Dopamine and noradrenaline efflux in the rat prefrontal cortex after classical aversive conditioning to an auditory cue
Abstract
We used bilateral microdialysis in the medial prefrontal cortex (PFC) of awake, freely moving rats to study aversive conditioning to an auditory cue in the controlled environment of the Skinner box. The presentation of the explicit conditioned stimuli (CS), previously associated with foot shocks, caused increased dopamine (DA) and noradrenaline (NA) efflux. This conditioned response was dependent on the immediate pairing of the two stimuli; in the pseudoconditioned group that received an equal number of both stimuli, but in an unpaired fashion, no conditioned increases in efflux were observed.
Introduction
Experimental studies in rodents and nonhuman primates have shown that both dopamine (DA) and noradrenaline (NA) afferents to the prefrontal cortex (PFC) are involved in cognitive and emotional processes (Arnsten, 1997; Aston-Jones et al., 1999; Broersen et al., 2000). Cortical DA and NA release, measured as in vivo efflux, is activated after many different stimuli such as arousing, stressful and rewarding (Feenstra, 2000). This led to the hypothesis that cortical DA release indicates whether a stimulus is salient or behaviourally significant, regardless of its value (Imperato et al., 1991; Cenci et al., 1992 and whether attentional and preparative processes are being activated (Bertolucci-D'Angio et al., 1990). A similar reactivity for NA has been suggested by Aston-Jones et al. (1999).
Stimuli that normally do not affect DA and/or NA efflux may acquire significance after association with either rewarding/appetitive or stressful/aversive unconditioned stimuli (US) in a classical conditioning procedure. They become conditioned stimuli (CS), predicting the US. Aberrant responses to such stimuli may underlie psychiatric disorders such as post traumatic stress disorder, panic disorder and addictive states (Siegel, 1979; Kolb, 1987). NA and DA have been suggested to be involved strongly in these processes (Bremner et al., 1996; Berke & Hyman, 2000), and it could be hypothesised that they are important in a possible role of the PFC in the extinction of conditioned responses (Morgan et al., 1993; Morrow et al., 1999).
It is not clear, however, whether activation of DA and NA efflux by conditioned stimuli (if present) indeed indicates salience irrespective of appetitive and aversive aspects or whether fundamental differences exist between these latter two processes. Comparisons are seriously hampered by the different experimental procedures, including the type of CS used. Context (i.e. the box in which the aversive US was presented) has been widely used as CS in aversive conditioning following the report by Herman et al. (1982). Recently also explicit CS (a noise or a light signal) have been used (Goldstein et al., 1996). The few reports on in vivo efflux available up till now appear to suggest activation of cortical DA by context (Wêdzony et al., 1996; Yoshioka et al., 1996) and of NA but not DA by explicit cues (Wilkinson et al., 1998; McQuade & Stanford, 2000). Using strongly different procedures, Bassareo & Di Chiara (1997) and Ahn & Phillips (1999) showed that complex, multimodal CS associated with an appetitive US were capable of activating DA efflux. To allow better comparisons and more comprehensive studies, there is a clear need for methods to determine prefrontal NA and DA efflux in a similar way after aversive or appetitive conditioning. We adapted the experimental set-up as used by Young et al. (1993) and report on NA and DA release during aversive conditioning to an explicit stimulus.
Experimental methods
All experiments were approved by the Animal Experimentation Committee of the Royal Netherlands Academy of Arts and Sciences (NIH 97.08/00). Upon arrival, male Wistar rats (Harlan, The Netherlands) were put on a 12-h light : 12-h darkness cycle with dimmed red light from 07.00 h to 19.00 h and white light from 19.00 h to 07.00 h. The animals were housed in large macrolon cages (four rats to a cage) with sawdust bedding and food and water supplied ad libitum. After 3 weeks, when their weight was 250–300 g, the rats were equipped with two microdialysis cannulae placed bilaterally in the medial PFC (A + 3.0; L ± 1.8; V −5.5 from bregma; 12° angle) as described previously (Feenstra et al., 1998). Surgery was conducted under dimmed light conditions and the rats' eyes were covered. The animals were replaced in individual perspex cages (25 × 25 × 32 cm; free access to food and water). Two days later, they were placed in a Skinner box (MED Associates, Georgia, VT, USA) with a dimmed house light. The Skinner box was placed in an outer wooden box, with a hole for the fluid lines and a window for behavioural observations. The two PFC cannulae were continuously perfused in series and the dialysate was automatically introduced on-line into the HPLC every 16 min for measurement of DA and NA concentrations in a single HPLC run (Feenstra et al., 1998). After stabilisation of DA and NA efflux, an aversive conditioning session was started in a first group of rats (AVERSIVE); a 10-s white noise (25–30 dB as measured at 10–20 cm distance) (CS) immediately followed by a 0.3 mA foot shock (US) was repeatedly presented (9 ×, with a variable interval of 60–120 s). This was followed after 2–3 h by a test session with presentation of only CS (9 × 10 s white noise). Another group (PSEUDO) was exposed to pseudoconditioning; the white noise was followed by the foot shock only after a variable interval (30–60 s; CS + US). A third group (CONTROL) served as controls with only CS (white noise) presentations.
Cannula placements were evaluated in thionine-stained cryostat sections of the brains after death. All data presented were obtained in rats with correctly placed cannulae.
Extracellular concentrations were calculated in pg/50 µL. The mean of the four baseline samples immediately preceding the first experimental sample of either session was taken as control (100%) value. Relative data were used for presentations in the figures and for statistical analysis per session (repeated measures anova with factors group and sample, followed by Student–Newman–Keuls for group differences). For each group a repeated measures anova was followed by contrast analysis of individual samples with the fourth basal sample as reference.
Maximum relative effects were calculated as the maximal stimulus-induced value (in the first or second sample after the stimulus) compared to baseline controls. Maximum increases were compared for various conditions using anova and Student– Newman–Keuls.
Results
During stimulus presentations the behavioural responses of the animals in the Skinner boxes were observed. In the CONTROL and PSEUDO groups, presentation of the CS (10 s white noise) initially led to an increased locomotor activity. This response waned with progressive testing and freezing was not observed. In the AVERSIVE group the paired presentation of CS + US resulted in a stronger increase in locomotion than observed in the other two groups. After a few paired presentations the CS reliably elicited freezing behaviour. This was also the case during the CS alone sessions.
Basal levels of DA and NA were detected in all rats and were 1.12 ± 0.18 pg/50 µL and 5.42 ± 0.32 pg/50 µL, respectively, for the AVERSIVE group (n = 8), 1.05 ± 0.25 pg/50 µL and 3.63 ± 0.70 pg/50 µL for the PSEUDO group (n = 4) and 1.84 ± 0.46 pg/50 µL and 6.16 ± 0.74 pg/50 µL for the CONTROL group (n = 6). A significant difference was detected for NA concentrations between PSEUDO and CONTROL groups (Student–Newman–Keuls).
Presentation of the CS + US in the first session produced main effects for sample for DA (F11,165 = 16.20) and NA (F11,165 = 11.81), both P < 0.001, and for group for DA (F2,15 = 7.92, P < 0.001; however, NA F2,15 = 2.31, P = 0.099) as well as for group × sample interactions for DA (F22,165 = 5.24) and NA (F22,165 = 3.73), both P < 0.001. Post hoc Student–Newman–Keuls tests revealed that CS + US presentation produced increases in DA and NA extracellular concentrations in the AVERSIVE and PSEUDO groups compared with the CONTROL group for samples 5–7 (DA) and 5 and 6 (NA) (Fig. 1). The increases in the PSEUDO group were longer lasting than in the AVERSIVE group (samples 7 and 8 for DA and 7 for NA). Maximum increases were not different in these groups, but were significantly different from CONTROL; 204 ± 22% for DA and 151 ± 7% for NA in the AVERSIVE group and 248 ± 12% for DA and 169 ± 14% for NA in the PSEUDO group (Fig. 2).
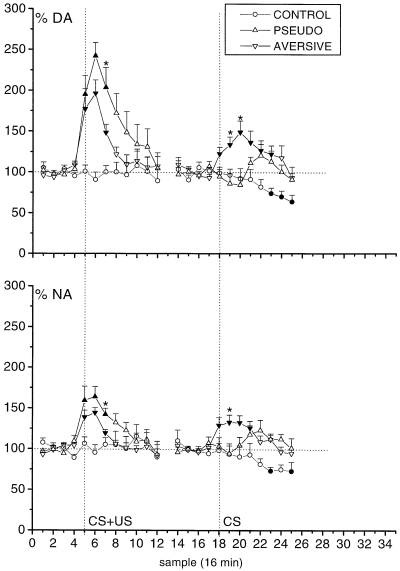
Time course of DA and NA efflux in the prefrontal cortex in the conditioning (AVERSIVE), pseudoconditioning (PSEUDO) and CONTROL groups. All data are mean ± SEM) values of 4–8 rats. The vertical dotted line indicates samples obtained during the CS + US and CS alone sessions. Filled symbols (▴,▾,●) indicate significant differences compared with baseline samples 4 or 17. *Significant differences between AVERSIVE and PSEUDO groups.
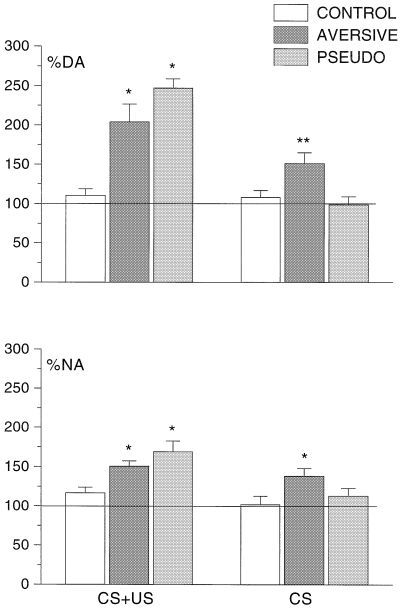
Maximal responses of DA and NA efflux in the prefrontal cortex in the CONTROL, conditioning (AVERSIVE) and pseudoconditoning (PSEUDO) groups upon presentation of CS + US or CS alone. All data are mean ± SEM) values of 4–8 rats. **Significantly different from both other groups in this session; *significantly different from CONTROL in this session.
Subsequent CS alone presentation in the second session again produced main effects for sample for DA (F11,165 = 2.66) and NA (F11,165 = 2.57), both P < 0.01, for group for DA (F2,15 = 6.31) and NA (F2,15 = 7.13), both P < 0.01, as well as for group–sample interactions for DA (F22,165 = 3.54) and NA (F22,165 = 2.88), both P < 0.001 (Fig. 1). Post hoc Student–Newman–Keuls tests revealed that CS alone presentation produced increases in DA and NA extracellular concentrations in the AVERSIVE group compared with the PSEUDO and CONTROL groups for samples 19 and 20 (DA) and 19 (NA). Maximum increases for the AVERSIVE group in the CS alone session were 152 ± 14% for DA and 139 ± 9% for NA. The DA effect was significantly different from both CONTROL and PSEUDO groups, the NA effect from the CONTROL group. (Fig. 2).
Discussion
The presentation of an explicit CS, previously associated with foot shocks, increased DA and NA efflux in the PFC. Our paradigm, with the rats continuously staying in the Skinner box and with white noise as the CS, allowed presentation of CS alone without effects on DA or NA efflux. The conditioned efflux was dependent on the immediate pairing of the two stimuli; unpaired presentation of an equal number of both stimuli did not lead to conditioned responses upon CS presentation, neither in behaviour, nor in DA/NA release.
In fear conditioning, re-exposure to a box where rats were exposed to foot shocks has been the classical procedure and has been shown to increase central NA turnover and metabolism (Tilson et al., 1975; Cassens et al., 1980). Subsequent studies in discrete brain regions suggested that DA metabolism was increased selectively in the PFC compared to other DA-innervated areas (Herman et al., 1982; Deutch et al., 1985), whereas NA metabolism was not activated in the PFC as it was in other areas (Dunn et al., 1986; Tsuda et al., 1986; Heinsbroek et al., 1990; Tanaka et al., 1990). More recent studies used a combination of contextual (a box) and explicit (a noise) stimuli, and showed that conditioned fear resulted in activation of both DA and NA metabolism in the PFC (Goldstein et al., 1996). In vivo studies apparently confirmed these findings re-exposure to the box increased prefrontal DA efflux (Wêdzony et al., 1996; Yoshioka et al., 1996), whereas exposure to an explicit CS (noise, light) previously associated with foot shock increased NA, but not DA efflux in the PFC (Wilkinson et al., 1998; McQuade & Stanford, 2000). Our present and previous results, however, do not confirm an apparent selective reactivity of NA and DA to explicit and contextual CS, respectively. Re-exposure to context associated with foot shocks activated NA but not DA efflux in the PFC (Feenstra et al., 1999). Now we present evidence for activation of both DA and NA afferents to the PFC upon presentation of an explicit cue associated with foot shocks. Apparently, both context and explicit CS are able to induce DA and NA efflux in the PFC, depending on the conditioning procedure.
Comparison to previously reported data is hampered by the large differences in experimental set-ups. We carried out our experiments in the active (dark) period of the day–night cycle. This is in contrast to other microdialysis studies (Wêdzony et al., 1996; Yoshioka et al., 1996; Wilkinson et al., 1998; McQuade & Stanford, 2000). Although we showed that the effects of US like novelty exposure are very similar in the dark and the light phase (Feenstra et al., 2000), it is not known whether the same applies to explicit stimuli.
A second experimental variable is handling of rats. The use of contextual stimuli almost inevitably leads to transfer between boxes, which may interfere with the assessment of conditioned neurochemical responses (Feenstra et al., 1999). The absence of a conditioned DA efflux, i.e. absence of a selective response to an explicit CS+ compared with CS–, in the study of Wilkinson et al. (1998) might be related to this.
Although comparison to other studies using aversive conditioning is difficult, a comparison to studies using appetitive conditioning is even more problematic. Bassareo & Di Chiara (1997) reported a conditioned increase in DA efflux using an experimental paradigm that was considerably different and included the introduction of a plastic box (CS) into the cage that predicted presentation of palatable food.
Therefore, we are presently using a paradigm for appetitive conditioning similar to the one described above for aversive conditioning, in order to identify the stimulus conditions that lead to enhanced efflux of cortical DA and/or NA (Mingote et al. in preparation).
Abbreviations
-
- anova
-
- analysis of variance
-
- CS
-
- conditioned stimuli
-
- DA
-
- dopamine
-
- HPLC
-
- high performance liquid chromatography
-
- NA
-
- noradrenaline
-
- PFC
-
- prefrontal cortex
-
- US
-
- unconditioned stimuli.




