Evolution of biometric and life-history traits in lizards (Gallotia) from the Canary Islands
Evolution von biometrischen und Lif-History-Daten bei Eidechsen (Gallotia) von den Kanarischen Inseln
Abstract
enThe aim was to study as to how biometric and life-history traits of endemic lacertids in the Canary Islands (genus Gallotia) may have evolved, and possible factors affecting the diversification process of this taxon on successively appearing islands have been deduced. To that end, comparative analyses of sexual dimorphism and scaling of different body, head and life-history traits to body size in 10 species/subspecies of Gallotia have been carried out. Both Felsenstein's independent contrasts and Huey and Bennett's ‘minimum evolution’ analyses show that male and female snout-vent length (SVL) changed proportionally (sexual size dimorphism not changing with body size) throughout the evolution of these lizards and all within-sex biometric traits have changed proportionally to SVL. Life-history traits (size at sexual maturity, clutch size, hatchling SVL and mass, and life span) are highly correlated with adult female body size, the first two being the only traits with a positive allometry to female SVL. These results, together with the finding that the slope of hatchling SVL to female SVL regression was lower than that of SVL at maturity to female SVL, indicates that larger females reach maturity at a larger size, have larger clutches and, at the same time, have relatively smaller hatchlings than smaller females. There was no significant correlation between any pair of life-history traits after statistically removing the effect of body size. As most traits changed proportionally to SVL, the major evolutionary change has been that of body size (a ca. threefold change between the largest and the smallest species), that is suggested to be the effect of variable ecological conditions faced by founder lizards in each island.
Zusammenfassung
deEs wurde untersucht, wie sich biometrische und life-history Merkmale endemischer Lacertiden der Kanarischen Inseln (Gattung Gallotia) entwickelt haben. Aus den Ergebnissen werden mögliche Faktoren abgeleitet, die den Diversifikationsprozess dieses Taxons auf den sukzessiv entstandenen Inseln beeinflusst haben. Phylogenetische Analysen zum Sexualdimorphismus und zu den Relationen verschiedener Körper-, Kopf- und life-history Merkmale zur Körpergröße bei zehn Arten/Unterarten der Gattung Gallotia wurden durchgeführt. Sowohl Felsensteins “independent contrasts”– als auch Huey und Bennetts ‘minimum evolution’-Analysen haben gezeigt, dass sich die Kopf-Rumpf-Länge (KRL) von Männchen und Weibchen proportional im Laufe der Evolution verändert hat (der sexualdimorphe Größenunterschied verändert sich nicht mit der Körpergröße) und dass sich alle innersexuellen metrischen Charaktere proportional zur KRL verändert haben. Life-history Merkmale (Größe bei Geschlechtsreife, Gelegegröße, Jungtier KRL und Masse, Lebenserwartung) korrelieren stark mit der Körpergröße adulter Weibchen. Allein die Gelegegröße zeigt eine positive Allometrie zur KRL der Weibchen. Das Ergebnis, dass die Steigung der Regressionsgeraden der KRL der Schlüpflinge zu der der Weibchen niedriger war als die der KRL bei Erreichen der Geschlechtsreife zu der der Weibchen, spricht dafür, dass größere Weibchen größere Gelege produzieren mit relativ kleineren Schlüpflingen als kleinere Weibchen. Es gab keine signifikante Korrelation zwischen jeweils zwei life-history Merkmalen, wenn die Körpergröße statistisch eliminiert wurde. Da sich die meisten Charaktere proportional zur KRL verändert haben, ist die Körpergröße die hauptsächliche evolutionäre Veränderung (ca. dreifache Veränderung zwischen der größten und der kleinsten Art). Dies wird auf den Effekt von variablen ökologischen Bedingungen zurückgeführt, denen Gründereidechsen auf jeder Insel ausgesetzt waren.
Introduction
Variation in a specific morphological trait is usually accompanied by variation in other morphological, physiological or behavioural traits, both within species (during development) and between species (Andrews 1982; Emerson and Arnold 1989; Bauwens et al. 1995). Moreover, as a certain set of morphological, physiological and behavioural traits may be correlated with ecological factors such as habitat type, covariation between different traits has been used to explore the relationships between morphological variation and ecology (Losos 1990a; Garland and Losos 1994). On the contrary, several traits may influence survival and reproductive output of the individuals and therefore life-history patterns are intimately coupled to trait variation (Blueweiss et al. 1978). Two life-history patterns have been described as the extremes of a wide range of patterns, at one end, species having a long life span usually have slow growth rates, late maturation and produce a few large young and, at the other end, species having opposite traits (Pianka 1970). These patterns may have many interrelated causal factors such as genetic, developmental, physiological or ecological influences. Comparative studies help to find correlations among life-history traits and associations with environmental variation (Ballinger 1983; Dunham et al. 1988).
Comparative studies of trait covariation have commonly used families or orders as the taxonomic units. The comparison between these higher taxa is usually difficult to interpret as, for example, many life-history differences between the families may obscure the observed relationships (Dunham and Miles 1985; Dunham et al. 1988). Therefore, lower taxonomic units have been considered in an attempt to reveal microevolutionary patterns in specific morphological, behavioural and life-history traits (Carothers 1984; Stearns 1992; Bauwens and Díaz-Uriarte 1997). Comparative analyses of different traits within the family Lacertidae exist (Bauwens et al. 1995; Bauwens and Díaz-Uriarte 1997). In the present contribution a phylogenetic comparison among species within the lacertid genus Gallotia is carried out in order to infer the evolution of biometric and life-history traits.
The genus Gallotia (Arnold 1973), endemic to the Canary Islands, is considered to be a basal group within Lacertidae (Harris et al. 1998). Until very recently, five extant species (G. atlantica, two subspecies; G. caesaris, two subspecies; G. galloti, four subspecies; G. stehlini and G. simonyi, one subspecies) and three extinct species (G. goliath bravoana, G. s. simonyi and G. s. gomerana) had been described (Hutterer 1985; Bischoff 1998). Gallotia stehlini and G. simonyi are large lizards [up to 270 mm snout-vent length (SVL)] and the other species are medium- to small-sized (60–120 mm SVL). Two new large species have recently been discovered, one in the north-west of Tenerife island (G. intermedia, maximum male SVL =150 mm; Hernández et al. 2000) that is genetically very close to the endangered G. simonyi machadoi from El Hierro island (Rando et al. 1997), and another in south-west of La Gomera (G. gomerana, maximum male SVL = 195 mm; Nogales et al. 2001). All species are considered to have a monophyletic origin and are closely related taking into account genetic distances between them (González et al. 1996). Gallotia spp. are heliothermic, have a mostly vegetarian diet supplemented by some insects. Their activity cycle includes highest activity during spring and summer, although they also are active on sunny days of autumn and winter. All species are ground-dwelling, may climb bushes to get food, and live in all types of habitats, from xeric lava fields to densely bush-vegetated areas. All species are sexually dimorphic and a polygynic mating system was suggested for some species (Molina-Borja et al., 1997). However, recent observations point to a polyginandrous system. All species are oviparous, with egg number increasing with female SVL.
The evolution of these lizards must have been tightly coupled to the temporal distribution of emergence of the Canary Islands from the ocean by volcanic activity. As a result of their proximity to Africa, the ancestors of Gallotia probably lived in the continent, and it is hypothesized that they successively colonized each island after its appearance (see Mayer and Bischoff 1991; Thorpe et al. 1994).
Given that only one lizard species (two at most) have lived on each island in the past, one could expect a lesser degree of differentiation between lizards in all islands than in the case of several species living together in each one of them. The latter case has been demonstrated to occur in Anolis lizards from Greater Antilles where many different ecomorphs have developed in islands inhabited by several species (Williams 1983; Losos 1990b; Losos et al. 1997). Another possibility is that several species with different body shapes and/or life-history patterns could have developed as a result of the effect of different ecological factors found by each lizard species on each island. Our general question is related to this matter. Did sexual size dimorphism (SSD), general body shape or life-history patterns change throughout lizard evolution on the islands? For example, SSD could be stronger in larger than in smaller species [as predicted by Rensch's rule (Rensch 1960)], or could be more developed in species inhabiting older islands than in those from younger islands, because of the longer evolutionary time in the first case. The analyses were also aimed at revealing if the Canarian lizards follow or do not follow the same pattern of life-history trait evolution as continental lacertids (Bauwens et al. 1995). Specific goals of the present study are to carry out phylogenetic-based statistical analyses of (1) SSD, (2) relationships between morphological traits and SVL within each sex, and (3) the association of some life-history traits to female SVL.
To test these ideas, we have inferred the way in which traits have evolved by carrying out between-species (phylogenetic) analyses of sexual dimorphism and scaling of several body, head and life-history traits to body size in 10 species/subspecies of Gallotia. Some morphological traits may be important in the behaviour of animals. For example, head size has been considered significant in intramale competition (Carothers 1984; Hews 1990), and hind-limb length (HLL) in running, climbing or antipredatory capacities (Huey et al. 1984; Bauwens et al. 1995). In G. galloti galloti, a significant relationship between some body traits of male contestants and intensity of aggression has been found (Molina-Borja et al. 1998).
To make a comparative study, phylogenetic information has to be taken into account (see Harvey and Pagel 1991; Martins and Hansen 1996). Several methods have been developed to address this problem. Independent contrasts (Felsenstein 1985) takes into account phylogenetic information and calculates weighed differences between trait values. These weighed differences (contrasts) are independent and can be used in standard statistics. We used Felsenstein's method because it provides more reasonable type I error rates when testing pairwise relationships than other methods (Cheverud and Dow 1985). The ‘inferred changes approach’ of Huey and Bennett (1987) (see Martins and Garland 1991; Martins and Hansen 1996) that permits the analysis of trait evolution considering the change from ancestor estimated trait values to that of current species have also been used.
Materials and Methods
Species, number of animals sampled and traits analysed
Specimens of the different species of Gallotia were collected from each island (Fig. 1) during the breeding period (March to July) of 1996–1999. Due to the restricted access to the area where they live and the protected status of the population, only a limited number of specimens of G. s. machadoi were measured.
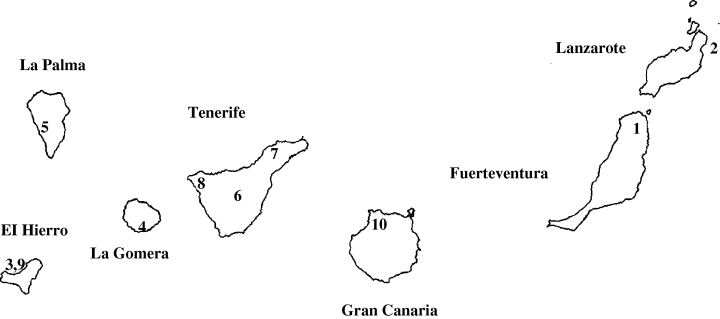
Distribution of the specimens analysed in the present study. (1) Gallotia atlantica mahoratae (Malpaís de la Arena), number of males (m) = 26 and females (f) = 26; (2) G. a. atlantica (Punta Mujeres), m = 25, f = 21; (3) G. caesaris caesaris (Guinea), m = 49, f = 49; (4) G. c. gomerae (Tecina), m = 23, f = 28; (5) G. galloti palmae (Tazacorte), m = 52, f = 30; (6) G. g. galloti (Teide's National Park), m = 44, f = 36; (7) G. g. eisentrauti (Bajamar), m = 29, f = 19; (8) G. intermedia (Teno), m = 17, f = 29; (9) G. simonyi machadoi (Fuga de Gorreta), m = 9, f = 9; (10) G. stehlini (Gáldar), m = 14, f = 16; T. dugesii dugesii (Funchal), m = 36, f = 38
All specimens were captured with tomato and banana baited traps. The following traits were measured in situ with a digital caliper (0.01 mm precision): SVL, pileus width (PW; distance between the rear borders of parietal scales), head depth (HD; height between parietal scale and lower jaw border), fore-limb length (FLL; distance between axilla and longer finger), and HLL (distance between groin and longest finger). After the measurements, all animals were released at the site of capture. Life-history data (clutch size, offspring size, age at maturity, adult life span, egg mass and hatchling mass) were gathered from similar sample sizes of the unpublished data of Molina-Borja and Rodríguez-Domínguez and from the literature (Bischoff 1974; Castanet and Báez 1991; Bannert 1998; Bosch and Bout 1998; Rodríguez-Domínguez and Molina-Borja 1998). For some species or subspecies, no data are presently available for some of the variables and therefore fewer than 10 species were included in some analyses. We considered SVL at sexual maturity for females as the minimum body size of those individuals with oviductal eggs. Sexual size dimorphism was calculated using the index of Lovich and Gibbons (1990): (mean adult male SVL/mean adult female SVL)−1 (see review of Fairbairn 1997). It has been argumented that SSD should be preferably based on asymptotic size (Stamps 1993) although it is often difficult to obtain reasonable estimates of this parameter for free living animals (Stamps and Andrews 1992). For the present analyses only animals above size at sexual maturity were used and, on the other hand, calculations of SSD based on estimates of asymptotic size did not differ appreciably from those obtained with the Lovich and Gibbons's formula.
Data
Phylogenetic analyses
In order to control for non-independence of the data obtained from related species, independent contrasts (Felsenstein 1985) were calculated for all morphological and life-history traits using a phylogenetic tree obtained by applying the neighbour-joining method (Saitou and Nei 1987) to a matrix of genetic distances calculated with DNAdis program (Phylip package, Felsenstein 1986–1993) from 307 mt ADN base pair sequences of different Gallotia species (González et al. 1996). The percentages of supported bootstrapped trees at each node were obtained using the SEQBOOT and CONSENSE programs (PHYLIP package). To estimate ancestral values for the traits and if their inferred changes are correlated along branches of the phylogenetic tree, we also carried out ‘minimum evolution’ analyses (Huey and Bennett 1987, revised in Martins and Garland 1991; example in Garland et al. 1991). We calculated ancestral values using sum-of-squared changes parsimony analysis (PDSQCHP program in Garland's PDAP package).
No genetic information is available yet for the recently discovered large lizard of La Gomera. Therefore, we carried out an extensive exploratory analysis of the relationships between different biometric and life-history traits in the species for which genetic information and biometric data are presently available. For G. intermedia there was only access to mean SVL and PW data (Hernández et al. unpublished data); therefore, this species was only included in analyses of these two traits. For all the other biometric traits, nine species instead of 10 were analysed. From all specimens captured, only sexually mature animals were considered (smallest male having easily evaginable hemipenes and smallest female having enlarged ovarian follicles) for the analyses (corresponding to the sample sizes specified in legend of Fig. 1). The resulting relationships were explored by considering: (1) different ways of calculating genetic distances between the species (Kimura 1980; Jin and Nei 1990); (2) both rooted and unrooted phylogenetic trees; (3) taking or not taking into account an outgroup species (we used data from Teira dugesii from Funchal; Abreu, unpublished data); (4) phylogenetic trees with variable or identical branch lengths (Brownian and punctuational model of evolutionary change, respectively). To reduce uncertainty inherent in using the only available phylogenetic tree, a set of 1000 trees were generated by computer simulation and confidence intervals for slopes of independent contrasts regressions were calculated (Martins 1996). An approximate robustness of the results were obtained in this manner.
Regression and correlation coefficients
The contrasts calculated for each trait were then used to perform regression analysis both within and between sexes. To analyse how different biometric traits scale to body size, regression slopes from standardized independent contrasts were calculated. Log10 transformation was applied to all variables before contrasts were obtained. Within and between sex trait correlations, taking into account the variation in SVL, were analysed calculating contrasts for the different traits and SVL first, and then calculating the residuals from the regressions of pairs of contrasts. To analyse relationships between some lizard and island traits, independent contrasts on body size and island diversity (height) or emergence time were also performed. All contrast regressions were forced through the origin as expressed by Garland et al. (1992). The slopes with the reduced major axis (RMA) method because of the error associated with the measurements being taken was calculated (McArdle 1988; LaBarbera 1989). Significance of the slopes with respect to theoretical values was obtained by the t-test described by Clarke (1980). Significance level was always set at p < 0.05. Ordinary least square (OLS) regression were also calculated for comparison. Independent contrast analyses were carried out with the programs compare (Martins 1997) and pdtree (Garland et al. 1999). Correlations between pairs of traits were calculated from standardized independent contrasts and inferred changes. As individual tests were used to evaluate the significance of correlations, the sequential Bonferroni method with α = 0.10 that is considered a more appropriate error rate when using multiple tests was used (Chandler 1995). pdsimul program (Garland's pdap package) was used to obtain scaled null distributions of correlation coefficients with which to calculate critical values for comparison with empirical correlations of the inferred changes. Non-phylogenetic statistical analyses were carried out with SPSS version 9.0 statistical package.
Results
Biometric and life-history traits showed a great variation range among species analysed (see Table 1). For example, mean male SVL ranged between 62 mm (G. a. mahoratae) and 185 mm (G. stehlini), and SSD index between 0.08 (G. c. caesaris) and 0.31 (G. a. atlantica).
| Species | Sex | Snout-vent length (mm) | SSD index | Pileus width (mm) | Head depth (mm) | Fore-limb length (mm) | Hind-limb length (mm) | Maximum length (mm) | Length at maturity (mm) | Hatchling length (mm) | Clutch size | Age at maturity (months) | Adult life span (months) | Egg mass (g) | Hatchling mass (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G. atlantica mahoratae | m | 62.76 | 7.41 | 7.86 | 20.53 | 33.54 | 69.0 | 52.6 | 24.02 | 1.72 | – | 502 | – | 0.32 | |
| f | 56.78 | 0.11 | 6.18 | 6.04 | 17.6 | 27.57 | 61.5 | 49.2 | |||||||
| G. caesaris caesaris | m | 73.33 | 8.84 | 9.16 | 25.56 | 38.58 | 87.0 | 62.0 | 34.0 | 2.5 | 23 | 61 | 1.21 | 0.94 | |
| f | 67.61 | 0.08 | 7.75 | 7.70 | 22.94 | 34.25 | 77.0 | 57.0 | |||||||
| G. a. atlantica | m | 85.92 | 10.02 | 11.48 | 27.58 | 45.78 | 96.0 | 59.5 | 24.5 | 2.49 | – | 61 | 0.6 | 0.6 | |
| f | 64.88 | 0.31 | 6.98 | 7.24 | 19.58 | 30.97 | 73.0 | 57.0 | |||||||
| G. c. gomerae | m | 96.34 | 11.6 | 11.32 | 32.25 | 48.88 | 111.0 | 77.11 | 33.12 | 3.22 | 23 | 61 | – | 1.022 | |
| f | 83.14 | 0.16 | 9.03 | 8.75 | 26.57 | 41.63 | 92.0 | 73.61 | |||||||
| G. galloti palmae | m | 103.29 | 12.11 | 14.28 | 25.81 | 48.46 | 120.0 | 82.61 | 35.0 | 3.0 | 35 | 73 | 1.42 | 1.1 | |
| f | 88.73 | 0.16 | 10.08 | 11.56 | 20.66 | 40.95 | 100 | 78.11 | |||||||
| G. g. galloti | m | 107.36 | 13.17 | 15.52 | 35.45 | 54.96 | 122.0 | 81.0 | 33.1 | 2.95 | 35 | 73 | – | 1.162 | |
| f | 88.45 | 0.21 | 10.16 | 10.96 | 29.84 | 46.21 | 102.0 | 77.0 | |||||||
| G. g. eisentrauti | m | 115.69 | 13.64 | 17.24 | 39.32 | 60.64 | 138.0 | 92.31 | 35.3 | 3.4 | 35 | 85 | 1.63 | 1.35 | |
| f | 92.34 | 0.25 | 10.52 | 11.93 | 30.01 | 47.73 | 99.0 | 80.61 | |||||||
| G. intermedia | m | 146.8 | 16.7 | – | – | – | 160.0 | 115.71 | 50.42 | 7.42 | – | 1252 | – | 2.552 | |
| f | 136.5 | 0.08 | 14.5 | – | – | – | 154.0 | 114.51 | |||||||
| G. simonyi machadoi | m | 161.44 | 16.02 | 17.77 | 54.91 | 75.28 | 194.0 | 135.0 | 52.9 | 8.6 | 35 | 133 | 5.2 | 3.95 | |
| f | 143.88 | 0.12 | 13.27 | 14.90 | 48.11 | 66.35 | 165.0 | 131.0 | |||||||
| G. stehlini | m | 184.93 | 20.55 | 25.37 | 61.23 | 87.39 | 220.0 | 146.0 | 52.1 | 9.8 | 35 | 145 | – | 2.12 | |
| f | 152.0 | 0.24 | 16.04 | 17.39 | 49.9 | 68.81 | 170.0 | 135.0 | |||||||
| T. dugesii dugesii 3 | m | 71.08 | 8.04 | 7.53 | 24.71 | 36.95 | 81.0 | – | 28.2 | 2.6 | – | – | – | – | |
| f | 60.94 | 0.17 | 6.55 | 5.49 | 20.41 | 29.94 | 67.0 | – |
- 1 Values extrapolated (but not included in the analyses) from the regressions based on independent contrasts between length at maturity and SVL or; 2 other traits to length at sexual maturity.
- 3 Species used as an outgroup in the phylogenetic tree for independent contrasts calculations.
Comparative analyses
Alternative phylogenies
As genetic distances calculated by the methods of Kimura (1980) and Jin and Nei (1990) did not differ, only the first method was used for the following analyses. Results based on rooted (Fig. 2) and unrooted phylogenies were very similar, independently of using original (variable) or unity branch lengths with any type of tree and including or not data from T. dugesii as an outgroup. No significant difference appeared between regression slopes of standardized contrasts based on trees with branch lengths set to 1 in comparison with that of those based on phylogenies with variable branch length, although the coefficient of determination (R2) was somewhat higher for the results from the first tree type. Analyses of confidence intervals for regression slopes (calculated from the computer generated 1000 trees and the method described in Martins 1996) of pairs of trait's independent contrasts showed that the regression models predicted reasonably well the variation of the dependent variables in relation to the independent variable (SVL). For example, mean regression slope of male SVL on female SVL was 0.9936 and 95% confidence interval (0.76 > b > 1.22), variances attributable to phylogenetic uncertainty (varP) and to deviations of the measured species data from the phylogenetic model (varS) were, 0.0138 and 0.0002, respectively. RMA and OLS regression slopes did not vary and regression slopes between any pair of trait contrasts were not significantly different when including or not data from T. dugesii. Considering the uniformity of results independently of the method of analysis used and that the general evolution of Gallotia lizards in the Canaries could be better represented by a punctuational more than a Brownian model pattern (see Discussion), only the results for the contrasts based on rooted phylogenies without an outgroup and with branch lengths set to 1 is presented in this study.
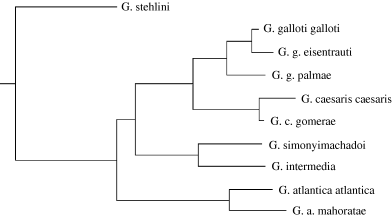
Rooted phylogenetic tree based on genetic distances (from 307 mtDNA base pair sequences) between Gallotia species. Figures at the nodes represent the percentage of supported bootstrapped trees
Contrast analyses and inferred changes of biometric traits
Correlation analyses of independent contrasts and inferred changes showed that all traits were significantly correlated with SVL both in males and females (Table 2a and b, Fig. 3). Scaling analyses showed that there was a positive relationship both between contrasts of male and female SVL (Fig. 4) and between contrasts from each trait and SVL within each sex (Table 2a and b). The slopes of all regressions did not differ significantly from 1 (expected value for isometry), except for male HLL that scaled negatively with SVL (Table 2a). When removing the effect of body size (calculating the residuals of the regression for each trait on SVL), no significant correlation was found between any pair of traits.
| Dependent variable | r | P r | b exp | b OLS | b RMA | P RMA | |
|---|---|---|---|---|---|---|---|
| a | |||||||
| Mean head width | FL1P | 0.984 | *** | 1 | 0.898 | 0.913 | ns |
| ME1P | 0.988 | ** | 1 | 0.894 | 0.904 | ns | |
| Mean head depth | FL1P | 0.974 | * | 1 | 1.039 | 1.068 | ns |
| ME1P | 0.976 | ** | 1 | 1.061 | 1.086 | ns | |
| Mean fore-limb length | FL1P | 0.996 | *** | 1 | 0.998 | 1.001 | ns |
| ME1P | 0.991 | ** | 1 | 0.995 | 0.998 | ns | |
| Mean hind-limb length | FL1P | 0.998 | ** | 1 | 0.880 | 0.881 | ** |
| ME1P | 0.998 | ** | 1 | 0.885 | 0.886 | ** | |
| b | |||||||
| Mean male SVL | FL1P | 0.973 | ** | 1 | 1.042 | 1.070 | ns |
| MElP | 0.990 | ** | 1 | 0.907 | 0.933 | ns | |
| Mean head width | FE1P | 0.978 | ** | 1 | 0.900 | 0.920 | ns |
| ME1P | 0.987 | ** | 1 | 0.907 | 0.932 | ns | |
| Mean head depth | FE1P | 0.981 | ** | 1 | 1.013 | 1.032 | ns |
| ME1P | 0.982 | ** | 1 | 1.03 | 1.049 | ns | |
| Mean fore-limb length | FE1P | 0.981 | ** | 1 | 1.049 | 1.058 | ns |
| ME1P | 0.991 | ** | 1 | 1.048 | 1.058 | ns | |
| Mean hind-limb length | FE1P | 0.995 | ** | 1 | 0.924 | 0.928 | ns |
| ME1P | 0.992 | ** | 1 | 0.93 | 0.938 | ns | |
| Adult life span | FL1P | 0.993 | ** | – | 1.044 | 1.051 | – |
| ME1P | 0.993 | ** | – | 1.041 | 1.048 | – | |
| SVL at maturity | FL1P | 0.999 | ** | 1 | 0.970 | 0.980 | ns |
| ME1P | 0.999 | ** | 1 | 0.950 | 0.953 | ns | |
| Clutch size | FL1P | 0.989 | ** | 0 | 1.665 | 1.683 | ** |
| ME1P | 0.990 | ** | 0 | 1.652 | 1.668 | ** | |
| Clutch size1 | FL1P | 0.973 | ** | 0 | 0.480 | 0.493 | * |
| ME1P | 0.977 | ** | 0 | 0.486 | 0.498 | * | |
| Hatchling SVL | FL1P | 0.946 | * | 1 | 0.800 | 0.845 | ns |
| ME1P | 0.94 | ** | 1 | 0.841 | 0.894 | ns | |
| Hatchling mass | FL1P | 0.945 | * | 3 | 1.833 | 1.939 | * |
| Hatchling mass1 | ME1P | 0.938 | ** | 1 | 0.547 | 0.583 | ** |
- r, correlation coefficient; Pr, significance of correlation coefficient; bexp, expected value of the regression slope under isometry relationship; bOLS, slope of ordinary least squares (OLS) regression; bRMA, slope of the reduced major axis (RMA) regression; PRMA, p-value of the difference between bexp and bRMA. 1Female BW as independent variable.
- *p < 0.05 ; **p < 0.01; ***p < 0.001.
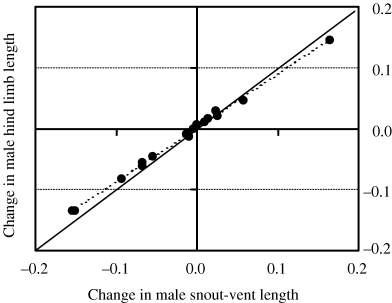
Scatterplot of inferred changes in male SVL and male hind-limb lengths. Points represent changes occurring along each of the branch segments in the phylogenetic tree. Dashed line, regression line adjusted to the points. Continuous line, at 45° angle, represents perfect coadaptation
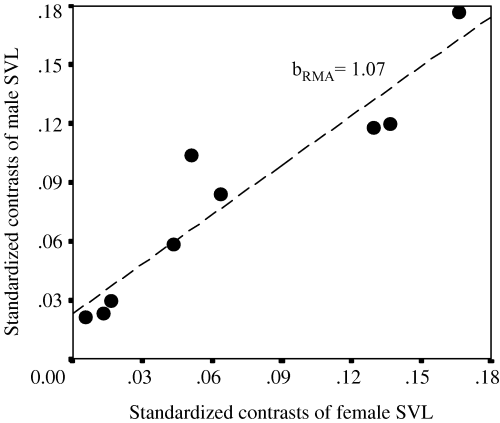
Relationship between standardized contrasts of male SVL and female SVL. Regression line obtained with the RMA method
Comparative analysis did not show any trend in SSD, the slope of male SVL on female SVL not being significantly different from 1 (Table 2b). Contrasts of male body size were correlated with those of island height, although not attaining significance (R2 = 0.24; F = 2.21, df = 7); a positive but non-significant correlation also appeared between contrasts of SSD and those of island emergence time (R2 = 0.32, F = 3.2, df = 7).
Life-history traits
Snout-vent length at maturity, adult life span, clutch size, hatchling size and hatchling mass were all significantly correlated with adult female size (Table 2b and Fig. 5). Corresponding regression slopes were not significantly different from the expected value, with the exception of those of clutch size to female SVL, which was significantly greater than the theoretical value. Moreover, the change in hatchling mass was correlated with a proportionally lesser change in female body mass. Although not significantly different from 1, the scaling exponents for hatchling size and mass to female SVL are <1 (Table 2b).
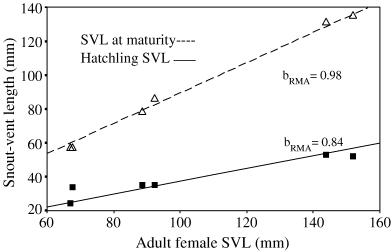
Relationship between hatchling body length, size at sexual maturity to adult female size. Data points are the original mean values for each species and the RMA lines are those obtained from independent contrast regressions
No significant correlation between any pair of traits was found when removing the effect of body size (residuals of these traits to female SVL) (lower cells of Table 3). The highest negative correlations were between SVL at sexual maturity and clutch size, and between the first trait to maximum life span. the highest positive correlations were between maximum life span and clutch size, and between hatchling mass and hatchling SVL.
| SVL at maturity | Clutch size | Hatchling SVL | Hatchling mass | Maximum life span | |
|---|---|---|---|---|---|
| SVL at maturity | – | 0.947** | 0.852 | 0.796 | 0.959** |
| Clutch size | −0.812 | – | 0.895* | 0.818* | 0.998** |
| Hatchling SVL | −0.493 | 0.478 | – | 0.869 | 0.880* |
| Hatchling mass | 0.157 | 0.309 | 0.554 | – | 0.791 |
| Maximum life span | −0.533 | 0.640 | 0.075 | 0.147 | – |
- *p < 0.05; **p < 0.01.
Discussion
Biometric traits
Male SVL changed proportionally to female SVL, that is SSD did not increase with body size, showing that Gallotia species do not follow ‘Rensch's rule’ (1960; review in Fairbairn 1997), agreeing with the results for other lacertids (Braña 1996). This suggests that factors affecting the magnitude of SSD have not changed with the evolution of these lizard species, although there is a moderate amount of variation around the regression line of the two variables (Fig. 4). The main factors considered to affect different body size in each sex are related to sexual selection in males and reproductive selection in females (see review of Fairbairn 1997), a phenomena that may have a differential importance in certain habitats (Butler et al. 2000). However, proximate factors such as sexual differences in growth rate may also explain the difference between male and female body size (Shine 1990). Possible restraining factors for the evolution of a comparatively bigger male body to female body size with increasing length could be increasing costs for developing very large male bodies and/or a selective limit for very small female size.
The interspecies analyses also show that change in all head and most body traits in both sexes have followed linearly the change in SVL (slopes of contrast regressions not significantly different from 1) undergone in Gallotia lineage. However, male HLLs scaled negatively with adult SVL, indicating that larger species did develop comparatively shorter hind limbs. Different HLL to SVL relationships have been found in different taxa and studies (see Table 4). The functional importance of longer hind limbs in lizards has been considered to have greater movement capacity, movement speed (Losos 1990a,b; Christian and Garland 1996), or mating ability (Lappin and Swinney 1999). Therefore, males with longer hind limbs would be better endowed to run faster or to have a greater capacity to fight an opponent. Results extrapolated from the only published data in four Gallotia species (Márquez and Cejudo 1997) show that species having relatively longer hind limbs to their SVL also have higher maximum sprint velocities. Moreover, males of G. galloti use their hind limbs to keep away their opponents during biting bouts of fighting contests (personal observation). On the contrary, hind limbs have been shown to reflect adaptations to specific substrates used by lizards: longer limbs in species of Anolis living on or near the ground and shorter limbs in species living on twigs (Losos et al. 1997). It is not clear as to how comparatively shorter hind limbs in the largest Gallotia are related to particular habitats, as they may walk and climb over different substrates, including plant branches and rocks. However, shorter limbs have been considered to reduce the muscle force needed to keep the joints at equilibrium (Christian and Garland 1996), which could be applied to the largest Gallotia.
| HLL to SVL relationship | Lizard taxa | References |
|---|---|---|
| Negative allometry (larger species have comparatively shorter HLL) | Terrestrial iguanids | Pounds et al. (1983) |
| Negative allometry | Australian agamids | Witten (1985) |
| Negative allometry | Lizards from different phylogenetic groups | Christian (1995) |
| Positive allometry (larger species have comparatively longer HLL) | 22 Species of varanids | Christian and Garland (1996) |
| Positive allometry | Leopard lizards (Crotaphytidae) | Lappin and Swinney (1999) |
| Negative allometry | Gallotia lizards | This study |
With the exception of HLL, a constant proportion between all the other measured traits and SVL has been maintained for each sex during the evolution of Gallotia species in the Canary Islands. The fact that no significant relationship persists between head and limb traits after statistically removing the effect of body size means that the variation of these traits are tightly associated with that of SVL.
Life-history traits: relationship with body size
A correlated evolution of life-history traits and female SVL (and body mass) in Gallotia is shown by our results, reflecting that body size is a major factor affecting these traits, as is the rule in many other species of vertebrates and invertebrates (Blueweiss et al. 1978; Stearns 1992; Marshall and Gittleman 1994).
Although all life-history traits show a positive relationship with female SVL (Table 2b), some relationships have important consequences. While SVL at maturity and adult female SVL change proportionally (similarly to other lacertids; Bauwens and Díaz-Uriarte 1997), clutch size increases disproportionately with female SVL; females of larger species have larger clutch sizes for their body size. However, clutch size increased proportionally less (slope = 0.493) than female mass (a better indication of female clutch capacity). As females from some small species may lay more than one clutch per year, the constraints imposed by small body size seems to have been overcome by increasing the number of clutches per season. An increase of clutch size with female SVL was found in a non-phylogenetic study of other lacertids (Bosch and Bout 1998), but not (slope = 0.233) in a phylogenetic analysis of some scleroglossa lizards (Clobert et al. 1998), indicates differing relationships between these traits within different scleroglossan groups.
On the contrary, the smaller scaling exponents (not significant probably because of the limited number of species in Gallotia) of hatchling size and mass to female body size with respect to their theoretical value (Table 2b) agrees with the general trend for other lacertids (Bauwens and Díaz-Uriarte 1997). Increases of clutch size with female SVL are accompanied by reductions in hatchling size. This is usually interpreted as females being restricted in energy allocation for reproduction, not being able to produce clutches with many offspring and the same hatchling size as those producing smaller clutches (Dunham et al. 1988; Berrigan 1991).
The fact that scaling exponent of sexual size at maturity to female SVL is higher (1.02) than that of hatchling size to female SVL (0.84) means that newborn size is a lower proportion of size at maturity in the larger species and, therefore, the increase in length between birth and maturity is proportionally greater in that species.
Removing the effect of female SVL
All significant correlations between life-history traits disappeared when the effect of female SVL was statistically removed (lower cells of Table 3), indicating that there is no trait covariation independent of body size. The absence of significance for the strongest correlations could be the result of the low number of existing species of Gallotia. The negative correlation between body size at sexual maturity and clutch size suggests that females attaining sexual maturity at a larger size would have a comparatively smaller egg number than those reaching sexual maturity at a smaller size, and this is in agreement with the negative correlation between age at maturity and clutch size found for other lizards (Dunham et al. 1988). The negative correlation of SVL at sexual maturity with hatchling size agrees with the above finding of a lower proportion of hatchling size relative to size at sexual maturity for larger females.
Our data for Gallotia species do not fit the ‘fast–slow’ gradient (Stearns 1983) of life-history traits for other taxonomic groups in the sense that the correlation between clutch size and adult life span is positive instead of negative as in other vertebrates (Harvey et al. 1989), thus agreeing with the results for other lacertids (Bauwens and Díaz-Uriarte 1997). The negative correlations between SVL at maturity and clutch size and between SVL and maximum life span agree with the patterns for mammals and birds (Harvey et al. 1989), but not with that of the other lacertids (Bauwens and Díaz-Uriarte 1997). In the latter case, as only a large species (Lacerta lepida) was included in the analyses, it could explain the above difference with the lizard samples of the present study, that included several large lizards. Large species with ‘slow’ life-history traits would have less ability to compete with smaller species of shorter life duration but relatively larger clutch sizes; this without taking into account other constraints as a need for greater food supply or higher predator pressure in larger species. This could be useful to understand the fate of large (slow life history) species as they were widely distributed in the western islands in the past (Hutterer 1985), but are presently under threat of extinction (except G. stehlini) having been replaced by smaller species. A slow life history has been shown to increase the probability of extinction in some species (Webb et al. 2002).
Final remarks
The results of the present study have shown that, independent of how the precise changes in body size have been throughout the evolution of the Gallotia species in these islands, there has been a correlated change of male and female SVL and of the different head and body traits to SVL in each sex. The evolution of SSD and body proportions may have been limited by high genetic correlation between all species. The primary change which is that of body size suggests that it could have been the main effect of ecological conditions faced by the lizards in the islands. The biggest species only evolved in the more ecologically diverse islands and not in the more xeric Lanzarote and Fuerteventura, possibly because of restricted food and refuge resources on these islands. The magnitude of SSD could be related to ecological factors such as island diversity (height of each island was used as an indirect measure of niche diversity) or evolutionary diversity: time passed since the emergence of each island, as more time would have been available for natural or sexual selection to occur. Although the correlations between contrasts of each of these two traits with SSD was positive, none of them reached significance.
The evolution of Gallotia in the Canaries may have produced different-sized species but with a similar body proportions. The fact that body size has been the main change does not necessarily imply that all the other traits follow its evolution; it could also be that body size is an epiphenomenon of the change in some life-history traits such as a delay in the age at maturity or of ecological features (Dunham and Miles 1985; Bauwens et al. 1995). Moreover, geographical variation analyses (Niewiarowski 1994) of biometric and life-history traits within each island and species could show some adaptations to local conditions as has been the case for other lizard species on islands (Losos et al. 1997).
To sum up, the main conclusions are: (1) the magnitude of SSD has been maintained throughout the evolution of Gallotia; (2) most body traits have changed proportionally to SVL in both sexes; (3) male HLL changed proportionally less than SVL, indicating that larger species have proportionally shorter hind limbs; (4) life-history traits scaled with female SVL, the only one having a significant positive allometry being clutch size; clutch size also increased with female body mass but in a lesser proportion; (5) larger females not only have larger clutches but also have comparatively smaller and lighter hatchlings than smaller females.
Acknowledgements
The authors thank Dirk Bauwens and Emilia Martins for their help with regression and phylogenetic analyses. They also made helpful comments on a first version of the manuscript. E. Martins, T. Garland Jr and J. Felsenstein provided the compare, pdap and phylip program packages, respectively. The authors also thank Dr M. Suárez for her help with the statistics. J.P. Pérez, M. Fleitas and M. Prunell helped in gathering data from G.s. machadoi, G. c. gomerae and G. atlantica, respectively. E. Hernández kindly provided us with data from G. intermedia, J.M. Abreu with data from T. dugesii of Funchal, and A.F. Francisco and N. Marichal contributed with the data obtained from a population of G. g. palmae, and W. Bischoff and Brigitte Bannert for providing unpublished data on hatchlings from G. stehlini and G. c. gomerae, respectively. The Viceconsejería de Medio Ambiente, Autonomous Canarian Government, especially F. Domínguez-Casanova and the Consejerías de Medio Ambiente of all island's Cabildos (local government institutions) are thanked for the corresponding permits to capture the endangered El Hierro's giant lizard, G. s. machadoi, and all the other lizard species, respectively. Guadalupe Acosta provided great help in everyday laboratory assistance and Neil Abrey corrected the English.




