What can we see with the endoscope? Present status and future perspectives
Abstract
The present status and future perspectives in new technologies of image processing and analysis, infrared ray endoscopy and autofluorescence endoscopy for gastrointestinal cancer are presented in this paper. Spectroscopic measurements using an endoscopic spectroscopic system are useful for distinguishing between benign and malignant gastric mucosal lesions, and the histological classification of early gastric cancer is possible on the basis of the spectroscopic characteristics. It is expected that adaptive hemoglobin index color enhancement would be useful for the qualitative diagnosis of early gastric cancer and for detecting specialized columnar epithelium in Barrett’s esophagus in combination with magnifying endoscopy. Our preliminary experience suggess that magnifying endoscopy with a narrow-band imaging system could predict the histological characteristics of gastric cancerous lesions with high accuracy. Recent studies revealed that infrared ray electronic endoscopy is very useful for diagnosing the depth of invasion in early gastric cancer. In addition, it is evident that specific antibodies tagged with the indocyanine green derivative can label cancer cells and can generate a fluorescent signal strong enough to detect small cancers using an infrared fluorescence endoscope. The future development and evaluation of autofluorescence endoscopy are discussed, and we propose a modification to the system, including the excitation lights.
Introduction
New endoscopic technology is needed to improve the diagnosis and treatment of gastrointestinal (GI) cancer. Unlike a fiberscope that directly captures light signals, an electronic endoscope obtains images in the form of electron signals captured through semiconducting elements. Thus, images obtained by electronic endoscopes can be subjected to various ways of image processing and analysis. The important roles of processing endoscopic images are to emphasize lesions for facilitating their detection and qualitative diagnosis, and to improve the accuracy of endoscopic diagnosis by converting color- and structure-based diagnostic information into more objective and quantitative indicators. A way of image processing aimed at emphasizing the features of a given image is called ‘image enhancement’, while that of image processing involving quantitative analysis of a captured image and extraction of its characteristics in numerical parameters for subsequent image reconstruction is called ‘image analysis’. This paper will outline the current status and future perspectives in new technologies of image processing and analysis, infrared ray endoscopy and autofluorescence endoscopy for GI cancer.
Quantification of colors
Efforts to develop endoscopes as a quantitative measurement of colors began in the 1980s. These attempts were primarily directed at combining a spectrometer with an endoscope, and analyzing images obtained using an electronic endoscope. The important requirements to be fulfillled by an endoscope for color diagnosis are: (i) capability for quantitative analysis of colors; (ii) capability for real-time representation of quantitative data; (iii) simplicity of the test method for use during routine endoscopy; and (iv) capability to discern normal from abnormal data.
The spectral characteristics of the GI mucosa are well known to be influenced essentially by blood flow and hemoglobin characteristics. In addition, it is thought that the mechanism producing mucosal color change in diseased tissues might be based on differences in angiogenesis, modulation of the microvasculature in the tissue and density of tissue structure. These changes might affect the scatter characteristics and cause changes of the spectral reflectance, which depend on wavelength. A new endoscopic spectroscopic system (ESS) has been developed by Olympus Optical (Tokyo, Japan) to evaluate the spectral characteristics of tissue, especially the reflectance of various lesions in the GI tract.1,2 The ESS is combined with a sequential electronic endoscope system and a recording system for digital images (Fig. 1). For spectroscopic measurements, the system generates halogen light through a small-diameter probe and guides the reflected light into the spectroscope (Fig. 2). The ESS is worked up within 0.5 s by using a gate signal in a dark period during irradiation of the red, green and blue lights in the sequential electronic endoscope.
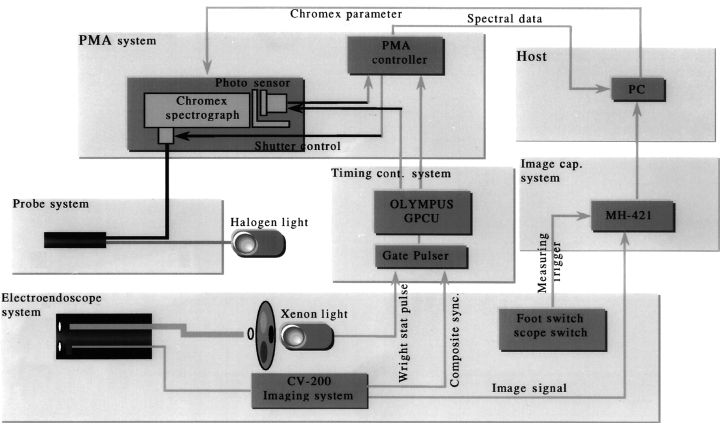
Fig. 1. Endoscopic spectroscopic system. The spectrometer is composed of a spectrograph and sensor controller that are connected to the probe system and the personal computer. The electronic endoscope and the spectrometer system are synchronized by the timing controller. The electric endoscope is connected to the electronic endoscope imaging system and the recorded images are stored in the personal computer as image data. GPCU, gate pulse control unit; MH-421, image compression unit (Olympus); PC, personal computer; PMA, multichannel spectroscope (Hamamatsu Photonics).
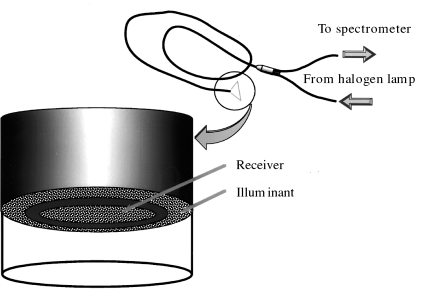
Fig. 2. Measurement probe for the endoscopic spectroscopic system. For spectroscopic measurements, the system generates halogen light through a small-diameter probe and guides the reflected light into the spectroscope.
We examined the feasibility of this system to distinguish between benign and malignant gastric mucosal lesions, and to histologically classify early gastric cancer in 83 cases of gastric mucosal lesions, including 26 cases of early gastric cancer.3 The study revealed significant differences in the spectroscopic characteristics between normal mucosa and early gastric cancer, and between mucosal erosions and type IIc early gastric cancer (P < 0.05). Furthermore, in cases of type IIc early gastric cancer, the spectroscopic characteristics differed markedly between those of well differentiated adenocarcinoma and poorly differentiated adenocarcinoma or signet-ring cell carcinoma, and between those of moderately differentiated adenocarcinoma and poorly differentiated adenocarcinoma or signet-ring cell carcinoma. In the analysis of the degree of differentiation and the wavelengths, not only the spectroscopic characteristics for the hemoglobin absorption range (530–600 nm), but also those for the short-wavelength range (380–450 nm), differed significantly among different histological types. These results indicate that endoscopic-spectroscopic measurements are useful in distinguishing between benign and malignant gastric mucosal lesions, and that the histological classification of early gastric cancer is possible on the basis of the spectroscopic characteristics of the lesions. In the future, we propose to investigate the wavelength characteristics of malignant lesions, and efforts to improve systems based on this knowledge can be expected to lead to the development of endoscope systems with further sophisticated diagnostic capabilities.
Image analysis has been conducted to investigate the vascular density and endoscopic color of early gastric cancer. Furukawa et al.4 quantified the density of mucosal blood vessels in 121 surgically resected cases of depressed-type early gastric cancer by means of computerized image analysis of tissue specimens immunologically stained with monoclonal antibody directed against CD34. They demonstrated that the density of blood vessels in the mucosa was closely related to the endoscopic color, and that differentiated adenocarcinomas often had a high vascular density and appeared red, while undifferentiated adenocarcinomas often had a low vascular density and discoloration. Takemura et al.5 used a similar method to investigate the relationship between the vascular density and the endoscopic color of elevated-type gastric neoplasms (32 early cancers and 26 adenomas). Their data showed that in cases of early cancer, the relative vascular density was very high and the lesions appeared red endoscopically, and that in cases of adenoma, the vascular density was comparable to that of the surrounding normal mucosa, but the lesions nevertheless sometimes appeared red or assumed discoloration due to the influence of the light intensity or other factors operative during endoscopy. It has been well recognized that the color of the GI tract observed during endoscopy is related to the hemoglobin level of the mucosa. Furthermore, as we will mention later in this paper, observation of capillaries on the superficial gastric mucosa has been conducted using a magnifying electronic endoscope, and the microvascular structure of tumorous lesions that is related to the endoscopic color of the lesions is being gradually elucidated.
Adaptive enhancement by image processing
Adaptive enhancement is a way of image processing to enhance some particular structural patterns of endoscopic images, such as those of the microstructure and of the blood vessels visible on the mucosal surface. Its aim is to improve the qualitative and quantitative information contained in endoscopic images by allowing a clearer delineation of changes in the mucosal patterns as represented by the pit pattern diagnosis and emphasizing minute irregularities of the mucosal surface. This function, which allows real-time observation with enhanced structure pattern elements, has already been adopted in the EVIP-230 system (Olympus Optical).
Previous studies2 on the detectability of the pit pattern of colorectal tumors showed that adaptive enhancement using image processing remarkably improved the detectability when compared to ordinary endoscopic observation techniques. Also, in previous studies of upper GI endoscopy, enhancement was found to be useful for the diagnosis of the extent of cancer invasion, evaluation of gastric ulcer scars, and more reliable evaluation of the red color sign of varices. The authors6 have attempted various image-processing techniques during pancreatoscopy using a sequential electronic endoscope and an image converter for the purpose of improvement of the image quality, and reported that adaptive enhancement by image processing emphasized the minute structure of tumors and the pancreatic duct wall, leading to a clearer delineation of the lesions.
IHb color enhancement
Hemoglobin index (IHb) color enhancement is supposed to enhance colors by emphasizing the uneven distribution of the volume of red blood cells, and to present even mild redness more clearly, based on the principle of organ reflectance spectrophotometry. This function has been adopted in the EVIP-230 system. Conventional techniques of IHb color enhancement are likely to make the entire image too reddish. As enhancement is performed uniformly for bright and dark parts, conventional techniques are likely to produce excessively red images if the enhancement is strong and, in addition, with further enhancement, noise is more likely to be produced in part of the image. With the recently developed adaptive IHb color enhancement technique, the intensity of enhancement can be selected for each pigment, corresponding to the brightness level of the pigment under study. With this technique, the intensity of enhancement can be suppressed for the dark parts that do not need to be enhanced intensely, while the appropriately exposed parts can be enhanced intensely. For parts showing halation, the intensity of enhancement might be slightly reduced to minimize halation as far as possible. As a result, by making use of the enhancement technology in which marked changes of contrast can be obtained even with weak enhancement, we can avoid the entire image from becoming excessively red, which facilitates easier observation and suppression of halation-caused white clipping. At present, the clinical significance of this image-processing technique is being evaluated objectively in cases of tumors and inflammatory lesions of the esophagus, stomach and colon.
At our facility, adaptive IHb color enhancement is used for magnifying endoscopy to identify areas of Barrett’s esophagus and to identify specialized columnar epithelium (Fig. 3). It has been shown that the use of adaptive IHb color enhancement produces a red-white contrast that delineates more clearly the superficial structure of Barrett’s esophagus. Specialized columnar epithelium in the esophagus, which has attracted much attention as a possible origin of carcinoma, is difficult to diagnose with an ordinary electronic endoscope. The use of a magnifying endoscope in combination with adaptive IHb color enhancement has been shown to be useful in detecting this epithelium.
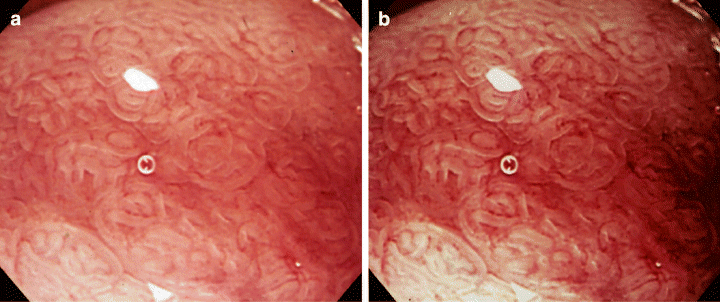
Fig. 3. Endoscopic pictures of specialized columnar epithelium in Barrett’s esophagus taken by magnifying endoscopy (a) and by that in combination with adaptive hemoglobin index (IHb) color enhancement (b). The microstructure and capillaries of the superficial mucosal layer can be observed more clearly by magnifying endoscopy in combination with adaptive IHb color enhancement.
We are also studying whether or not adaptive IHb color enhancement would be useful during ordinary endoscopy for determining the extent of invasion in cases of early gastric cancer. This technique was shown in one study to be quite useful in the diagnosis of the extent of cancer invasion in 67% (10/15) of all cases of depressed-type undifferentiated adenocarcinoma showing discoloration.7 In these cases, the extent of the lesion as determined on the image was identical to the histopathologically determined extent of invasion. In Japan, the incidence of intestinal metaplasia of the gastric mucosa is expected to decrease, accompanied by a decrease in the percentage of well-differentiated adenocarcinoma and increase in the percentage of undifferentiated adenocarcinoma among all gastric cancers. Considering the recent proposal for expanding the indications of endoscopic mucosal resection (EMR) to include undifferentiated adenocarcinoma of 20 mm or smaller in size without ulceration, it is essential for undifferentiated adenocarcinoma to be detected in the early stage and for the extent of tumor invasion to be accurately evaluated. It is therefore expected that adaptive IHb color enhancement would be useful for detecting discolored areas and for the qualitative diagnosis of cancer.
Mucosal hemodynamic imaging
Evaluation of the hemodynamics of the GI mucosa is important. Methods currently available for endoscopic observation of blood flow through the GI mucosa include organ reflectance spectrophotometry and laser flowmetry. In the former, a fiberoptic probe is inserted through the working channel, and the characteristics of the scattered and reflected light at the point where the probe comes in contact with the mucosal surface is analyzed. With these methods, measurement is possible only at the single point where the probe comes into contact with the mucosa. For examination of the 2-D distribution of blood flow, measurements need to be repeated at multiple points. From this point of view, efforts have been made to develop a functional endoscope that would allow visualization of mucosal hemodynamics by the processing of images collected with an electronic endoscope.
It is thought that changes in the mucosal blood flow are closely related to the onset and healing process of mucosal lesions, and that the hemodynamics at the microcirculatory level might be altered by the presence of tumorous lesions. Because the functions of tumorous and non-tumorous blood vessels differ under both physiological and non-physiological conditions, pharmacoendoscopy has been performed, paying attention to the reactivity of the blood vessels to the application of vasoactive substances. The authors followed the time-course of the pharmacodynamic changes in the capillaries of 25 patients with early gastric cancer during magnifying endoscopy after spraying 0.005% norepinephrine onto the mucosa.8 In cases of gastric cancer, the images of the contracted and dilated capillaries in the mucosal layer differed from those of the capillaries in the surrounding normal mucosa, which provided valuable information for qualitative diagnosis. Observation of the capillaries near the lamina propria and muscularis mucosa was useful for the diagnosis of the depth of invasion in five cases of early gastric cancer associated with ulceration in which such diagnosis was difficult by conventional methods.
Attempts have also been made to perform autofluorescent endoscopic diagnosis by spraying 0.02% norepinephrine onto the region affected by gastric cancer.9 The normal mucosa became paler than the tumor after spraying norepinephrine, thereby clarifying the tumor boundary in the autofluorescent image. The combined use of pharmacoendoscopy with light-induced fluorescence endoscopy is a promising means of improving the detection of gastric malignant tumors because it enhances the boundaries of these lesions.
Image processing with a narrow-band imaging system
The narrow-band imaging (NBI) system is composed of a sequential combination of an electronic endoscope and a source of light equipped with new narrow-band filters corresponding to red, green and blue (Fig. 4). The use of this system in combination with a magnifying endoscope yielded highly clear images of the capillaries on the mucosal surface.10 The characteristics of the filters used are: red 485–515 nm, green 430–460 nm, blue 400–430 nm. When needed, these filters might be further adjusted so as to be optimal for observation of the microstructure. The scattering of light in vivo tends to become less as the wavelength of the light gets longer. This implies that light of short wavelengths permeates tissues less deeply, undergoes scattering and absorption near the surface, and is observed as reflective light. On the other hand, light of long wavelengths permeates tissues deeply. The rays in the short-wavelength blue range are absorbed well by hemoglobin in vivo. The NBI system utilizes these characteristics.
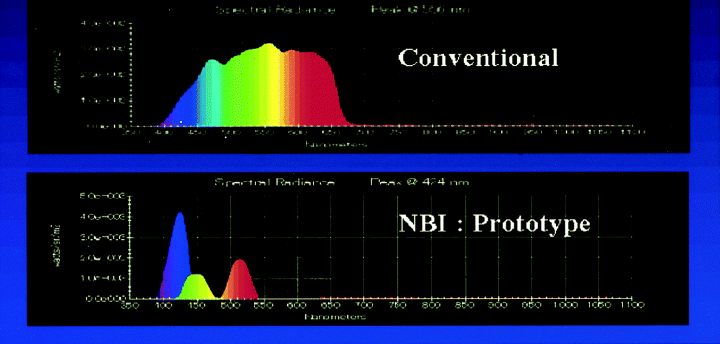
Fig. 4. Spectrum data of narrow-band imaging filters corresponding to red, green and blue in comparison with conventional filters. NBI, narrow-band imaging.
In actual practice, observation with the NBI system is preceded by normal-scale and magnified image observations of the lesion using a magnifying endoscope GIF-Q240Z (Olympus Optical). For magnifying endoscopy, a transparent hood is attached to the tip of the endoscope with the 2-mm top of the hood protruding from the endoscope tip. We previously pointed out that when performing magnifying-endoscopic diagnosis of early gastric cancer, it is important to detect changes in the capillaries in the superficial mucosal layers, as well as those in the microstructure of the mucosa.11 When conventional magnifying endoscopic images and NBI images of type IIc early gastric cancer were compared, the capillary network in the depressed surface was more evident on the NBI images (Fig. 5). Depressed differentiated adenocarcinoma is often characterized by capillary patterns assuming a fine network, while undifferentiated adenocarcinoma is mostly characterized by an irregular corkscrew pattern. These patterns might be capillary patterns specific to each histological type of carcinoma affecting the mucosal layer.12 Both CD31 and CD34 are specifically taken up by the vascular endothelium. Three-dimensional images of microvessels with a laser-scanning microscope from histological specimens are in the process of being compared with endoscopic images with the goal of establishing a new method of diagnosis based on microvascular image analysis.
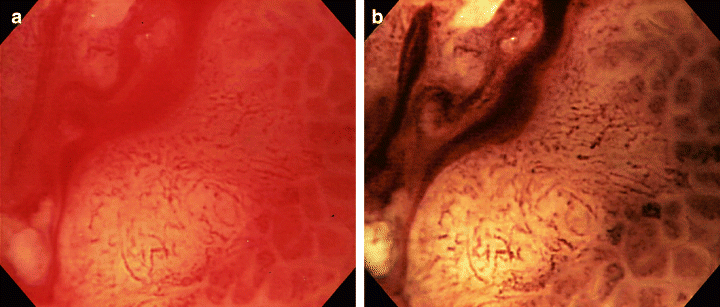
Fig. 5. Endoscopic pictures of type IIc early gastric cancer taken by magnifying endoscopy (a) and by the narrow-band imaging system (b). The lesion was diagnosed histologically as signet-ring cell carcinoma and the depth of cancer invasion was limited to the mucosa.
We have been using the NBI system for: (i) early detection and diagnosis of dysplasia and adenocarcinoma developing from Barrett’s esophagus; (ii) determination of the degree of differentiation, extent of invasion and depth in cases of early gastric cancer; (iii) assistance in the diagnosis of pit patterns of colorectal tumors; and (iv) classifying the stage of inflammatory bowel disease.
Infrared ray electronic endoscopy
The infrared ray electronic endoscopy (IREE) system is composed of a light source, special filters, distally mounted charge-coupled device (CCD) and a forward-viewing electronic endoscope (GIF-Q200 IR; Olympus Optical). Light produced by a xenon lamp first passes through either an infrared cut filter or an 805-nm band-pass filter. The filters can be selected with a switch on the front panel of the light source. Light received by the CCD is converted into electric signals. The signals are then processed by the image analysis equipment and displayed on a color monitor. To more clearly visualize vessels in the mucosa and submucosa, indocyanine green (ICG) is used as a contrast medium. It absorbes light maximally at a wavelength of approximately 805 nm in human serum. After intravenous injection of ICG, deep submucosal vessels can be observed very clearly under near-infrared illumination. In Japan, IREE has been evaluated as one of diagnostic methods such as discrimination of esophageal varices and determination of effectiveness after endoscopic injection sclerotherapy, the depth of invasion in esophageal and gastric carcinoma and so on.13 Iseki et al. evaluated its usefulness for assessing the depth of invasion of 61 depressed or ulcerated gastric cancers.14 As a result, the accuracy for depth of cancer invasion was 89% for mucosal cancers and 89% for submucosal or deeper cancers.
Recently, a new type of IREE with two wavelengths of light source has been developed that penetrates infrared rays from 790 to 960 nm. The new type of IREE shows the presence of ICG and submucosal vesssels more vividly than conventional IREE. Nagao et al. reported that the color after intravenous administration of ICG is related to endoscopic appearance, histology and the depth of invasion, and thereby that the new type of IREE is very useful for diagnosing the depth of invasion in early gastric cancer.15
If a lesion is labeled with a substance that can be detected by an electronic endoscope and if the electronic signals are processed by a computer, advanced endoscopic diagnosis of minute lesions might be expected. To establish such a diagnostic technique, it would be necessary to develop a fluorescent labeling material that would be safe for use in vivo. To this end, ICG N-hydroxysulfosuccinimide ester (ICG-sulfo-OSu) was chosen to serve as the infrared fluorescent-labeling material.16 This substance is a derivative of ICG that can be administered safely to the living body. It has been confirmed that this substance has a protein-binding group, and emits fluorescence when excited by infrared rays. Infrared-fluorescence observations of freshly resected gastric-cancer specimens using anti-carcinoembryonic antigen antibody labeled with this substance have already succeeded.17,18 However, as the intensity of fluorescence emitted from this substance is not adequate, another infrared fluorescent-labeling material, namely, 3-indocyanine-green-acyl-1,3-thiazolidine-2-thione (ICG-ATT) has been developed.19 The same group successfully bound the tumors in vivo with an anti-mucin 1 antibody, as subsequently confirmed by performing immunohistochemistry with a secondary antibody. The antibody labeled with an ICG derivative might therefore be clinically useful in detecting GI microcarcinoma by videoendoscopy.20
Autofluorescence endoscopy
The light-induced fluorescence endoscopy of the gastrointestinal tract (LIFE-GI) system, developed jointly by Olympus Optical and Xillix Technologies (Vancover, Canada), was introduced, and its usefulness in the early diagnosis of GI cancer has been evaluated.21–23 The LIFE-GI is composed of a mercury lamp, a blue excitation filter, a white light source, two high-sensitivity CCD cameras, a color CCD camera for white light, an endoscope and a monitor. First, 437 nm wavelength blue light from the mercury lamp is filtered through the excitation filter and is used to illuminate the tissue through the endoscope. The autofluorescence generated by the tissue is amplified by the high sensitivity CCD camera attached to the end of the endoscope. This camera has two built-in high-sensitivity imaging elements that can capture autofluorescence in the green range (490–560 nm) and in the red range (over 630 nm). The captured fluorescence is processed by the image processor and displayed in colors on the monitor on a real-time basis. The signals are processed so that healthy areas are displayed as light blue. We used the LIFE-GI in 52 patients with 54 lesions (33 early gastric cancers, 21 benign lesions) to assess its ability to detect early gastric cancer.24 Comparing the images with the histological findings, 21 of the 33 carcinomas appeared dark red, 10 had a mixed pattern of dark red and white, and two could not be detected. In 85% of the cancer lesions (28/33), cancer extension was correctly detected. The sensitivity and specificity were 94 and 86%, respectively.
A new generation LIFE instrument system (LIFE II system) is being developed that has a smaller and lighter camera head, comparable with existing fiberscopes, and with higher resolution, contrast and sensitivity. The illumination bundle is attached to a 270-W metal halide lamp filtered to provide the required spectrum of light for each mode. Movement of a single lever on the camera changes modes by switching the filter in the light source and the imaging detectors. Haringsma et al. tested the feasibility of detecting upper and lower early stage neoplastic lesions with the LIFE II system that were not evident by white-light endoscopy (WLE).25 Although there are few lesions that are truly undetectable by WLE, LIFE can highlight subtle lesions without magnification or dye spraying and could therefore significantly reduce the time required for endoscopy. In patients with multiple lesions, the ability to distinguish dysplastic and hyperplastic lesions would allow prioritization of endoscopic therapy, again with an increase in efficiency. In addition, LIFE should also make it possible to target biopsy specimens more accurately as a result of the enhanced visual contrast between a lesion and surrounding normal mucosa. The importance of these advantages should increase with the emergence of new tests for screening for early stage malignancy in the colon.
The background mucous membrane of the stomach is complex and artifacts associated with atrophic gastritis make it difficult to detect and to delineate the extent of minute cancers, even with dye spraying. Well-differentiated carcinomas of the stomach often emitted dark red fluorescence, as in the esophagus and colon, while undifferentiated adenocarcinomas with fibrotic change emitted white fluorescence. The reason for the absence of fluorescence emission in some cases with undifferentiated adenocarcinoma remains unknown. This is a problem that remains to be resolved in the use of fluorescence endoscopy for the screening of gastric cancer. A preliminary study was recently conducted to identify the excitation wavelength at which normal mucosa can be distinguished most easily from cancerous areas, with the ultimate goal of developing a new autofluorescence endoscope.26 Using an imaging spectroscopy system (Olympus Optical), which is a new autofluorescence endoscopy system, the fluorescence spectra was measured at multiple excitation wavelengths in cases of gastric cancer. The spectra were measured serially at multiple points, which seemed to be lying in the borders between normal and affected mucosa. The excitation wavelengths of the light used for this system were 360, 390 and 440 nm. When calculating the data, the fluorescence spectrum for each excitation ray was divided by the reflective spectrum (610 nm). In cases of differentiated adenocarcinoma, good contrast was obtained in both ultraviolet range (360 nm) and with 440 nm blue light used for the LIFE-GI. This finding is consistent with previous reports. Among the cases of undifferentiated adenocarcinoma, the contrast was poor in some cases when 440 nm blue light was used, while better contrast was obtained with ultraviolet excitation light (360 nm). These results suggest that the combined use of conventional blue excitation light (440 nm) and ultraviolet excitation light (360 nm) is useful for the diagnosis of undifferentiated adenocarcinoma, in which contrast enhancement has conventionally proved difficult. It is suggested to modify the system based on these findings.
Conclusion
The present status and recent trends in technologies of image processing and analysis, infrared ray endoscopy and autofluorescence endoscopy for GI cancer have been presented in this paper. A common issue is how to convert subjective, sense-relying endoscopic diagnosis into objective, quantitative endoscopic diagnosis. Some of those technologies are near commercialization while others are still on preliminary trial. It is essential that instrumentation development and clinical evaluation of instruments be promoted efficiently through the joint efforts of both the industrial and academic sectors.
Acknowledgment
This work was supported by a Grant-in Aid (10–37) for Cancer Research from the Minisry of Health, Labour and Welfare of Japan.




