Serial follow-up study of serum testosterone and antisperm antibodies in patients with non-obstructive azoospermia after conventional or microdissection testicular sperm extraction
Summary
Testicular sperm extraction (TESE) combined with intracytoplasmic sperm injection is becoming a first-line treatment even for non-obstructive azoospermia. The current focus of TESE is the identification of seminiferous tubules that contain spermatozoa and minimization of testicular damage. Although microdissection TESE has been introduced as a preferred procedure for sperm retrieval, no serial follow-up studies of testicular damage have been reported. In the present study, we assayed serum testosterone concentrations and for the presence of antisperm antibodies (ASA) for 1 year after conventional multiple TESE or microdissection TESE and compared postoperative testicular damage between procedures. Thirteen patients who underwent conventional multiple TESE and 12 patients who underwent microdissection TESE were included in this study. Serum total and free testosterone concentrations were evaluated before operation and 1, 6 and 12 months after TESE. Serum ASA was also evaluated before and 12 months after TESE. Serum total and free testosterone concentrations in all patients in both groups showed no significant postoperative decrease. A comparison between the two groups of serum total and free testosterone concentrations showed no significant difference (total testosterone, p = 0.2477; free testosterone, p = 0.3098). No incidence of new ASA formation was identified in the present study. In conclusion, TESE procedures cause neither a decrease of serum testosterone nor formation of ASA. Serum testosterone concentration are similar between patients in the conventional multiple TESE and microdissection groups. Therefore, microdissection TESE is safe with respect to testicular damage, particularly for patients with hypogonadism.
Introduction
Testicular sperm extraction (TESE) for intracytoplasmic sperm injection (ICSI) was first introduced for the treatment of obstructive azoospermia in 1993 (Craft et al., 1993; Schoysman et al., 1993). TESE combined with ICSI is becoming a first-line treatment even for non-obstructive azoospermia (NOA). Several TESE procedures have been used, including needle biopsy, open biopsy to obtain one sample (simple TESE) or multiple samples (multiple TESE) and microsurgical operation (microdissection TESE). The current focus of TESE is the identification of seminiferous tubules that contain spermatozoa. In this regard, microdissection TESE should be the best procedure for patients with NOA, as the testis in these patients is known to be very small and heterogeneous (Schlegel, 1999; Amer et al., 2000; Silber, 2000; Okada et al., 2002; Tsujimura et al., 2002). Direct visualization with an operating microscope is a great advantage, because larger, more opaque, whitish tubules, presumably containing more intratubular germ cells with active spermatogenesis, can be identified. Indeed, we have reported an improved spermatozoa retrieval rate by microdissection TESE (42.9%) rather than by multiple TESE (35.1%) for patients with NOA (Tsujimura et al., 2002).
Another current focus of TESE is to minimize testicular damage. Testicular scars, impaired blood flow and devascularization after TESE procedures have been reported (Schlegel & Su, 1997). A decrease in testosterone blood concentrations after TESE procedures have also been reported (Manning et al., 1998; Schill et al., 2003). Testicular damage may be minimized by use of needle biopsy rather than open biopsy. However, in difficult NOA cases, needle biopsy is much less likely than open biopsy to result in the acquisition of rare foci of spermatogenesis (Craft et al., 1997). In one report, 14% of patients with NOA had successful sperm recovery by needle biopsy, whereas 63% of patients had successful sperm recovery by open biopsy (Ezeh et al., 1998). Microdissection TESE has been also reported to be advantageous with respect to postoperative complications, because direct visualization can minimize tissue excision and the risk of inadvertent vascular injury (Silber, 2000). Indeed, microdissection TESE was reported to cause significantly fewer acute and chronic complications than conventional procedures (Amer et al., 2000; Okada et al., 2002). A postoperative endocrinological study also showed that a testosterone decline was more common in patients who underwent conventional TESE than in those who underwent microdissection TESE (Okada et al., 2002). However, no long-term serial follow-up studies of serum testosterone after microdissection TESE have been reported.
Antisperm antibodies (ASA) can be found in fertile and infertile men and in women. Sperm are shielded from exposure to the immune system by the blood–testis barrier. Breach of this barrier by trauma, surgery or infection may result in the formation of ASA, which are formed to antigens on the sperm surface. Although the presence of serum ASA as cause of infertility in humans is somewhat controversial, it has been associated with decreased sperm count, lower motility and decreased normal sperm formation (Menge & Beitner, 1989). Thus, the formation of ASA may be considered a marker of immunological testicular impairment.
In the present study, we investigated the serum testosterone concentration, as well as the prevalence of serum ASA after TESE procedures. In addition, we compared these results between NOA patients who underwent multiple TESE vs. microdissection TESE.
Materials and methods
Patients
Twenty-five patients who underwent TESE procedure at Osaka Police Hospital between January 2000 and April 2002 were included in this study. Thirteen patients underwent conventional multiple TESE and 12 patients underwent microdissection TESE. All patients were diagnosed with NOA on the basis of a complete history, physical examination and endocrinological profile and were scheduled for TESE with sperm freezing. Of the microdissection TESE group, two patients (16.7%) had a 47 XXY karyotype (Klinefelter syndrome). Preoperative patient characteristics are shown in Table 1. Patient age was 35.4 ± 6.4 years (range 29–49 years) for the multiple TESE group and 31.8 ± 4.7 years (range 27–42 years) for the microdissection TESE group. Although testicular size measured by orchidometer did not show a significant difference between groups, Johnsen's score count (JSC), which was evaluated from histological diagnostic specimens obtained at the time of TESE, was significantly lower in the microdissection group (3.0 ± 2.2) than that in the multiple TESE group (5.1 ± 2.3; p = 0.0240). Furthermore, serum follicle-stimulating hormone concentration was significantly higher in the microdissection TESE group (28.1 ± 13.5 mIU/mL) than in the multiple TESE group (14.8 ± 10.3 mIU/mL; p = 0.0108). Likewise, that of testosterone was significantly lower in the microdissection TESE group (2.6 ± 1.1 ng/mL) than in the multiple TESE group (3.5 ± 1.0 ng/mL; p = 0.0429). These histological and endocrinological differences caused a difference of outcome of TESE procedure; testicular spermatozoa were found in seven of 13 patients by undergoing multiple TESE (53.8%) and two of 12 patients (16.7%) undergoing microdissection TESE. In addition, TESE was performed unilaterally for two of 13 patients by undergoing multiple TESE and one of 12 patients by undergoing microdissection TESE, because enough testicular spermatozoa for ICSI were obtained from one testis.
| Multiple (13 patients) | Microdissection (12 patients)a | p-value | |
|---|---|---|---|
| Age (years) | 35.4 ± 6.4 | 31.8 ± 4.7 | NS |
| Testicular size (mL) | 10.1 ± 6.6 | 9.4 ± 5.2 | NS |
| Patient with varicocele (case/%) | 1/7.7 | 4/33.3 | NS |
| Endocrinological profile | |||
| LH (mIU/mL) | 4.7 ± 2.2 | 8.4 ± 6.6 | NS |
| FSH (mIU/mL) | 14.8 ± 10.3 | 28.1 ± 13.5 | 0.0108 |
| PRL (ng/dL) | 7.4 ± 7.8 | 7.1 ± 4.5 | NS |
| E2 (pg/dL) | 21.6 ± 5.4 | 21.4 ± 4.2 | NS |
| TT (ng/dL) | 3.5 ± 1.0 | 2.6 ± 1.1 | 0.0429 |
| Free T (pg/dL) | 12.7 ± 3.1 | 10.6 ± 4.0 | NS |
| Johnsen's score count | 5.1 ± 2.3 | 3.0 ± 2.2 | 0.0240 |
- LH, luteinizing hormone; FSH, follicle-stimulating hormone; PRL, prolactin; E2, oestradiol; TT, total testosterone; free T, free testosterone; NS, not significant. aIncluding two patients with Klinefelter syndrome.
Surgical approach
Multiple TESE. Multiple TESE was generally performed under spinal or local anaesthesia. Through a small vertical incision in the median scrotal raphe, the skin, dartos muscle and tunica vaginalis were opened to expose the tunica albuginea. The tunica albuginea was incised for approximately 4 mm at the upper pole near the head of the epididymis, the midline of the testis and the lower pole opposite the rete testis. Gentle pressure was applied to the testis to extrude a sufficient volume, which was excised with sharp scissors. If no spermatozoa were identified in samples of one testis according to the procedure of spermatozoa retrieval described below, subsequent samples were obtained from the contralateral testis. All testicular samples were approximately the same size (approximately 50 mg per site). The procedure was terminated when spermatozoa were retrieved or when all three samples from upper, middle and lower sites per testis had been examined for the presence of testicular spermatozoa. The tunica albuginea was closed with 3-0 Vicryl, and then scrotal layers were closed separately.
Microdissection TESE. Microdissection TESE was performed under general anaesthesia according to a procedure reported previously (Silber, 2000; Tsujimura et al., 2002). After the tunica albuginea was opened widely along the antimesenteric border, direct examination of the testicular parenchyma was performed at ×20 to ×40 magnifications of an operating microscope. An attempt was made to identify individual seminiferous tubules that were larger than other tubules in the testicular parenchyma. The examination was carried out to include as much of the testicular parenchyma as possible. Small samples (10–15 mg) were excised sharply from the larger, more opaque tubules. The procedure was terminated when spermatozoa were retrieved or when further biopsy was believed likely to jeopardize the testicular blood supply. In all patients undergoing microdissection TESE, six to eight samples were retrieved from one testis.
Methods
Serum total testosterone and free testosterone concentrations were evaluated before TESE and 1, 6 and 12 months after TESE in all patients. Serum ASA also was evaluated before and 12 months after TESE in all patients. Blood samples were collected between 0900 and 1100 h. Free testosterone was measured by analogue ligand radioimmunoassay, and ASA was measured by sperm immobilization assay. The ratio of sperm motilities (%) in the control serum (C%) and the test serum (T%) was calculated and called as the sperm immobilization value (SIV = C/T). When the patient's SIV was more than 2.0, he was judged to have positive ASA. Alterations of serum total and free testosterone concentrations after TESE were compared between the multiple TESE and microdissection TESE groups. In addition, the formation of new ASA after TESE was assayed.
Statistical analysis
Statistical analysis of patient backgrounds between the multiple TESE group and the microdissection TESE group was performed by an unpaired Student's t-test or chi-squared analysis as appropriate (Table 1). Statistical analysis of alterations of serum total and free testosterone concentrations was performed by Friedman analysis. To compare alterations of serum testosterone between groups, statistical analysis was performed by repeated measures anova (Fig. 1). A p-value <0.05 was considered to be significant.
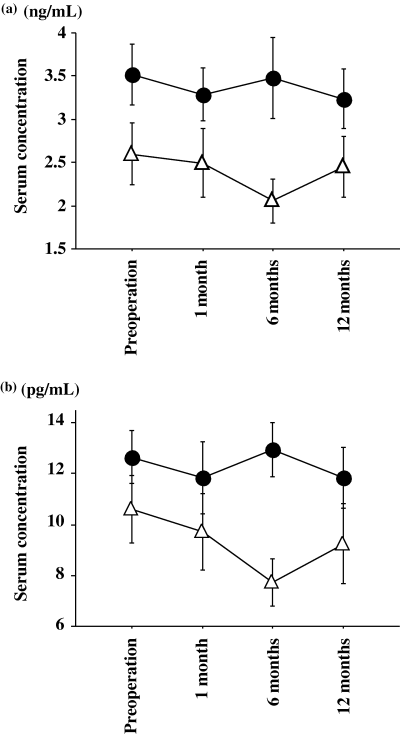
Postoperative alteration of serum total testosterone (a) and free testosterone (b) concentrations in the multiple TESE group and the microdissection TESE group. Significant serum alterations after TESE were not identified in either group (multiple, p = 0.3780; microdissection, p = 0.2625). For free testosterone, significant alterations after TESE were not identified either (multiple, p = 0.6823; microdissection, p = 0.0762). Comparison between the two groups showed no significant differences (total testosterone, p = 0.2477; free testosterone, p = 0.3098). Filled circle, multiple TESE; open triangle, microdissection TESE.
Results
Serum total and free testosterone concentrations in all patients showed no significant postoperative decrease (total testosterone, p = 0.4173; free testosterone, p = 0.5485). Profiles from both groups are shown in Fig. 1. For total testosterone, significant alterations after TESE were not identified in either group (multiple, p = 0.3780; microdissection, p = 0.2625). For free testosterone, significant alterations after TESE were not identified either (multiple, p = 0.6823; microdissection, p = 0.0762). A comparison between the two groups showed no significant differences (testosterone, p = 0.2477; free testosterone, p = 0.3098). Results of the ASA assay are shown in Table 2. None of 16 patients without testicular spermatozoa formed ASA pre- or postoperatively, and two of nine patients with testicular spermatozoa, who were found to have ASA preoperatively, maintained positivity postoperatively. Seven of nine patients with testicular spermatozoa showed no ASA preoperatively, and none of these patients formed antibodies postoperatively.
| Testicular spermatozoa by TESE (case) | Antisperm antibody | |
|---|---|---|
| Preoperation (case) | 1 year after TESE (case) | |
| Present (9) | Positive (2) | Positive (2) |
| Negative (7) | Negative (7) | |
| Absent (16) | Positive (0) | Positive (0) |
| Negative (16) | Negative (16) | |
- TESE, testicular sperm extraction.
Discussions
Currently, several TESE procedures are performed routinely. The complications of these procedures must be evaluated because this procedure, along with ICSI, has become a standard treatment for patients with NOA. Schlegel & Su (1997) used ultrasonography and reported the incidence of testicular scars, impaired blood flow and devascularization in subjects with NOA 3 months after open testicular biopsy. A decrease in testosterone blood concentrations after conventional TESE has also been reported (Manning et al., 1998; Schill et al., 2003). Thus, TESE appears to effect postoperative testicular damage. Recently, microdissection TESE has been introduced as a preferred procedure because of the high testicular spermatozoa retrieval rate and low incidence of operative complications. The excision of testicular tissue is limited, with maximized yield of spermatozoa, and incisions can be made in an avascular region of the tunica albuginea because the subtunical vessels can be identified and avoided by the use of microscope (Schlegel, 1999; Silber, 2000). These advantages decrease the incidence of postoperative testicular damage. Indeed, a comparative study based on testicular ultrasonography within 6 months of TESE showed that microdissection TESE was less invasive than conventional TESE (Amer et al., 2000). Likewise, a significant decrease of serum testosterone was identified in NOA patients who underwent conventional TESE (5.0%) 6 months after operation, but no decrease was identified in the microdissection TESE group (Okada et al., 2002). However, the postoperative observation period in these reports was relatively short, and postoperative investigation was performed only one time at 6 months after operation.
In the present study, we evaluated serum total and free testosterone concentrations several times in NOA patients who underwent and compared the concentrations between the multiple TESE and microdissection TESE groups. Our data clearly show no severe endocrinological damage in these patients. This data is consistent with a previous report based on the long-term follow-up study in terms of serum total testosterone for patients who underwent conventional TESE procedure (Manning et al., 1998). Furthermore, we found that a postoperative decrease in serum testosterone was not identified, regardless of the TESE method used (Fig. 1). In the present study, preoperative testicular impairment was more severe in the microdissection TESE group than in the multiple TESE group; JSC and preoperative serum testosterone concentrations were significantly lower in the former group. Thus, postoperative endocrinological testicular damage is not induced by microdissection TESE, even in patients with severely damaged testicular tissue. Therefore, our data emphasize the safety of the microdissection TESE procedure. In addition, we opened the tunica albuginea antimesenterically at microdissection TESE, as opposed to the transverse incision made by Schlegel (1999). Transverse incision appears to avoid vascular injury because the testicular artery enters the testis posteriorly, beneath the epididymis at the mid-pole, continues inferiorly to the lower pole and then courses superiorly along the anterior surface, where it gives rise to transverse branches that supply the parenchyma. However, a wide antimesenteric incision as reported by Silber (2000) allows extensive visualization of the testicular tubules at microdissection TESE. We believe that it is possible to avoid vascular injury by visualization and careful investigation of subtunical vessels by microscopy, even with the use of an antimesenteric incision of the tunica albuginea.
Correlation between the presence of ASA and infertility remains controversial, although a relation has been reported (Menge & Beitner, 1989). Several aetiologies of ASA formation have been described, including cystic fibrosis (Vazquez-Levin et al., 1994), vasectomy (Jarow et al., 1994), childhood inguinal herniorrhaphy (Matsuda et al., 1992), varicocele (Gilbert et al., 1989) and genital tract infection (Mazumdar & Levine, 1998). It was previously reported that needle or open testicular biopsy did not result in the formation of new or increased levels of ASA in the seminal fluid or serum when patients were tested 6 months postoperatively (Harrington et al., 1996). In the present study, two of 25 patients (8.0%) were found to have ASA preoperatively, and this rate is consistent with that in previous reports (Harrington et al., 1996; Westlander et al., 2001). None of the patients without testicular spermatozoa formed ASA pre- or postoperatively and patients with testicular spermatozoa who were found to have ASA preoperatively maintained positivity postoperatively. Seven patients with testicular spermatozoa, who were not found to have ASA preoperatively, did not form ASA postoperatively, indicating that TESE does not induce the formation of ASA postoperatively. Thus, both the multiple TESE and microdissection TESE procedures are considered to be safe with respect to immunological testicular damage.
In conclusion, TESE procedures cause neither a decrease of serum testosterone nor formation of ASA. Alterations of serum testosterone concentrations are similar between the conventional multiple TESE group and the microdissection TESE group. Microdissection TESE in which the tunica albuginea is opened antimesenterically is a safe method, particularly for those patients with low serum testosterone concentration.




