Nonactivated microglia promote oligodendrocyte precursor survival and maturation through the transcription factor NF-κB
Abstract
We demonstrate a role for nonactivated rat microglia in the survival and maturation of oligodendrocyte precursor cells (OPCs). Media conditioned by nonactivated microglia increase the number of surviving galactocerebroside+ (GalC+) oligodendrocytes in vitro at 48 h by inhibiting the apoptosis of OPCs and stimulating their maturation to GalC+ oligodendrocytes. These effects are not observed with medium conditioned by microglia activated with interferon-γ (IFN-γ). Conditioned medium from nonactivated microglia is associated with upregulation in OPCs of nuclear factor of kappa binding (NF-κB) p65 subunit. The use of antisense to the inhibitor of kappa binding (IκB) induces p65 subunit activation in OPCs and, in common with medium conditioned by nonactivated microglia, also inhibits OPC apoptosis and promotes cell maturation. Anti-platelet-derived growth factor (PDGF) antibody abolishes this effect even though PDGF-A chain is expressed at similar levels within both nonactivated and IFN-γ-activated microglia and both conditioned media have similar levels of PDGF-A chain bioactivity. However, only conditioned medium from nonactivated microglia recruit phosphatidyl-3-inositol (PI-3) kinase to the PDGF-α receptor and synergise with endogenous PDGF-A chain to increase NF-κB activation. These results suggest that, dependent on their state of activation, microglia produce soluble factors that promote oligodendrocyte development through an effect on the PDGF-α receptor-signalling pathway.
Introduction
Survival and differentiation of the oligodendrocyte lineage are dependent on paracrine signals provided through the release of soluble survival factors (Barres et al., 1992; Richardson et al., 1996). Following receptor ligation, these initiate signal transduction events that lead ultimately to the activation of multiple transcription factors (Hill & Treisman, 1995). The downstream consequences of individual transcription factors including nuclear factor of kappa binding (NF-κB) (Sen & Baltimore, 1986) determine cellular fate. Translocation of NF-κB, especially the p65 subunit, has been linked to cell survival and maturation (Baldwin, 1996). Phosphatidyl-3-inositol (PI-3) kinase is an enzyme also involved in the survival cascade mediating effects of oligodendrocyte growth factors (– & McMorris, 1996), which can in turn activate NF-κB (Romashkov & Makarov, 1999).
Astrocytes are an important source of growth factors for oligodendrocytes, secreting platelet-derived growth factor (PDGF; Noble et al., 1988; Richardson et al., 1988), fibroblast growth factor-2 (FGF-2) (McKinnon et al., 1990), insulin-like growth factor-1 (IGF-1) (Barres et al., 1992) and neurotrophin-3 (NT-3; Barres et al., 1993). Neurons also influence oligodendrocyte proliferation, survival and differentiation by the release of these and other factors such as glial growth factor (Canoll et al., 1996). Interest in the involvement of microglia in CNS pathology (reviewed by Kreutzberg, 1996), together with evidence for oligodendrocyte toxicity in vitro through a contact dependent mechanism (Zajicek et al., 1992; Merrill et al., 1993) has tended to displace ideas on the role they may play during oligodendrocyte precursor cell (OPC) development through the release of soluble factors. Yet microglia are known to synthesise multiple cytokines (Giulian et al., 1988; Frei et al., 1989; Ganter et al., 1992), and nerve growth factor (Mallat et al., 1989; Elkabes et al., 1996), NT-3 (Elkabes et al., 1996), FGF-2 (Presta et al., 1995) and the IGFs (Breese et al., 1996) that could potentially influence the development of macroglia. Here, we describe contrasting effects of nonactivated and interferon-γ (IFN-γ) -activated microglia on survival and differentiation of the oligodendrocyte lineage.
Materials and methods
Cell culture
Mixed glial preparations were prepared as described previously (McCarthy & DeVellis, 1980; Zajicek et al., 1992). Cultures were incubated in Dulbecco's Modified Eagle's Medium (DMEM, Gibco, UK) + 10% foetal calf serum (Gibco, UK) + 1% penicillin and streptomycin (Gibco, UK) (DFP) at 37 °C in 7% CO2 and the medium was changed after 24 h.
Microglia were prepared from 6-day mixed glial cultures after shaking for 2 h at 200 r.p.m. After centrifugation, the cell pellet was resuspended in DFP, counted and plated onto sterile glass coverslips or plastic for 15 min. The nonadherent cells were then removed by washing twice in HBSS. The remaining microglia which were > 96% CD11b positive (Ox42, Serotec, UK) were cultured in N2B3 medium ± 100 units/mL IFN-γ (Serotec, UK) for 20 h to produce resting or activated conditioned media, respectively. N2B3 consisted of DMEM ± 0.5% foetal calf serum, 1 mm glutamine, 50 U/mL penicillin and streptomycin and 1 mL Sato mix per 100 mL basal medium leading to a final concentration of 100 µg/mL bovine serum albumin, 60 ng/mL progesterone water soluble, 16 µg/mL putrescine, 40 ng/mL sodium selenite, 40 ng/mL thyroxine, 40 ng/mL triiodothyronine, 5 µg/mL insulin and 10 µg/mL transferrin (all Sigma, UK). Conditioned media were collected from microglial cultures, filtered (0.2 µm) and incubated with oligodendrocytes at a 1 : 2 dilution unless otherwise stated or tested using the L929 bioassay for tumour necrosis factor (TNF)α as described in Zajicek et al. (1992).
Oligodendrocytes were obtained by shaking 7-day mixed glial cultures that had previously had the microglia removed as above, at 160–180 r.p.m. for 12–16 h. The cells were resuspended in N2B3 and contaminating microglia removed by differential adhesion to an 80 cm2 flask (37 °C for 30 min). The nonadherent oligodendrocytes were resuspended in fresh N2B3 before plating onto sterile coverslips coated in 0.01% w/v poly lysine (Sigma) and incubated at 37 °C in 7% CO2. These mixed oligodendrocyte lineage cultures containing 30% A2B5+ (Eisenbarth et al., 1979), 60% GalC+ (Ranscht et al., 1982), 8% Ox42+ and 2% glial fibrillary acidic protein+ (GFAP+, Dako, UK) cells immediately after plating were incubated in conditioned media for 48 h. Where appropriate, mixed oligodendrocyte lineage cultures were incubated either with microglial conditioned medium ± neutralising growth factor antibodies specific for human PDGF, human FGF-2 (R & D Systems Europe Ltd, UK), human IGF-1 (Upstate Biotechnology, USA) and rat ciliary neurotrophic factor (CNTF; R & D Systems) that all showed species crossreactivity, or antisense TGGCTGAAACATGGC and mis-sense oligonucleotides TAGTTGGAACACGGC (PE Applied Biosystems, UK) to mouse inhibitor of kappa binding (IκB; Tewari et al., 1992; Barger et al., 1995). To control for the effect of IFN-γ, oligodendrocytes were separately cultured with unconditioned medium or IFN-γ alone. DNA incorporation and cell viability within these cultures were quantified using immunocytochemistry to identify cell type in conjunction with bromodeoxyuridine (BrdU) incorporation, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) or TUNEL assays.
MTT, BrdU and TUNEL assays
Oligodendrocyte and microglial cultures were incubated with 0.5 mg/mL MTT (Sigma) for 1 h at 37 °C. The cells were then blocked with 10% rabbit serum prior to incubation. Oligodendrocytes were incubated with 1 : 2 dilutions of hybridoma supernatants to A2B5 or GalC followed by 1 : 100 dilutions of goat anti-mouse IgG3-TRITC (Harlan Sera-Lab, UK) and/or goat anti-mouse IgM-FITC (Harlan Sera-Lab). Microglia were stained with 10 µg/mL mouse anti-CD11b (Ox42, Serotec) followed by 10 µg/mL anti-mouse IgG-TRITC (Sigma). All cells were then fixed in 2% paraformaldehyde. Contaminating astrocytes within oligodendrocyte cultures were identified after permeabilization with 0.1% Triton X-100 in DFP at 4 °C for 1 h. The cultures were then incubated with 10 µg/mL rabbit anti-GFAP followed by 10 µg/mL anti-rabbit IgG-TRITC (Sigma). The coverslips were washed and mounted in Vectashield H-1000 (Vector Laboratories Inc., USA) and viewed at 200 × magnification. For the BrdU assay, oligodendrocyte coverslips were incubated with 1 µg/mL BrdU (Gratzner, 1982) for a 6-h pulse at 12 or 24 h after plating. Coverslips were fixed in 2% paraformaldehyde before adding ice-cold methanol followed by 2 m HCl (Fisons Scientific Equipment Ltd, UK). After washing in 0.1 m borate buffer and DFP, the cells were incubated with a 1 : 2 dilution of A2B5 hybridoma supernatant followed by mouse anti-BrdU-FITC (Boehringer Mannheim, Germany) and 10 µg/mL anti-mouse IgM-TRITC (Harlan Sera-Lab) for 1 h at 4 °C. A fluorescein-based TUNEL assay kit (Boehringer Mannheim) was used, in accordance with manufacturer's instructions, in association with morphologically intact A2B5+ cells to identify apoptotic OPCs. All coverslips were mounted prior to counting the number of MTT+ GalC+, A2B5+, GFAP+ or Ox42+ cells. Results are based on a minimum of three separate experiments. Cells were counted in a preset pattern from each coverslip. Statistical analysis was carried out using Students t-test on the total population sampled per coverslip.
[3H]Thymidine incorporation
Incorporation of DNA in L929 cells was measured by adding 0.8 µCi of [3H]thymidine (10 Ci/mmol) to monolayers for the last 4 h in culture. The cells were trypsinized and harvested onto glass-fibre filter paper (Printed Filtermat A, Wallac, UK); scintillant was added and thymidine incorporation measured in a β-counter (1205 Betaplate, Wallac, UK).
Analysis of NFκB and signal transducer and activator of transcription (STAT) transcription factors
Nuclear extracts were prepared and incubated with 32P-γATP radiolabelled double-stranded oligonucleotides containing consensus binding sequences for STATs or NF-κB as described in Girdlestone & Wing (1996). The reaction was performed on a nondenaturing 6% acrylamide gel in 0.5 x TRIS-borate : EDTA, which was then dried and autoradiographed. Optical density was calculated using NIH image software (Washington, USA) and statistical analysis carried out using the paired t-test. Identity of the transcription factor was confirmed by the inhibition of band formation with affinity purified rabbit antiserum to STAT1, 2, 3, NF-κB p50 and p65 (Santa Cruz, USA). Intracellular staining for NF-κB was carried out after fixing and subsequent cell permeabilization using 0.1% Triton X-100 in DMEM + 10%FCS at 4 °C for 1 h. After three washes, the cells were incubated at 4 °C with 10 µg/mL affinity-purified rabbit anti-NF-κB p50 subunit, anti-NF-κB p65 subunit or an irrelevant rabbit control for 1 h. The cells were washed, incubated with anti-rabbit IgG-FITC (Sigma) for 1 h at 4 °C, mounted and viewed at 1000 × magnification under oil.
RNA preparation and reverse transcriptase-polymerase chain reaction
Cytoplasmic RNA was extracted according to the methods of Sambrook et al. (1989). RNA (1 µg) was heated to 65 °C for 15 min and made up to 9.5 µL (or 10.5 µL where no reverse transcriptase used) with water, 4.0 µL 5 x first strand buffer, 2 µL 0.1 m DTT, 30 U RNAsin, 2.5 µL 10 mm dNTP, 1 µL 50 µm oligo d(T)16 primer and 1 µL M-MLV reverse transcriptase (200 U/µL) (all Boehringer Mannheim, Germany). The mixture was made up to 100 µL after incubation at 37 °C for 60 min and then 95 °C for 3 min. Fivefold dilutions of reverse transcriptase (RT) product were amplified with primers for PDGF-A chain (GAGATAGACTCCGTAGGGAGTGAG, GGACAGCTTCCTCGATGCTTCTCT) and glyceraldehyde-3-phosphate dehydrogenase (CAAAGTTGTCATGGATGACC, CCATGGAGAAGGCTGGGG) according to the methods of Funa et al. (1996) and analysed on a 2% agarose gel stained with ethidium bromide and photographed to confirm the expected sizes.
Immunoprecipitation and quantification of PDGF-α receptor tyrosine phosphorylation
Oligodendrocytes were solubilized in 50 mmβ-glycerophosphate pH 7.3, 2 mm EDTA pH 8.0, 1 mm EGTA pH 8.0, 100 mm NaCl, 1% Triton X-100, 5 mm 5-mercaptoethanol, 0.2 mm NaVO4, 1 mm benzamidine, 0.1 mm PMSF, 20 µg/mL leupeptin, 1 µm pepstatin A, 1 µm aprotinin and 0.05 µm okadaic acid before preclearing with protein G (all Sigma, UK). After centrifugation 1 µg/mL anti-PDGF-α receptor (R & D, UK) and protein G were added. The immunoprecipitated antigen was washed and run in duplicate on a 10% reduced SDS/PAGE gel before electroblotting (PVDF) in Tawbin buffer pH 8.4. Nonspecific protein binding was blocked in 1% gelatine in PBS, before dividing the blot and probing with either 2 µg/mL anti-PDGF-α receptor, anti-phosphotyrosine or anti-PI-3 kinase (crossreactive to p85 and p110 subunits) (both Santa Cruz, USA). The blot was then probed sequentially with anti-rabbit/mouse IgG biotin and developed using immunoperoxidase (Vectastain ABC kit, UK) according to the manufacturers instructions. The optical density of the immunoprecipitated PDGF-α receptor and its tyrosine phosphorylation were analysed from six experiments.
Results
Medium conditioned by nonactivated microglia promotes GalC+ oligodendrocyte survival
Mixed oligodendrocyte lineage cultures plated at the same density were incubated for 48 h in 1 : 2 dilution of serum and insulin-free conditioned media. A total of 25% of GalC+ oligodendrocytes plated in unconditioned medium ± IFN-γ were alive at 48 h (Fig. 1A, column 1). Incubation for 48 h with medium conditioned by nonactivated microglia significantly increased the percentage of surviving GalC+ oligodendrocytes compared to unconditioned medium (Fig. 1A, column 2). The survival effect of medium conditioned by nonactivated microglia on GalC+ oligodendrocytes was first evident after 48 h. The effect on GalC+ oligodendrocyte survival was no longer observed at a dilution of 1 : 8 (data not shown). There was no increase in the survival of GalC+ oligodendrocytes using medium conditioned by IFN-γ-treated microglia (Fig. 1A, column 3). The effect was cell specific: medium conditioned by IFN-γ-treated but not nonactivated microglia increased astrocyte survival by stimulating proliferation (data not shown). The secretion of TNF-α secretion rose from 1.14 ± 0.45 in nonactivated to 3.46 ± 1.46 U/mL in IFN-γ-treated microglial conditioned medium (six experiments, mean ± SEM, P < 0.02) confirming microglial activation.
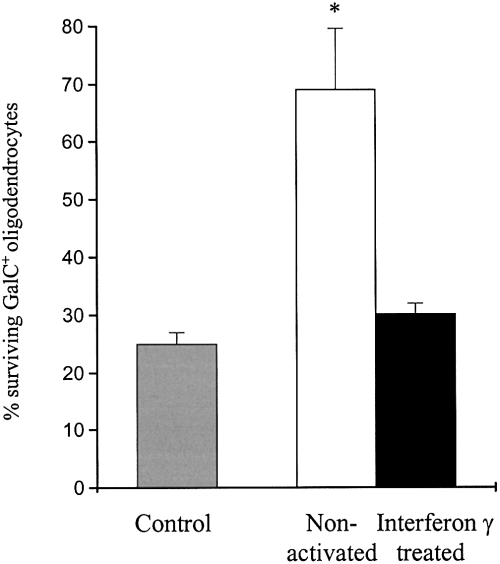
Serum and insulin-free media conditioned by nonactivated microglia reduce GalC+ oligodendrocyte loss at 48 h compared with unconditioned media and media conditioned by IFN-γ-treated microglia (*P < 0.02, four experiments, mean ± SEM).
Medium conditioned by nonactivated microglia reduces OPC apoptosis and enhances maturation
The low number of surviving OPCs (three cells per field of view) after 48 h in serum and insulin-free media did not provide an adequate baseline for assessing the effect of conditioned media and the number of OPCs was therefore increased using insulin and serum-containing medium. Under these conditions, medium conditioned by nonactivated microglia reduced OPC apoptosis (Fig. 2A). There was no difference in the percentage of GalC+ oligodendrocytes undergoing apoptosis in mixed oligodendroglial cultures treated with medium conditioned by nonactivated or IFN-γ-treated microglia, or in cultures containing < 2% OPCs and an equal number of mature (i.e. GalC+) oligodendrocytes (data not shown). The incorporation of BrdU in OPCs was also not significantly different in the presence of either conditioned medium.
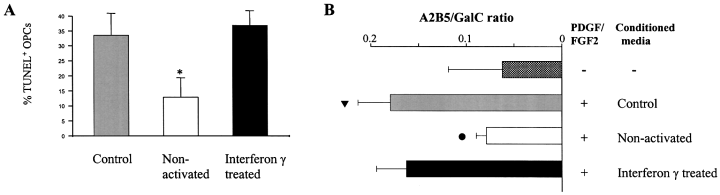
In the presence of serum and insulin, the percentage of TUNEL+ OPCs is reduced in the presence of medium conditioned by nonactivated microglia compared to unconditioned media and medium conditioned by IFN-γ-treated microglia after 48 h in culture (A; *P < 0.01, 4 experiments, mean ± SEM, % of ∼ 130 OPCs per coverslip). The addition of PDGF-AA and FGF-2 (both at 10 ng/mL) increases the A2B5 : GalC ratio at 24 h delaying the maturation of OPCs cultured in insulin and serum-free medium (B; rows 1 and 2. tP < 0.0002, three experiments, mean ± SEM). In the presence of these growth factors, media conditioned by nonactivated microglia but not IFN-γ-treated microglia reduce the A2B5 : GalC ratio after 24 h in culture producing a shift towards more mature oligodendrocytes (B; rows 3 and 4, lP < 0.0005).
Because medium conditioned by nonactivated but not IFN-γ-treated microglia reduced OPC survival at 48 h, and culture in insulin and serum-free conditions yielded low numbers of OPCs, the interaction of conditioned media and the growth factors PDGF-AA and FGF-2 (both 10 ng/mL) was arrested at 24 h when cell numbers were equal. At this timepoint, PDGF-AA and FGF-2 act to increase OPC survival and partially inhibit maturation, increasing the ratio of A2B5 : GalC from ∼ 0.05 to 0.155 (Fig. 2B, rows 1 and 2). By contrast, the addition of medium conditioned by nonactivated microglia but not IFN-γ-treated microglia (Fig. 2B, rows 3 and 4) significantly reduced the A2B5 : GalC ratio despite the continued presence of PDGF-AA and FGF-2. The increase in surviving GalC+ oligodendrocytes was accompanied by a reduction in the number of OPCs but with no overall change in cell number. These results indicate that enhanced OPC maturation contributes to the increase in survival of GalC+ oligodendrocytes. Together, reduction in OPC death and the promotion of maturation in the presence of medium conditioned by nonactivated microglia account for the survival effect shown in Fig. 1.
NF-κΒ expression is induced in OPCs by medium conditioned by nonactivated microglia
Nuclear extracts were obtained from purified mixed oligodendrocytes incubated with conditioned media for 2 h. A double-stranded NF-κB consensus sequence was added to reveal the presence of NF-κB.
NF-κB exponentially declined during culture of oligodendrocytes incubated for 48 h with serum and insulin-free medium (data not shown) but was significantly upregulated compared to unconditioned medium ± IFN-γ in oligodendrocytes exposed to medium conditioned by nonactivated microglia at both 2 (n = 8; P < 0.05 paired t-test) and 24 h (n = 5; P < 0.05 paired t-test) (Fig. 3A). The expression of NF-κB was reduced by medium conditioned with interferon γ treated but not nonactivated microglia (n = 12, P < 0.02 paired t-test). No changes in NF-κB expression were seen at 48 h in GalC+ cultures containing < 2% OPCs after a 2-h exposure to conditioned media. The upregulation of transcription factors was specific for NF-κB because there was no effect on IFN-γ-induced STAT1 binding to a STAT consensus sequence (data not shown). The oligodendrocyte NF-κB band was lost with anti-NF-κB p65 but not p50 (Fig. 3B). The identity of the translocating subunit was confirmed as NF-κB p65 translocated from the cytoplasm to the nucleus in a proportion of A2B5+ cells following incubation with medium conditioned by nonactivated microglia (Fig. 3C–F).
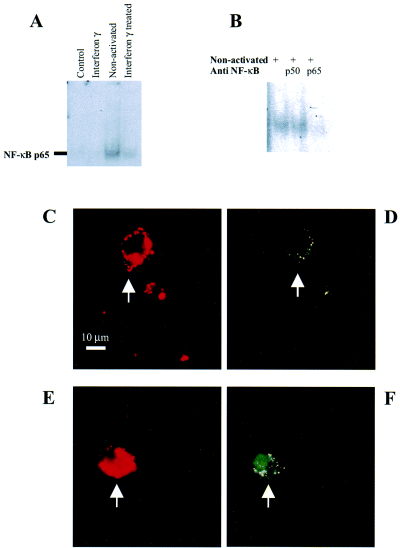
At 2 and 24 h (illustrated) exposure to medium conditioned by nonactivated microglia induces binding to an NF-κB consensus sequence in oligodendrocyte cultures (A; P < 0.02, 12 experiments, compared with control). NF-κB binding is prevented by anti-NF-κB p65 but not anti-NF-κB p50 (B). A 2-h exposure of A2B5+ oligodendrocyte precursors (C–E) to medium conditioned by nonactivated microglia stimulates the translocation of NF-κB p65 from the cytoplasm in unconditioned medium (D) to the nucleus in the presence of medium conditioned by nonactivated microglia (F).
Activating NF-κB using antisense to IκB promotes the survival of GalC+ oligodendrocytes by reducing OPC apoptosis and enhancing their maturation
To explore further the relationship between NF-κB expression and oligodendrocyte differentiation, NF-κB was upregulated by antisensing its natural inhibitor IκB. The NF-κB consensus sequence was added to nuclear extracts derived from oligodendrocytes cultured with IκB antisense or scrambled mis-sense oligonucleotides. This demonstrated a dose-dependent increase in the activation of NF-κB with antisense but not the scrambled mis-sense oligonucleotides (Fig. 4A). Antisense oligonucleotides at nontoxic concentrations increased the number of GalC+ oligodendrocytes compared with cultures containing mis-sense in insulin and serum-free medium (Fig. 4B).
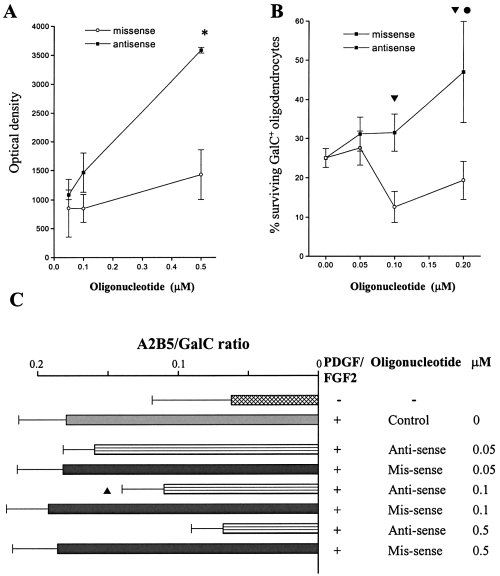
Oligodendrocytes were incubated with anti- or mis-sense oligonucleotides to IκB at concentrations between 0.05 and 0.5 µm. Antisense oligonucleotides induce NF-κB consensus sequence binding compared to scrambled mis-sense control oligonucleotides (A; *P < 0.001 at 0.5 µm antisense > mis-sense, four experiments, mean ± SEM). At concentrations between 0.05 and 0.2 µm antisense oligonucleotides produce a dose dependent reduction in GalC+ oligodendrocyte loss above that of scrambled mis-sense control at 48 h (B; 0.1–0.2 µmtP < 0.01, four experiments, mean ± SEM). Further GalC+ oligodendrocyte survival at antisense concentrations of 0.2 µm is elevated above that seen at concentrations of 0.05 µm (lP < 0.05). Antisense to IκB produces a dose-dependent shift in the A2B5 : GalC ratio towards GalC+ oligodendrocytes (C; sP < 3.3 × 10−6 at 0.1 µm, θP < 6 × 10−10 at 0.2 µm, three experiments, mean ± SEM).
Antisense to IκB reduced the percentage of apoptotic OPCs (20 ± 2%) compared to scrambled mis-sense controls (32 ± 3%) after 48 h in the presence of serum and insulin containing medium (P < 5.5 × 10−7, four experiments, mean ± SEM). Using PDGF and FGF-2 (both 10 ng/mL) to increase precursor cell number, the addition of anti- but not mis-sense oligonucleotides produced a dose-dependent (0–0.5 µm) reduction in the A2B5 : GalC ratio after 24 h (Fig. 4C). In turn, this was associated with an increase in NF-κB activation in a bandshift assay (data not shown). There was no difference in the percentage of GalC+ oligodendrocytes undergoing apoptosis with either antisense or mis-sense oligonucleotides in cultures containing < 2% OPCs with equal number of mature (i.e. GalC+) oligodendrocytes (data not shown).
PDGF antibody blocks the enhanced GalC+ oligodendrocyte survival induced by medium conditioned by nonactivated microglia
Neutralising antibodies to IGF-1, PDGF, FGF-2 and CNTF were added to oligodendrocyte cultures that had been supplemented with media conditioned by microglia. Antibodies to PDGF, but not IGF-1, FGF-2 or CNTF blocked the effect of medium conditioned by nonactivated microglia on GalC+ oligodendrocytes at concentrations of 1 µg/mL. Anti-PDGF antibody had no effect in the presence of medium conditioned by IFN-γ-treated microglia (Fig. 5A). The lack of any toxic effect of medium conditioned by IFN-γ-treated microglia with anti-PDGF antibody blockade implied that this conditioned medium did not adversely affect survival of GalC+ oligodendrocytes. Antibody blockade using up to 100 µg/mL IGF-1, 100 µg/mL FGF-2 or 10 µg/mL CNTF did not influence responses to medium conditioned by IFN-γ-treated microglia.
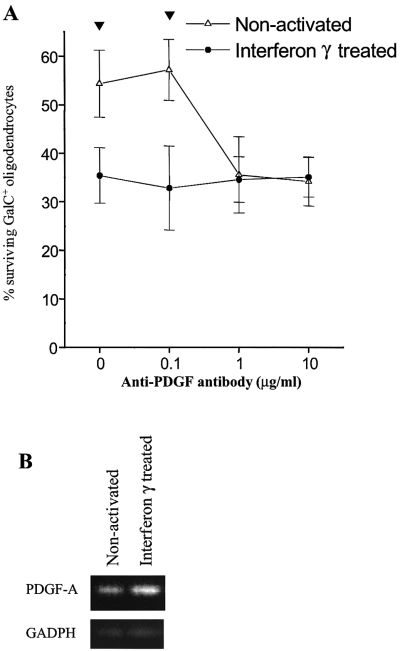
In the presence of medium conditioned by nonactivated microglia, addition of anti-PDGF antibody at concentration of > 1 µg/mL blocks the reduction in GalC+ oligodendrocyte loss seen after 48 h in culture (A; tP < 0.002, four experiments, mean ± SEM). PDGF-A chain and GADPH RT-polymerase chain reaction products, derived from microglia incubated with media conditioned by nonactivated microglia or IFN-γ-treated microglia, were run on a 2% agarose gel confirming the presence of PDGF-A chain within microglia in the presence of equal levels of GADPH (B).
PDGF-A chain is secreted by microglia and has equal bioactivity within medium conditioned by nonactivated and IFN-γ-activated microglia
Anti-PDGF (10 µg/mL) was used to immunoprecipitate PDGF from media conditioned by nonactivated and IFN-γ-treated microglia prior to incubation with mixed oligodendrocyte cultures. Loss of the survival effect of medium conditioned by nonactivated microglia established that PDGF was present in the conditioned medium and not produced directly by oligodendrocytes or in response to soluble microglial factors. The RT-polymerase chain reaction confirmed PDGF-A chain mRNA within nonactivated microglia and equal if not greater expression within IFN-γ-treated cells (Fig. 5B). To assay the bioactivity of PDGF-AA present within media conditioned by microglia, we quantified tyrosine phosphorylation of the PDGFα receptor after a 2-h exposure to conditioned media. At this time, OPC PDGF-α receptor expression was equal under both nonactivated and IFN-γ-activated conditions. The receptor was equally phosphorylated indicating similar levels of PDGF-AA activity within both conditioned media (six experiments).
Medium conditioned by nonactivated microglia synergise with the PDGF-α receptor pathway to increase the NF-κB response in oligodendrocyte precursors
The effect of medium conditioned by nonactivated microglia was blocked by anti-PDGF antibody, despite no change in the expression of PDGF-A chain in nonactivated and IFN-γ-treated microglia and PDGF-AA failed to reproduce the observed effect. Therefore, we looked for evidence of synergy between PDGF-AA and conditioned media, utilising the association of NF-κB with both survival and maturation. Mixed oligodendrocyte cultures were incubated for 4 h with unconditioned or conditioned media before exposure to PDGF-AA for a further 2 h. The addition of low (0.2 and 1 ng/mL) concentrations of PDGF-AA had no effect on NF-κB activation in unconditioned medium and that conditioned by IFN-γ-treated microglia. However, after preincubation with medium conditioned by nonactivated microglia, these concentrations of PDGF-AA significantly increased NF-κB activation in oligodendrocytes (three experiments, P < 0.02, Fig. 6A).
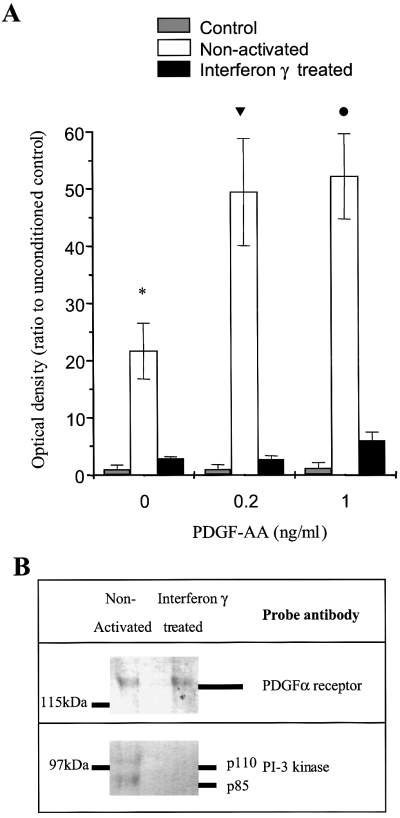
Significant NF-κB activation is still evident after incubating oligodendrocytes with medium conditioned by nonactivated microglia for 4 h and then with control medium for 2 h (A; *P < 0.01, three experiments, mean ± SEM). Exposure to control medium and then PDGF-AA (0.2 and 1 ng/mL) for 2 h does not affect NF-κB. Incubation with medium conditioned by nonactivated microglia for 4 h before a 2-h incubation with PDGF-AA (0.2 and 1 ng/mL) significantly induces NF-κB (tP < 0.03, 0.2 ng/mL, lP < 0.02, 1 ng/mL) over and above that seen in the absence of PDGF-AA. Immunoprecipitation of the PDGF-α receptor after a 2-h exposure to conditioned medium confirms similar levels of receptor expression (see also Fig. 3B); however, only in the presence of medium conditioned by nonactivated microglia is PI-3 kinase coimmunoprecipitated (B).
Medium conditioned by nonactivated microglia mediate their effects through PI-3 kinase and recruit its active subunit to the PDGF-α receptor in oligodendrocyte precursors
The increased survival of GalC+ oligodendrocytes in the presence of medium conditioned by nonactivated microglia was dose-dependently inhibited in the presence of the PI-3 kinase inhibitor, wortmannin (0–50 nm). No effect was seen with medium conditioned by IFN-γ-activated microglia or vehicle alone (0.1% DMSO). The PDGF-α receptor and PI-3 kinase coimmunoprecipitated in oligodendrocytes cultured with medium conditioned by nonactivated but not interferon γ treated microglia (Fig. 6B), further implicating an action of microglial-derived factors at the level of the PDGF-α receptor.
Discussion
Here we demonstrate in vitro a novel role for microglia on cells of the oligodendrocyte lineage. Medium conditioned by nonactivated but not IFN-γ-treated microglia increase the number of GalC+ oligodendrocytes in mixed oligodendrocyte lineage cultures due both to the promotion of precursor survival and their subsequent maturation under the influence of PDGF-AA and additional factors that remain to be characterised. By observing NF-κB activation on exposure to medium conditioned by nonactivated microglia and activating the transcription factor NF-κB within these cultures using antisense to IκB, we show that factors released by nonactivated microglia act in part by enhancing the PDGF-α receptor signalling pathway. We provide evidence that this may occur at the level of receptor–PI-3 kinase interaction.
Activation of NF-κB by IκB and resting microglial supernatants is associated with oligodendrocyte precursor survival and maturation
NF-κB is constitutively activated in freshly isolated mixed oligodendrocyte lineage cultures and within OPCs. The NF-κB complex can be upregulated after 2 h exposure to medium conditioned by nonactivated but not IFN-γ-treated microglia and is still maintained above control levels at 24 h. This pattern of increased activation of a constitutively expressed NF-κB subunit is also seen with NGF stimulation of rat sympathetic neurons (Maggirwar et al., 1998) where it is associated with neuronal survival. NF-κB up-regulation by medium conditioned with nonactivated microglia appears to represent a specific event, since the transcription factor STAT has a different pattern of activation compatible with the expression of IFN-γ receptors on oligodendrocytes and the presence of recombinant IFN-γ in conditioned medium (Torres et al., 1995).
The transcription factor NF-κB, originally identified in association with the immune system, can be induced within stressed cells (Baldwin, 1996) of many different origins to form part of a protective response (Baichwal & Baeuerle, 1997). Several studies have implicated NF-κB and the p65 subunit (Rel A) in cellular maturation and survival (reviewed in Baeuerle & Baltimore, 1996), acting through the transcription of protective genes such as A20 and TTG that prevent apoptosis and stimulate differentiation (Ferran et al., 1998; Xie et al., 1998). Activated NF-κB plays a role in the protection from apoptosis afforded by NGF in the PC12 neuronal cell line (Taglialatela et al., 1997) and in primary sympathetic neurons (Maggirwar et al., 1998). Though activated NF-κB has been associated with cell survival and maturation, complexity of the cellular response means that the outcome is not wholly determined by NF-κB. Indeed NF-κB has been implicated in glutamate- and β-amyloid protein-stimulated neuronal death (Behl et al., 1994; Grilli et al., 1996) and p75NTR receptor stimulation is associated with NF-κB activation but not survival in mature oligodendrocytes (Carter et al., 1996; Ladiwala et al., 1998; Yoon et al., 1998).
In an attempt to define the role of NF-κB activation in OPCs, we increased the pool of biologically active NF-κB using antisense to IκB (Barger et al., 1995). IκB inhibits NF-κB function by sequestering NF-κB dimers within the cytosol. The phosphorylation and subsequent degradation of IκB reveals a nuclear localisation signal for NF-κB permitting translocation and initiation of transcription of multiple genes including IκB. Because NF-κB is constitutively expressed in OPCs, NF-κB dependent IκB synthesis is readily inhibited with an antisense oligonucleotide to IκB. Directly activating NF-κB in OPCs using antisense to IκB blocked apoptosis and stimulated maturation to mature oligodendrocytes as we had previously observed using medium conditioned with nonactivated microglia. Whilst no changes in STAT activation were observed using antisense/mis-sense oligonucleotides to IκB, we cannot exclude the possibility that other transcription factors are also modified.
Nonactivated microglia promote oligodendrocyte survival through regulation of the PDGF-α receptor signalling pathway
PDGF-AA is an essential requirement for OPC survival and development. At low concentrations (< 1 ng/mL), PDGF-AA promotes survival through blockade of apoptosis without stimulating DNA incorporation (Barres et al., 1992). Although we have demonstrated the involvement of PDGF-AA in mediating the biological properties of conditioned media, it is apparent that PDGF-AA does not explain the differential effect of medium conditioned by nonactivated and IFN-γ-treated microglia on OPC survival and maturation. First, there was no obvious difference in the concentration of PDGF-AA in each condition; and, secondly, exogenous PDGF-AA failed to reproduce the maturation properties of medium conditioned by nonactivated microglia.
The presence of PDGF-A chain within microglia and bioactive PDGF-AA within freshly isolated microglial conditioned medium is expected as PDGF-A mRNA has previously been demonstrated in peritoneal macrophages, a cell of related lineage. In keeping with our results, PDGF-A chain expression in these cells is also not modified by IFN-γ activation. However the constitutive secretion of PDGF-A is downregulated in peritoneal macrophages after 6 days in culture (Kodelja et al., 1997) and this may explain in part why previous studies using 4-day microglia-conditioned media have not shown any effect on oligodendrocyte survival (Gard et al., 1995). Another factor contributing to the previously reported lack of an effect in previous work may lie in the observation that microglia acquire an activated phenotype after 2 days in culture (Becher & Antel, 1996) making media conditioned from 4-day microglia comparable to our interferon γ treated conditions.
Taken together, these findings argue for the presence of an additional IFN-γ-regulated factor(s) secreted by microglia, which modulates the PDGF-α receptor survival pathway within the OPC and promotes its maturation. Direct supporting evidence comes from our observation that medium conditioned by nonactivated microglia synergistically increases the activation of NF-κB when OPCs are subsequently stimulated with limiting concentrations of exogenous PDGF-AA. Indirect evidence is provided by the demonstration of differential PI-3 kinase recruitment to the PDGF-α receptor in the presence of media conditioned by nonactivated and IFN-γ-treated microglia. Activation of PI-3 kinase has been implicated in mediating the survival response to exogenous stimuli of multiple cell types, including oligodendrocytes (Vemuri & McMorris, 1996;Franke et al., 1997) and PI-3 kinase activates a cascade involving Akt kinase, IκB kinase and NF-κB (Romashkov & Makarov, 1999). We therefore examined whether PI-3 kinase is associated with the PDGF-α receptor in the presence of conditioned supernatants. Modulation of PI-3 kinase has previously been demonstrated for the PDGF-β receptor in 3T3 fibroblasts (Yu et al., 1998). We showed a significant difference in the degree of PI-3 kinase recruitment compatible with an interaction at this level but cannot exclude effects at other points in the intracellular pathway. Additional factor(s) produced by nonactivated microglia titrate between concentrations of 1 : 2 and 1 : 8 possibly reflecting biological instability. Preliminary data have revealed an as yet unidentified 40 kDa protein that is differentially expressed on two-dimensional gels.
In summary, we show that dependent on their state of activation microglia modulate sensitivity of the PDGF-α receptor pathway in OPCs. Others have postulated that inhibition of the PDGF-α receptor pathway in OPCs underlies their loss of mitotic responsiveness to PDGF-AA (Hart et al., 1989). We demonstrate a potential mechanism occurring at the PDGF-α receptor–PI-3 kinase interaction. Our in vitro results address the potential homeostatic role for microglia in regulating survival of differentiated oligodendroglia.
Acknowledgements
We thank Sarah Stevens for technical assistance, David Clayton for help with statistical analysis and Bill Blakemore for helpful discussions. This work was supported in part by a grant from SmithKline Beecham.
Abbreviations
-
- BrdU
-
- bromodeoxyuridine
-
- CNTF
-
- ciliary-derived neurotrophic factor
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- DFP
-
- DMEM + 10% foetal calf serum + 1% penicillin and streptomycin
-
- FGF-2
-
- fibroblast growth factor-2
-
- GalC
-
- galactocerebroside
-
- IFN-γ
-
- interferon-γ
-
- IGF
-
- insulin-like growth factor
-
- IκB
-
- inhibitor of kappa-binding
-
- MTT
-
- 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
-
- PDGF
-
- platelet-derived growth factor
-
- PI-3 kinase
-
- phosphatidyl-3-inositol kinase
-
- NF-κB
-
- nuclear factor of kappa-binding
-
- NT-3
-
- neurotrophin-3
-
- OPC
-
- oligodendrocyte precursor cell
-
- STAT
-
- signal transducer and activator of transcription.




