which also inhibits feeding upon ICV infusion into rats (Sasaki et al., 1991), has been shown to regulate dopamine uptake in cultured rat astrocytes (Inazu et al., 1999b).
It is also possible that there is a temporal specificity to the pathway via which FGF-1 suppresses feeding behaviour. A previous study showed that an ICV FGF-1 infusion inhibited feeding in rats, and that this suppression occurred within 2 h of the infusion and was sustained for 24 h (Hanai et al., 1989). We observed induction of HSP27 expression in periventricular astrocytes 2–6 h after FGF-1 infusion. On the other hand, earlier studies showed that 18 h after — but not 5 h after — infusion of 125I-FGF-1 or 125I-FGF-2 into the LV of rats, neurons were labelled directly in the lateral hypothalamic area and elsewhere in the hypothalamus, including the paraventricular nucleus, ventromedial hypothalamus and arcuate nucleus (Ferguson & Johnson, 1991). Thus, we hypothesize that the feeding suppression observed following FGF-1 administration results from an integration of the effects of FGF-1: one induces an early response, which may be mediated by astrocytes in the periventricular zone (Suzuki et al., 2001), and another a late response in which FGF-1 affects lateral hypothalamus neurons. It is anticipated that future analyses of direct induction or inhibition of HSP27 expression in hypothalamic astrocytes will shed additional light on the molecular mechanism by which FGF-1 regulates feeding behaviour.
Acknowledgements
The work was supported partly by a Competitive Research Grant of AIST to T.I. A.-J. L. is a NEDO postdoctoral fellow. Dr William Goldman at MST Editing Co. edited the text.
Abbreviations
-
- BSA
-
- bovine serum albumin
-
- CNTF
-
- ciliary neurotrophic factor
-
- CSF
-
- cerebrospinal fluid
-
- FGF-1
-
- fibroblast growth factor-1
-
- FGF-1/SeV
-
- SeV harbouring the FGF-1 gene
-
- GAPDH
-
- glyceraldehyde-3-phosphate dehydrogenase
-
- GFAP
-
- glial fibrillary acidic protein
-
- GFP
-
- green fluorescent protein
-
- HSP
-
- heat shock protein
-
- HSP27
-
- heat shock protein 27
-
- ICV
-
- intracerebroventricular
-
- i.p.
-
- intraperitoneal
-
- LV
-
- lateral ventricle
-
- MAP2
-
- microtubule-associated protein 2
-
- PBS
-
- phosphate-buffered saline
-
- PCR
-
- polymerase chain reaction
-
- SeV
-
- Sendai virus
-
- 3V
-
- third ventricle.




