Synthesis of progesterone in Schwann cells: regulation by sensory neurons
Abstract
In peripheral nerves, progesterone synthesized by Schwann cells has been implicated in myelination. In spite of such an important function, little is known of the regulation of progesterone biosynthesis in the nervous system. We show here that in rat Schwann cells, expression of the 3β-hydroxysteroid dehydrogenase and formation of progesterone are dependent on neuronal signal. Levels of 3β-hydroxysteroid dehydrogenase mRNA and synthesis of [3H]progesterone from [3H]pregnenolone were low in purified Schwann cells prepared from neonatal rat sciatic nerves. However, when Schwann cells were cultured in contact with sensory neurons, both expression and activity of the 3β-hydroxysteroid dehydrogenase were induced. Regulation of 3β-hydroxysteroid dehydrogenase expression by neurons was also demonstrated in vivo in the rat sciatic nerve. 3β-hydroxysteroid dehydrogenase mRNA was present in the intact nerve, but could no longer be detected 3 or 6 days after cryolesion, when axons had degenerated. After 15 days, when Schwann cells made new contact with the regenerating axons, the enzyme was re-expressed. After nerve transection, which does not allow axonal regeneration, 3β-hydroxysteroid dehydrogenase mRNA remained undetectable. The regulation of 3β-hydroxysteroid dehydrogenase mRNA after lesion was similar to the regulation of myelin protein zero (P0) and peripheral myelin protein 22 (PMP22) mRNAs, supporting an important role of locally formed progesterone in myelination.
Introduction
Progesterone (PROG), produced by the ovaries and adrenal glands, is known to regulate reproductive behaviour and gonadotropin release (McEwen, 1991; Chappell et al., 1997; Schumacher et al., 1999). PROG can also be synthesized by glial cells and some types of neurons (Jung-Testas et al., 1989; Guennoun et al., 1997), and may thus be considered as a ‘neurosteroid’. This term refers to steroids which can be synthesized within the central and peripheral nervous systems (Baulieu, 1991; Robel et al., 1999). In the peripheral nervous system, PROG is synthesized by Schwann cells and plays an important role in myelination. This function of PROG has been demonstrated in both the regenerating and ageing rodent sciatic nerve and in dorsal root ganglia (DRG) explant cultures (Koenig et al., 1995; Chan et al., 1998; Melcangi et al., 1998; Notterpek et al., 1999). One mechanism by which PROG may promote myelination is by activating the expression of genes coding for specific myelin proteins (Désarnaud et al., 1998). Its actions in peripheral nerves may be autocrine, as Schwann cells not only synthesize PROG, but also express an intracellular receptor for the steroid, as has been demonstrated by RT-PCR, immunocytochemistry and ligand binding studies (Jung-Testas et al., 1996).
As PROG has such an essential role in the peripheral nervous system, the regulation of its synthesis in Schwann cells may be important for the normal functioning and regeneration of peripheral nerves. PROG is synthesized from pregnenolone (PREG) by the enzyme 3β-hydroxysteroid dehydrogenase (3β-HSD). The presence and activity of this enzyme have been demonstrated in Schwann cells isolated from embryonic rat DRG (Guennoun et al., 1997), and its functional implication in myelination has been demonstrated in the regenerating mouse sciatic nerve by using the 3β-HSD inhibitor trilostane (Koenig et al., 1995).
Several Schwann cell genes and functions have been shown to be under the control of neuronal signals, requiring either direct contact between cells or involving diffusible molecules. For example, neurons regulate genes coding for growth factors, cytokines, transcription factors and myelin proteins in Schwann cells (Bolin & Shooter, 1993; Reynolds & Woolf, 1993; Snipes & Suter, 1994; Murphy et al., 1996). The possibility that PROG synthesis in Schwann cells may also be under neuronal control has been investigated in the present study. In this study we show that mRNA expression and activity of 3β-HSD are induced in Schwann cells by sensory neurons. This was demonstrated in vitro, using different coculture paradigms of DRG neurons and Schwann cells, and in the regenerating rat sciatic nerve after different types of lesions (cryolesion, nerve transection). Thus, steroid synthesis in the nervous system is regulated by cellular interactions.
Materials and methods
Surgical procedures for lesion of the sciatic nerve
Adult male Sprague–Dawley rats (200–250 g; Iffa Credo, France) were deeply anaesthetized with an intraperitoneal injection of 150 mg/kg of ketamine (Imalgène, Rhône Mérieux, France) and 3.7 mg/kg of acepromazine (Vetranquil, Sanofi, France). The right sciatic nerve was exposed and two types of lesions were performed. For cryolesion, which allows regeneration of the axons, the nerve was injured by local freezing (Mira, 1981). A non-resorbable thread (11-0 nylon, Ethicon, France) was tied in the epineurium to mark the lesion site. The nerve was submitted to six cycles of freezing-thawing by using a copper cryode (diameter 0.5 mm) cooled in liquid nitrogen. The extent of the lesion was about 1 mm (Ferzaz et al., 1989). The second type of lesion, which does not allow axonal regeneration, corresponded to transection of the nerve. To avoid fibre regrowth, each end of the nerve was sutured. Different times after lesion, animals were killed by decapitation and the segments of the sciatic nerves distal to the lesion were sampled, immediately frozen in liquid nitrogen and stored at −80 °C until RNA extraction. For immunohistochemistry, sciatic nerves were fixed in situ with 4% ice-cold paraformaldehyde for 10 min and postfixed for 1 h.
All experiments were carried out in accordance with the French decree (A 94120) from 1991 and associated guidelines of the EEC directive (86/609/EEC; 1986).
Schwann cell cultures from neonatal rat sciatic nerves
Purified Schwann cells were prepared as previously described (Brockes et al., 1979). Briefly, cells from 4-day-old (P4) Sprague–Dawley rats were dissociated with trypsin (0.25%; Bio Media, France) and collagenase type 1A (0.1%; C-9891; Sigma, St Louis, MO, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM; Bio Media) containing 10% fetal calf serum (FCS; Eurobio, France), penicillin (100 IU/mL) and streptomycin (100 µg/mL) on dishes coated with poly l-lysine hydrobromide (MW > 300 kDa, Sigma). Contaminating fibroblasts were partially eliminated by treating the cultures for 48 h with 10 µm of the antimitotic cytosine arabinoside (Sigma). Schwann cells were induced to proliferate in the presence of the mitogens forskolin (2 µg/mL) and insulin (5 µg/mL) (Schumacher et al., 1993). After cell confluence was reached, residual fibroblasts were eliminated by treatment with an antibody against the membrane protein Thy-1.1 (clone T11 D7e, Cedarlane, Ont., Canada) and Lox-Tox rabbit complement (Cedarlane). The cells were then further expanded in insulin and forskolin. The purity of Schwann cell cultures was assessed by double immunofluorescence labelling with antibodies to S100 to identify Schwann cells, and to Thy-1.1 (OX-7, Caltag, CA, USA) to detect fibroblasts.
Schwann cell cultures from embryonic dorsal root ganglia explants
DRG were taken from 18-day-old (E18) rat embryos and were cultured in DMEM-Ham's F12 (50 : 50, v/v) containing 10% FCS and nerve growth factor (NGF-7S, 30 ng/mL, Sigma) on collagen prepared from rat tails, as previously described (Kleitman et al., 1991). The explants were treated twice for 48 h with cytosine arabinoside (10 µm) to eliminate cells other than the not-dividing sensory neurons and the slowly dividing Schwann cells. After 4 weeks in culture, when Schwann cells fully populate the neurites which have grown out of the explants, the ganglia containing the sensory neurons were cut out with a microscalpel under a dissecting microscope. The remaining Schwann cells were removed from the dishes with trypsin/EDTA (0.25% trypsin, 0.3 mm EDTA) and were plated on new poly l-lysine-coated culture dishes and cultured in DMEM + 10% FCS. Photographs were produced with a Leitz (Germany) Diavert inverted microscope equipped with phase contrast illumination and a Wild (Germany) Photoautomat MPS 45 camera using Kodak Technical Pan film. Pictures were then digitized using a FilmScan 200 (Epson, France).
Cocultures of Schwann cells and sensory neurons
Pure cultures of sensory neurons were prepared from DRG of E18 rat embryos (Guennoun et al., 1997). They were dissociated with trypsin (0.25%) and collagenase type 1A (0.1%) and grown either on collagen-coated culture dishes or collagen-coated microporous membranes from Millicell inserts (0.4 µm pore size, Millipore, Bedford, MA, USA) in DMEM-Ham's F12 (50 : 50, v/v) containing 10% FCS and NGF (30 ng/mL). Neurons were kept for 1 week to allow their purification by cytosine arabinose treatment (two 24-h pulses). The complete elimination of Schwann cells and fibroblasts was assessed by immunocytochemistry with antibodies against S-100 (Z 311, Dako, Denmark) and GFAP (G-A-5, Sigma) for Schwann cells and against Thy-1.1 (OX-7, Caltag) to detect fibroblasts.
Two types of cocultures were used: Schwann cells and neurons were grown in close contact or were separated by a microporous membrane. In the first case, Schwann cells purified from neonatal sciatic nerves were seeded on dissociated sensory neurons. Under these conditions, Schwann cells grow along the neurites only and do not attach to the collagen support. In the second case, Schwann cells were grown in poly l-lysine-coated, six-well culture dishes 1 mm below neurons growing on a collagen-coated microporous membrane.
Culture of other cell types
Human hepatocarcinoma cell line
HepG2 (Knowles et al., 1980) was grown in DMEM-Ham's F12 (50 : 50, v/v) containing 10% FCS and NGF (30 ng/mL).
Fibroblasts
Cells isolated from neonatal rat sciatic nerves after dissociation by trypsin and collagenase, as described earlier, were seeded on uncoated plastic dishes. Under these conditions, only fibroblasts attach to the substrate as Schwann cells require poly l-lysine coating (unpublished observation). Pure cultures of fibroblasts were grown in the same medium.
Metabolism of [3H]pregnenolone
Schwann cells in culture were incubated in the presence of 100 nm[3H]PREG (Amersham, UK; specific activity 21.1 Ci/mmol, diluted to 10 Ci/mmol with cold PREG from Sigma) at 37 °C. Culture medium without cells, incubated with the same labelled substrate, was used as a control. After 24 h of incubation, culture medium was collected and stored at −20 °C. The number of cells was counted and DNA content was determined, as previously described (Groyer & Robel, 1980). Steroids were extracted from the culture medium three times with 10 mL of ethyl acetate/isooctane (1 : 1, v/v) and were then separated on silica gel plates (60F254, Merck, Germany) in chloroform/ethyl acetate (4 : 1, v/v). Quantification of radioactive areas on the thin-layer chromatography (TLC) plates was performed with an automatic TLC linear analyser (Berthold Wallac, Finland). To help in the identification of the [3H]PREG metabolites, the relative migration solvent front (Rf) of standard steroids (known metabolites of PREG and PROG) was also determined in the same chromatographic conditions using either radioactive or UV absorbent steroids.
The [3H] metabolites were further characterized by high-performance liquid chromatography (HPLC) on a Lichrospher 100-RP-18 (5 µm) column (Hibar, Merck). The mobile phase was a methanol/water gradient (60–80% in 30 min) at a flow rate of 1 mL/min. Fractions (1 mL) were collected and their radioactivity evaluated. Their identity was also confirmed by recrystallizations to constant specific activity (Guennoun et al., 1997).
RNA extraction
Total RNA was extracted from cultured Schwann cells or from frozen sciatic nerves by using a commercial reagent ‘RNA Now’ (Biogentex, TX, USA) according to the instructions of the manufacturer. Schwann cells were lysed directly with the RNA Now solution, whereas nerves were powdered in liquid nitrogen before RNA extraction.
Northern blot analysis
Total RNA (6 µg/sample) was separated on 1.2% formaldehyde-agarose gels and blotted onto nylon membrane using standard conditions. Filters were prehybridized and hybridized in a solution containing 50% formamide, 5 × SSC, 1 × Denhardt's solution (100 × Denhardt's solution: 0.2% BSA, 0.2% ficoll and 0.2% polyvinylpyrrolidone), 0.1% SDS and 100 µg/mL sheared herring sperm DNA at 42 °C overnight. The filters were washed in 1% SSC and 0.1% SDS at 50 °C. The protein zero (P0) cDNA probe was an insert of 1.8 kbp recovered by digestion with EcoR1 from the plasmid pSN63 (rat P0 cDNA cloned into the EcoR1 site of pUC18, Lemke & Axel, 1985). The peripheral myelin protein 22 (PMP22) cDNA probe was an insert of 1.7 kbp recovered by digestion with XhoI from the plasmid pCDM8 (complete rat PMP22 cDNA cloned into pCDM8). The ribosomal 18S probe used as an internal control was a 386-bp fragment derived by RT-PCR from rat cDNA sequence (obtained from GenBank) using the following primers: 18S forward, 5′-CTACCACATCCAAGGAAGCG-3’ and 18S reverse, 5′-CTCGGGCCTGCTTTGAACAC-3’. The probes were radiolabelled with [α-32P] dCTP using the rediprime kit (Amersham Pharmacia Biotech, UK). The blots were hybridized successively with the radiolabelled probes and exposed to film for 1 day (P0 and PMP22) or 1 h (18S).
Reverse transcriptase polymerase chain reaction (RT-PCR)
cDNA templates for PCR amplification were synthesized from 3 µg of total RNA using SuperScript II RNase H reverse transcriptase (Life technologies, Invitrogen, UK) for 2 h at 42 °C in the presence of random hexamer primers. Aliquots of cDNA corresponding to 1 µg of initial total RNA were subjected to PCR amplification. To amplify whichever isoform of 3β-HSD, we used a set of primers complementary to sequences common to the four isoforms of the enzyme. A forward primer 5′-CTGAATGTTACTGGCGAATTCTC-3′ and a reverse primer 5′-TGTAAAATGGATCC-AGCAGGA-3′ (corresponding to nucleotides 805–827 and 1099–1076 according to rat 3β-HSD type I) were used for amplification. Control reactions were performed in the absence of cDNA. The 18S ribosomal RNA was amplified in parallel in separate reactions for normalization of the results by using a forward primer (5′-CTACCACATCCAAGGAAGCG-3′) and reverse primer (5′-CTCGGGCCTGCTTTGAACAC-3′) corresponding to nucleotides 465–485 and 851–831. Each PCR reaction contained cDNA template, Taq DNA polymerase buffer, 0.5 µm of forward and reverse primers, 200 µm of each dNTP and 2 U Taq DNA polymerase (Qbiogene, France) in a total volume of 100 µL and consisted in 30 cycles at 94 °C for 1 min, 60 °C for 1 min and 72 °C for 1 min. Following amplification, to examine the PCR products, a 20-µL aliquot of each reaction and 100-bp DNA ladder were separated on a 1.2% agarose gel, visualized by ethidium bromide staining. The gels were subsequently quantified using BioRad's Image Analysis System and Molecular Analyst for Macintosh software. The relative levels of gene expression were measured by determining a ratio between the products generated from the target gene and the endogenous internal standard (18S RNA) in separate reactions (Horikoshi et al., 1993).
Immunohistochemistry
Sensory neurons, grown on microporous membranes or Schwann cells grown on plastic support, were washed with PBS, fixed in acetic methanol for 10 min, dehydrated in ascending concentrations of ethanol and then rehydrated. An indirect method with the avidin-biotin-peroxidase Elite kit (Vector Laboratories, CA, USA) was used (Hsu et al., 1981). The sensory neurons were stained with NR4 monoclonal antibody recognizing 67-kDa neurofilaments (Sigma) diluted at 1 : 200. Schwann cells were stained with G-A-5, a monoclonal antibody to GFAP (Sigma) diluted at 1 : 100.
Semithin sections of the membranes on which sensory neurons were growing were performed. After immunolabelling, the membranes were dehydrated in graded alcohols and were embedded in epon resin. After polymerization at 60 °C, they were cut transversally and mounted on glass slides. Photographs were produced with a Zeiss (Germany) Axioskop 20 microscope equipped with a MC 100 camera using a Kodak T-MAX-100 film and then digitized with a FilmScan 200 (Epson).
For nerve regeneration experiments, the nerves were dehydrated and embedded in Paraplast (Sherwood Medical, MO, USA). Longitudinal sections (7 µm) were mounted on glass slides and used for immunofluorescence labelling. Axons were stained with the monoclonal antibody to neurofilaments (NR4) diluted at 1 : 100. We used a fluorescein-labelled goat antiserum to mouse IgG as a second antibody (Jackson ImmunoResearch, PA, USA). Slides were examined with an Axiovert 135 m (Zeiss) using a ×40 objective. Digital picture files were produced with a LSM 410 confocal system (Zeiss) equipped with an argon laser.
Statistical analysis
Differences between two means were analysed by an unpaired, two-tailed t-test. Differences between multiple means were determined using one-way anova followed by Newman-Keuls's multiple comparison tests.
Results
Highly purified Schwann cells convert only very low amounts of [3H]pregnenolone to [3H]progesterone
Levels of PROG are elevated in the rodent sciatic nerve, even after removal of the steroidogenic endocrine glands by combined castration and adrenalectomy (Koenig et al., 1995). This observation strongly suggests a local synthesis of this neurosteroid. Schwann cells could be a source of PROG in peripheral nerves. However, confluent cultures of highly purified Schwann cells prepared from P5 rat sciatic nerves produced only very low amounts of [3H]PROG (0.16 ± 0.03 pmol/µg DNA/24 h, mean ± SEM, n = 10) when incubated for 24 h in the presence of 100 nm of [3H]PREG.
Astrocytes isolated from neonatal rat brain convert [3H]PREG to [3H]PROG, but only when cultured at low density (Akwa et al., 1993). To determine whether cell density also influences the synthesis of PROG by Schwann cells, they were grown at low (1200 cells/cm2) or high (32 000 cells/cm2) density for 3 days and then incubated for an additional 24 h in the presence of [3H]PREG (100 nm). Under both culture conditions, the conversion of [3H]PREG to [3H]PROG remained very low (low cell density, 0.14 ± 0.02; high cell density, 0.17 ± 0.04 pmol/µg DNA/24 h, mean ± SEM, n = 6) and no other steroid metabolites were formed.
Schwann cells grown in contact with sensory neurons show increased progesterone synthesis and 3β-hydroxysteroid dehydrogenase expression
Schwann cell activity and gene expression are influenced by neuronal signals (Snipes & Suter, 1994). To determine whether the synthesis of PROG in Schwann cells is also regulated by neurons, we compared the conversion of [3H]PREG to [3H]PROG between two types of culture: (i) purified cultures of Schwann cells prepared from neonatal rat sciatic nerves and maintained in the absence of neurons for 4 weeks; (ii) Schwann cells grown in contact with neurons for 4 weeks, obtained from E18 rat DRG explant cultures. After excision of the neuronal cell bodies from these cultures, the remaining Schwann cells, which had grown on the neurites, were trypsinized and axonal fragments were eliminated by centrifugation. Purified Schwann cells were seeded in new culture dishes and immediately incubated in the presence of 100 nm[3H]PREG for 24 h (Fig. 1).
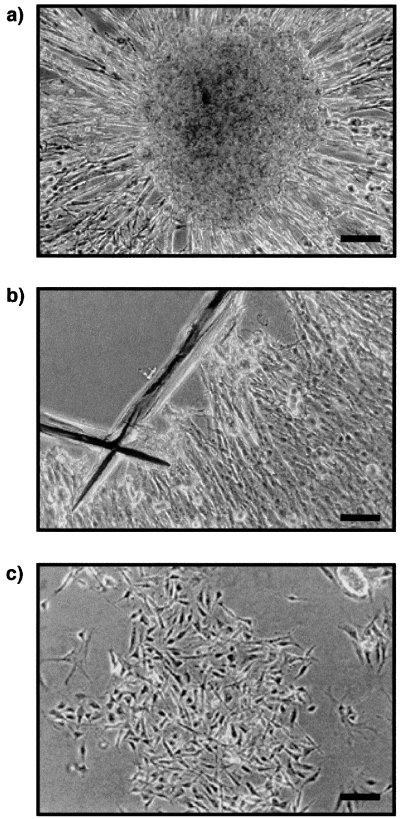
Isolation of Schwann cells from dorsal root ganglia (DRG) explant cultures. DRG explants from 18-day-old embryos (E18) from rats, from which fibroblasts had been removed by antimitotic treatment, were cultured for 4 weeks. (a) Schwann cells populated the neurites which grew out of the explants. (b) The sensory neurons cell bodies were then cut out with a microscalpel. (c) The remaining Schwann cells were trypsinized, purified and seeded into new culture dishes. Scale bar, 40 µm.
Schwann cells grown in close contact with DRG neurons produced much larger amounts of [3H]PROG than purified Schwann cells which were cultivated alone (Fig. 2a). They also expressed higher mRNA levels of the 3β-HSD enzyme, as shown by semiquantitative RT-PCR (Fig. 2b). In addition to PROG, Schwann cells isolated from the DRG explants produced the 5α-reduced metabolites 5α-dihydroprogesterone (5α-DH PROG; 1.4 ± 0.4 pmol/µg DNA/24 h, mean ± SEM) and 3α,5α-tetrahydroprogesterone (3α,5α-TH PROG; 0.8 ± 0.2 pmol/µg DNA/24 h, mean ± SEM). The identity of the metabolites separated by thin-layer chromatography was verified by HPLC and recrystallizations to constant specific activity. When [3H]PREG was incubated in the absence of cells, neither PROG nor its reduced metabolites were formed. Moreover, in the presence of the 3β-HSD inhibitor trilostane (5 µm), no [3H]PROG was formed from [3H]PREG and the 5α-reductase inhibitor L685-273 (1 µm) blocked the formation of 5α-reduced metabolites.
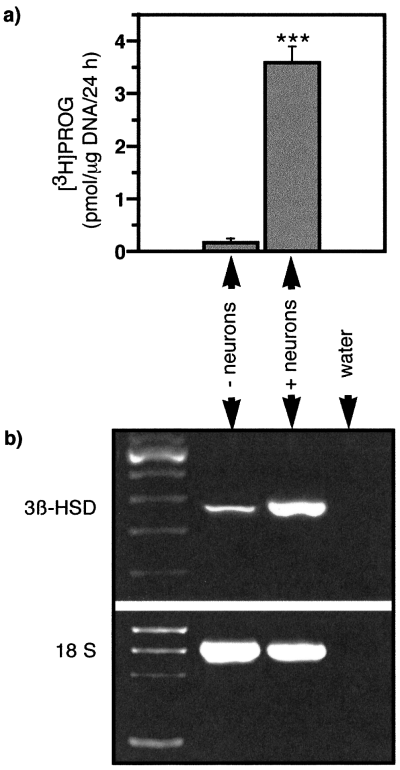
Progesterone synthesis and 3β-HSD mRNA expression in Schwann cells are increased in the presence of sensory neurons. (a) Schwann cells grown for 4 weeks in contact with sensory neurons and isolated from DRG explants (+ neurons) produced larger amounts of [3H]PROG when incubated in the presence of [3H]PREG than Schwann cells purified from neonatal sciatic nerves and cultured in the absence of neurons (– neurons) (mean ± SEM; ***P < 0.001; n = 6). (b) Similarly, RT-PCR analysis of 3β-HSD transcripts in Schwann cells showed increased levels in the presence of sensory neurons. The 18S ribosomal RNA was amplified in parallel and used for normalization of the results. Ratio 3β-HSD : 18S, 0.45 ± 0.03 (– neurons); 0.98 ± 0.06 (+ neurons), mean ± SEM, n = 3).
These results suggest that 3β-HSD expression and PROG synthesis in Schwann cells may be dependent on the presence of a neuronal signal. Alternatively, the different origin of the Schwann cells used in the previous experiment, neonatal sciatic nerve vs. embryonic DRG, could have explained the observed differences in [3H]PREG metabolism. To distinguish between these two possibilities, Schwann cells purified from neonatal sciatic nerves were cultured alone or on dispersed sensory neurons in DMEM-Ham's F12 medium containing 10% FCS and NGF (30 ng/mL). Neurons were prepared from E18 DRG and the absence of contaminating Schwann cells of DRG origin and fibroblasts was verified by immunocytochemistry with antibodies to S-100, GFAP and Thy-1.1. After 2 weeks of coculture, the sensory neurons were eliminated by hypoosmotic shock (2 × 5 min in 10 mm Tris buffer containing 1 mm MgCl2, pH 8, on ice). Schwann cells, which are resistant to this treatment, were immediately incubated for 24 h in the presence of 100 nm of [3H]PREG for 24 h. Those which had grown in contact with neurons produced higher amounts of [3H]PROG (mean ± SEM, 2.46 ± 0.31 pmol/µg DNA/24 h) than those which had grown alone (0.23 ± 0.04 pmol/µg DNA/24 h; P < 0.01; n = 4). Thus, sensory neurons can also induce PROG synthesis in Schwann cells from the sciatic nerve.
Direct contact with sensory neurons is not required for the induction of progesterone synthesis in Schwann cells
Regulation of Schwann cell functions by neurons has been shown to involve either direct contact or diffusible molecules (Bolin & Shooter, 1993). To determine whether diffusible neuronal factors are responsible for the induction of PROG synthesis in Schwann cells, we used a coculture system in which DRG sensory neurons and Schwann cells from neonatal sciatic nerves were separated by a microporous membrane. The neurons were grown on top of a collagen-coated membrane 1 mm above the Schwann cells, which were plated on Petri dishes coated with poly l-lysine. Using microscopy on serial semithin sections of the membrane, we confirmed that neurons and their processes did not cross the 0.4 µm pores (Fig. 3).
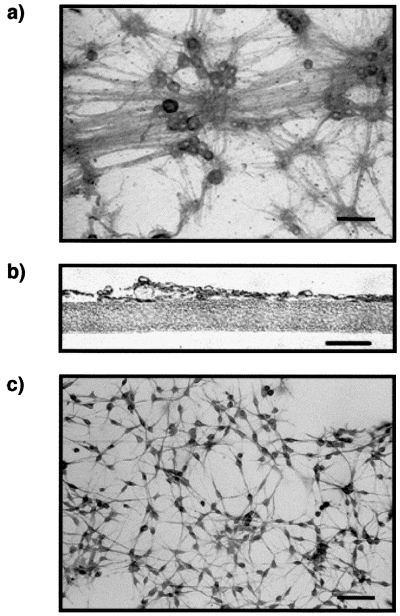
Coculture system in which sensory neurons and Schwann cells are separated by a microporous membrane. (a) Sensory neurons grown on a microporous membrane were stained with antineurofilament antibody. Scale bar, 40 µm. (b) Neurites do not cross the microporous membrane, as shown by serial semithin sections stained with the antineurofilament antibody. Scale bar, 10 µm. (c) Schwann cells grown 1 mm below the membrane were stained with an anti-GFAP antibody. Scale bar, 40 µm.
After different times of coculture (6–30 days), the membrane inserts with the neurons were removed and Schwann cells were incubated in the presence of 100 nm[3H]PREG for 24 h. As shown in Fig. 4a, the synthesis of [3H]PROG from [3H]PREG was significantly enhanced in Schwann cells cocultured with neurons for 12 days. The increase in PROG formation was maximal after 18 days and remained elevated at least until day 30. These results show that diffusible neuronal molecules are sufficient to induce PROG synthesis in Schwann cells.
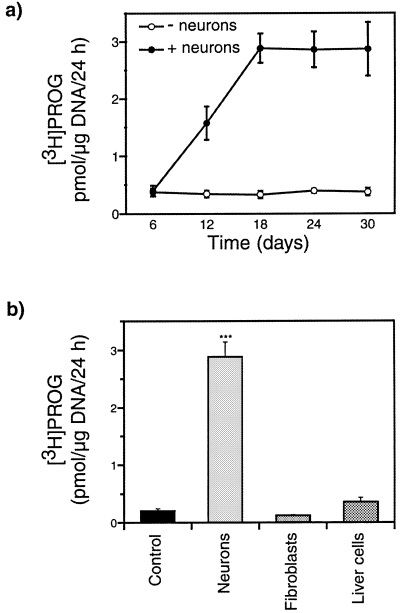
Induction of progesterone synthesis in Schwann cells by diffusible neuronal factors. (a) Formation of [3H]PROG from [3H]PREG by Schwann cells cultured for different times alone (– neurons; F4,15 = 0.18; P > 0.9) or in the presence of sensory neurons grown on a microporous membrane (+ neurons; F4,15 = 12.8; P < 0.001; 6 vs. 12 days P < 0.05; 6 vs. 18, 24 or 30 days P < 0.01) (n = 4). (b) The cell-specificity of this induction was verified by culturing either sensory neurons, fibroblasts or the liver cell line HepG2 on top of the membrane (F3,20 = 178; P < 0.001; ***P < 0.001 compared with control cultures). Means ± SEM; n = 6.
To test whether the induction of PROG formation in Schwann cells is neuron-specific, the same coculture paradigm was used, but Schwann cells were this time cultured for 2 weeks in the presence of sensory neurons, fibroblasts or a human liver cell line, grown on top of the membranes. Fibroblasts isolated from neonatal sciatic nerves are abundant in peripheral nerves, where they produce diffusible signalling molecules such as growth factors. Liver cells (the HepG2 human liver cell line) secrete a large set of diffusible factors. Under these culture conditions, only the DRG sensory neurons induced PROG synthesis in the Schwann cells (Fig. 4b).
Schwann cells grown in the presence of neurons further convert PROG to its reduced metabolites. However, whereas Schwann cells grown in direct contact with neurons produced both 5α-DH PROG and 3α,5α-TH PROG (Fig. 5b), Schwann cells separated from the neurons by a microporous membrane formed only 5α-DH PROG (Fig. 5c).
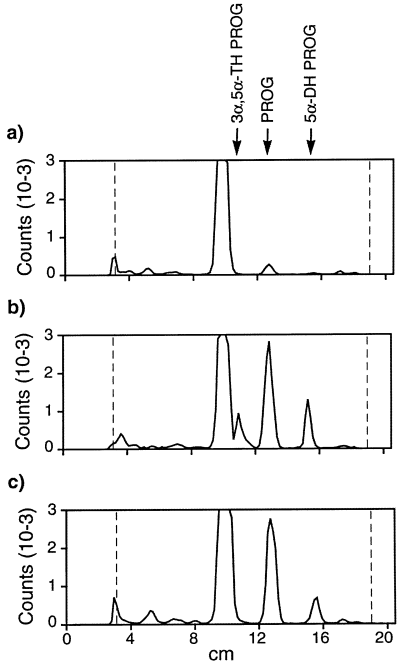
Formation of 3α,5α-TH PROG by Schwann cells is dependent on direct contact with sensory neurons. Metabolites formed from [3H]PREG were extracted from the culture medium and were analysed by thin-layer chromatography (TLC). Chromatograms were quantified with an automatic TLC linear analyser. The relative migration solvent fronts of 3α,5α-TH PROG, PROG and 5α-DH PROG were 0.49, 0.62 and 0.75, respectively. The identity of the TLC peaks was verified by HPLC and further confirmed by recrystallizations to constant specific activity. (a) Schwann cells grown in the absence of neurons converted only very low amounts of [3H]PREG to [3H]PROG. (b) When cultured in direct contact with sensory neurons for 2 weeks, Schwann cells produced significant amounts of [3H]PROG, [3H]5α-DH PROG and [3H]3α,5α-TH PROG. (c) When Schwann cells were separated from the sensory neurons by a microporous membrane, they formed only [3H]PROG and [3H]5α-DH PROG (representative chromatograms of three experiments shown).
Expression of the 3β-hydroxysteroid dehydrogenase in the sciatic nerve is dependent on a neuronal signal
The in vitro experiments described earlier show that 3β-HSD expression in Schwann cells is increased in the presence of neurons. To determine whether the enzyme is also expressed and regulated by neurons in vivo, 3β-HSD mRNA levels were analysed by semiquantitative RT-PCR in the intact sciatic nerve of adult male rats or different times after two types of lesion: cryolesion, after which axons regenerate, and nerve transection, after which no axonal regeneration takes place. The processes of axon degeneration and regeneration were monitored by neurofilament staining (Fig. 6a).
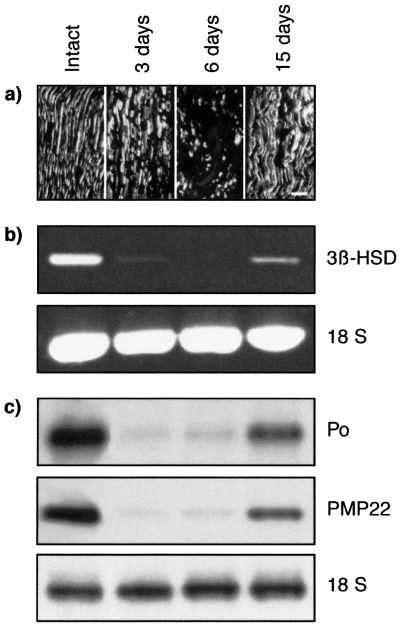
Analysis of 3β-HSD mRNA expression in intact rat sciatic nerves and after cryolesion. (a) Longitudinal sections, taken 10 mm distal to the lesion, were stained with an antineurofilament monoclonal antibody (NR4) to monitor the fate of axons by confocal microscopy. Three and 6 days after cryolesion, axons had degenerated and neurofilament immunoreactivity was restricted to axonal fragments. By day 15, axons had regenerated. Scale bar, 40 µm. (b) RT-PCR analysis of 3β-HSD transcripts in intact nerves and 3, 6 and 15 days after cryolesion in the part distal to the lesion site. Ratio 3β-HSD : 18S: intact, 0.72 ± 0.09; 3 days, 0; 6 days, 0; 15 days, 0.35 ± 0.05 (mean ± SEM; n = 3). (c) Northern blot analysis of P0 and PMP22 mRNA in the intact nerve and after cryolesion. Expression of 3β-HSD, P0 and PMP22 mRNA decreased dramatically 3 and 6 days after lesion and was re-induced after 15 days. Results were compared with ribosomal 18S RNA.
In the intact nerve, where Schwann cells are in contact with axons, 3β-HSD mRNA was present (Fig. 6b). Three and 6 days after cryolesion, when axons had degenerated, 3β-HSD mRNA levels were strongly reduced in the part of the nerve distal to the lesion site. By 15 days, when Schwann cells had made new contacts with the regrowing axons, the 3β-HSD was re-expressed. Changes in the expression of 3β-HSD mRNA after lesion paralleled those of mRNA coding for the myelin proteins P0 and PMP22 (Fig. 6c). In contrast with cryolesion, the 3β-HSD was not re-expressed 6 or 15 days after nerve transection in the part of the nerve distal to the section, where Schwann cells remained present and proliferate (Fig. 7a). Similarly, P0 and PMP22 mRNA were not re-expressed after nerve transection (Fig. 7b).
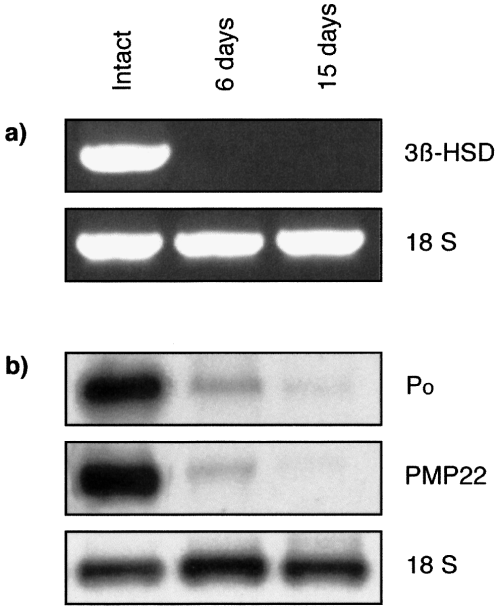
Analysis of 3β-HSD mRNA expression in intact rat sciatic nerves and after transection. RT-PCR analysis of 3β-HSD transcripts (a) and Northern blot analysis of P0 and PMP22 mRNA (b) in the intact nerve 6 and 15 days after nerve transection. Expression of 3β-HSD, P0 and PMP22 mRNAs decreased dramatically 6 days after lesion and were not restored 15 days after nerve transection. Results were compared with ribosomal 18S RNA. Ratio 3β-HSD : 18S: intact, 0.94 ± 0.09; 3 days, 0; 6 days, 0; 15 days, 0 (mean ± SEM; n = 3).
Discussion
Our results demonstrate that expression of the 3β-HSD enzyme and PROG synthesis in Schwann cells are both dependent on a diffusible signal produced by DRG sensory neurons. A direct contact of Schwann cells with axons is not required for the induction of the enzyme, as shown by the use of a two-compartment coculture system, in which Schwann cells were separated from neurons by a permeable membrane. The induction of 3β-HSD expression and activity in Schwann cells shows cell specificity, as other cell types including fibroblasts isolated from sciatic nerves and a hepatocarcinoma cell line had no effect.
The induction of PROG synthesis in Schwann cells by neurons required several days, suggesting a complex process involving multiple steps. Similarly, induction of the gene coding for the myelin P0 and repression of the message coding for the low-affinity NGF receptor p75NGFR by diffusible neuronal molecules have been reported to take several days (Bolin & Shooter, 1993). The diffusible factor(s) involved in the regulation of P0, p75NGFR and 3β-HSD mRNAs still need to be identified. We tried to identify the diffusible neuronal factor. Schwann cells were grown for 2 weeks in the absence and presence of forskolin (5 µm), an activator of the adenylate cyclase. Indeed, many of the effects of axons can be mimicked in vitro by elevating levels of cAMP in Schwann cells (Jessen & Mirsky, 1991) and it has been shown that cAMP upregulates 3β-HSD expression and activity in endocrine glands (Keeney & Mason, 1992). However, a prolonged elevation of intracellular cAMP levels in cultured Schwann cells by forskolin was ineffective in inducing 3β-HSD expression under our experimental conditions (unpublished observation). In addition, the effects of several peptide growth factors, which can be synthesized by neurons (GGF-II, bFGF, PDGF-BB, IGF-I and BDNF), and of neuropeptides, which stimulate steroidogenesis in endocrine glands (ACTH, β-endorphin and angiotensin II), were tested on Schwann cells grown in the absence or presence of forskolin. They were added daily to the culture medium for 2 weeks (10 ng/mL for the growth factors and 10−8 m for the neuropeptides). Under these culture conditions, none of the tested molecules induced PROG synthesis in Schwann cells (unpublished observations).
The influence of neurons on 3β-HSD expression in Schwann cells could also be demonstrated in vivo in the rat sciatic nerve. Present in the intact nerve, 3β-HSD mRNA could no longer be detected by RT-PCR after nerve transection, when Schwann cells lose their contact with axons. After cryolesion, a type of injury which allows axonal regeneration, 3β-HSD mRNA is not present when axons have degenerated and is again expressed when Schwann cells contact the regrowing axons. The time-course of the disappearance and re-induction of 3β-HSD mRNA after cryolesion is similar to the one observed for mRNA coding for the myelin proteins P0 and PMP22. This finding suggests that similar factors may be involved in the regulation of 3β-HSD and myelin protein gene expression, and is in agreement with the idea of an important role of locally formed PROG in myelination. The importance of PROG synthesis in myelin formation has previously been demonstrated by inhibiting the 3β-HSD in the regenerating sciatic nerve with trilostane (Koenig et al., 1995) and by showing that the expression of the enzyme is increased during peak myelin formation in cocultures of Schwann cells and neurons (Chan et al., 1998).
The presence of elevated levels of 3β-HSD mRNA (this study) and of PROG (Koenig et al., 1995) in the intact sciatic nerve suggest strongly that PROG may be involved not only in the formation of new myelin sheaths during regeneration, but may also play an important role in the maintenance of myelin. The maintenance of myelin sheaths may indeed be a dynamic process, as suggested by the observation that components of the myelin sheaths, such as lipids, have a high turnover (Bizzozero & Good, 1991). In agreement with this view, levels of mRNA coding for the major myelin proteins are also elevated in intact nerves.
Whereas diffusible factors are sufficient for the induction of PROG biosynthesis in Schwann cells, direct axonal contact appears to be necessary for 3α,5α-TH PROG formation. This strongly suggests that different steps of neurosteroid biosynthesis may be differentially regulated in Schwann cells. A recent study has shown that 3α,5α-TH PROG is able to increase expression of the myelin proteins P0 and PMP22 in the sciatic nerve of aged rats and in cultured Schwann cells, implying a role for the reduced metabolites of PROG in myelination (Melcangi et al., 1999).
The regulation of neurosteroid biosynthesis in the nervous system remains largely unexplored, except for the first step, the conversion of cholesterol to PREG by the mitochondrial cytochrome P450scc. Indeed, PREG synthesis in the brain and in glial cells is increased by ligands of the peripheral-type benzodiazepine receptor (PBR), which is involved in the regulation of cholesterol transport into the mitochondria (Korneyev et al., 1993; Papadopoulos, 1993). The PBR and its endogenous ligands, the diazepam binding inhibitor-derived peptides, are present in Schwann cells and are increased in the rat sciatic nerve during regeneration, when they may promote PREG and PROG biosynthesis (Lacor et al., 1999). Preliminary findings suggest that PREG may be synthesized from cholesterol in peripheral nerves (Akwa et al., 1993).
In summary, present findings demonstrate a role for cellular interactions in the regulation of PROG synthesis in the peripheral nervous system and provide further support for a physiological role of locally synthesized PROG in normal nerve functions and during regeneration.
Acknowledgements
This work was supported partly by the Association Française Contre les Myopathies (AFM), by a postdoctoral fellowship from the Myelin Project (USA) and Projet Myéline (France) to F. D. and by the European Community (Biomed 2, contract BMH4-CT97-2359). We thank Brigitte Delespierre and Monique Gouézou for excellent technical assistance. We are indebted to Dr Gregg Lemke (Salk Institute, San Diego, CA, USA) for providing the plasmid containing the rat P0 cDNA and Dr Ueli Suter (ETH, Zurich, Switzerland) for the plasmid containing the rat PMP22 cDNA.
Abbreviations
-
- [3H]3α,5α-TH PROG
-
- tritiated 3α,5α-tetrahydroprogesterone
-
- [3H]5α-DH
-
- PROG tritiated 5α-dihydroprogesterone
-
- [3H]PREG
-
- tritiated pregnenolone
-
- [3H]PROG
-
- tritiated progesterone
-
- 3β-HSD
-
- 3β-hydroxysteroid dehydrogenase/5ene-4ene-isomerase
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- DRG
-
- dorsal root ganglia
-
- E18
-
- 18-day-old embryo
-
- FCS
-
- fetal calf serum
-
- NGF
-
- nerve growth factor
-
- P0
-
- protein zero
-
- PBR
-
- peripheral-type benzodiazepine receptor
-
- PMP22
-
- peripheral myelin protein 22
-
- PREG
-
- pregnenolone
-
- PROG
-
- progesterone
-
- Rf
-
- relative migration solvent front
-
- RT-PCR
-
- reverse transcriptase polymerase chain reaction
-
- TLC
-
- thin-layer chromatography.




