Ethnic-specific Pathways to Obesity-related Disease: The Hispanic vs. African-American Paradox
Obesity is a well-known risk factor for multiple chronic diseases, including diabetes, heart disease, fatty liver disease, and some forms of cancer. The mechanisms of these relationships are unclear and may be specific for each disease. The most likely explanations of why increased body fat causes multiple health problems include the mediating roles of hyperinsulinemia and insulin resistance, body fat distribution, including ectopic fat deposition in peripheral tissues, and inflammatory mediators. Prior work in this area suggests that there are distinct ethnic differences in these obesity-related phenotypes that are evident early in life, and may help explain ethnic differences in the incidence and prevalence of obesity-related disease outcomes. The purpose of this article is to summarize these differences specifically in Hispanics and African Americans. The focus on these two ethnic groups is based on (i) they are the two largest ethnic minority groups in the United States and (ii) they share a similar propensity for obesity and insulin resistance, but markedly different profiles for obesity-related disease outcome, creating an informative comparative contrast. These comparisons yield two different profiles that could represent different pathways to obesity-related diseases.
The “Hispanic vs. African-American Paradox”
The tendency for Hispanics to be at lower risk of chronic diseases has been long known and has led to the term “Hispanic paradox” (1). This terminology has been used to describe the tendency for Hispanics to have lower than average rates of some chronic illnesses despite the fact that many live in relatively poor social or economic conditions (1). Reference to the “Hispanic paradox” originated from a 1986 article by Markides and Coreil, The health of Hispanics in the southwestern United States: an epidemiologic paradox (2). The main focus to date on examining the basis of this concept has been on aspects of culture, socioeconomic status, and the environment, but increasing evidence points to a possible biological basis.
Examination and interpretation of the “Hispanic paradox” is becoming increasingly relevant to ethnic disparities in obesity-related diseases. For example, Hispanics have lower all-cause and cardiovascular mortality than African Americans and whites, despite similar propensity for diabetes and obesity. In Table 1 we have summarized some key epidemiological data from whites, African Americans, and Hispanics. However, some studies and meta-analysis have challenged the basis of the Hispanic Paradox. For example, in a meta-analysis (3), the lower mortality in Hispanics was explained by migratory factors and other experimental confounders such as selection bias and reporting errors. In another analysis from the San Antonio Heart Study, there was no evidence to support lower mortality in Mexican Americans compared to non-Hispanic whites (4). In addition, mortality estimates by ethnicity may be inaccurate due to misclassification of ethnicity on death certificates and differences in data from foreign-born and native-born Hispanics (4).
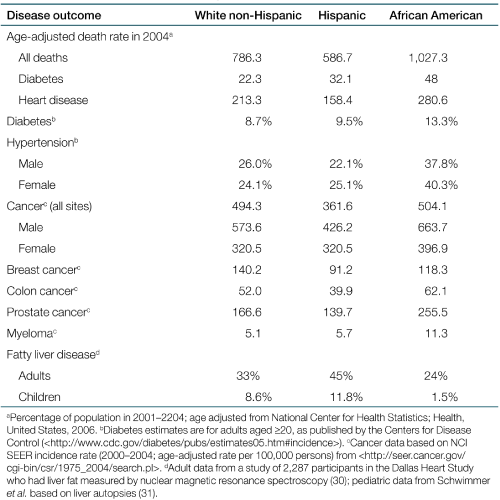 |
A major limitation in both of the abovementioned studies that challenged the notion of the “Hispanic paradox” is that the comparison groups were non-Hispanic whites. Non-Hispanic whites differ significantly from Hispanics in both rates of obesity and degree of insulin resistance. Therefore, a more appropriate comparison group might be African Americans, who have similar propensity for overweight and insulin resistance. Although only a few studies have directly compared these two groups, they highlight key differences as summarized below. In addition, epidemiological observations, summarized on Table 1, support the concept of distinct differences in obesity-related disease outcomes between Hispanics and African Americans despite the fact that both groups have increased propensity for obesity and similar degrees of insulin resistance (5). Thus, at least in terms of the discussion of obesity-related observations, it may be more useful to describe this paradox as a “Hispanic vs. African American paradox.” As reviewed in Table 1, African Americans have increased risk of certain forms of obesity-related cancers and hypertension, whereas for some outcomes, Hispanics appear “protected.” The two most striking disparities that emerge from this comparison are: (i) the higher prevalence of prostate cancer in African Americans whereas Hispanics appear “protected” and (ii) fatty liver disease which reflects an opposite pattern, with Hispanics at higher risk and African Americans appearing to be “protected” (Table 1).
Prior work from our group, and others, points to a potential biological basis that might explain why obesity-related outcomes differ dramatically between Hispanics and African Americans. Prior work in this area focusing on ethnic differences in children has been especially useful because it has allowed an examination of ethnic differences in the absence of some of the usual confounders that may complicate studies in adults, including, but not limited to, smoking, alcohol consumption, and sex steroid hormone differences. In the following sections, we summarize some of the key ethnic differences in obesity-related phenotypes between African Americans and Hispanics that suggest a possible biological basis to explain the ethnic disparities that exist in obesity-related disease outcomes in these two ethnic groups.
Insulin resistance, hyperinsulinemia, and type 2 diabetes
Obesity is the strongest factor contributing to insulin resistance and hyperinsulinemia, and this is evident early in life (6,7,8,9). One of the leading hypothesis explaining why “fat is bad” relates to the role of insulin resistance and hyperinsulinemia as the mediating link between obesity and disease risk. Insulin is a critical hormone for regulating metabolism and its concentration in the circulation is carefully coordinated. Its levels vary acutely in response to glucose and meal consumption and therefore assessment and interpretation of fasting levels is extremely limiting. It has long been known that African Americans tend to be hyperinsulinemic (at least compared to whites), and this is also evident during childhood (10). This greater hyperinsulinemia in African Americans is even more striking when insulin is examined in response to glucose. In previous studies in children we have shown that the insulin response to either oral (11) or intravenous (12) glucose administration is two to three times higher in African Americans compared to whites. These observations are independent of any ethnic difference in body composition or fat distribution and demonstrate profoundly higher levels of insulin in the circulation after oral or intravenous glucose administration, and presumably meal ingestion as well. We have previously shown that this elevated insulin response is partly due to higher insulin secretion from β-cells and lower insulin clearance from the liver in African Americans (13). In turn, we have also shown that the higher insulin in African Americans is also associated with higher levels of estradiol in the circulation (14).
We have also previously shown in children that African Americans and Hispanics are equally more insulin resistant than whites, independent of differences in adiposity (5). Interestingly, though, the compensatory response to this similar degree of insulin resistance is quite different in African Americans vs. Hispanics (5). In response to the same degree of insulin resistance, African Americans increase circulating insulin levels by both an increase in β-cell secretion (first-phase secretion in response to intravenous glucose) and a reduction in liver insulin clearance. Hispanics on the other hand rely solely on β-cell compensation to increase insulin through a secretory response (especially the second-phase insulin response). These observations raise the question as to whether this difference in compensation to obesity-related insulin resistance demonstrates a mechanism whereby African Americans can conserve β-cell function as a mechanism to protect β-cells from prolonged exposure to insulin resistance. This compensation however comes at the expense of higher insulinemia in African Americans and does not necessarily protect this group from type 2 diabetes (prevalence of type 2 diabetes is higher in African Americans compared to Hispanics; Table 1). Thus, there may be separate pathways from obesity to type 2 diabetes, one involving an eventual overwhelming of β-cell compensation leading to a failure in insulin secretion (more likely in Hispanics) and another involving an eventual failure of the liver to reduce liver clearance to maintain high insulin levels (more likely in African Americans).
The IGF-1 pathway and risk of obesity-related cancers
Obesity and hyperinsulinemia are associated with increased risk of certain types of cancer, especially breast (postmenopausal), endometrial, prostate, and colon (15). Although the mechanism of this connection is not known, one of the leading candidates is the pathway leading from obesity to hyperinsulinemia and the IGF-1 system (16). IGF-1 is a growth factor that is strongly related to growth hormone levels and has insulin-like properties and functions. IGF-1 also stimulates the differentiation and proliferation of myoblasts and therefore has been implicated in carcinogenesis. Given some striking examples of disparities in some obesity-related cancers between Hispanics and African Americans (Table 1), we have become interested in exploring obesity-related differences in IGFs across these ethnic groups. In agreement with the other studies (17), we have shown that the IGF profile during childhood maturation points to a higher free IGF-1 through childhood growth and pubertal development in African Americans compared to whites (18). In adults, we have shown that in African Americans there is a progressive increase in IGF-1 levels with increasing obesity status compared to a decline in IGF-1 with increasing obesity in Hispanics (19). Although there are mixed findings in the literature when examining the relationship between IGF-1 levels and obesity (20), there is a distinct inverse relationship in Hispanic children (21). These observations raise some interesting questions: (i) is there an obesity-protective mechanism in Hispanics whereby IGF is reduced with increasing adiposity? (ii) Is the higher IGF-1 profile in African Americans a result of the chronic hyperinsulinemia, especially in response to glucose as discussed above, and does this in turn possibly explain the higher incidence of some forms of cancers in African Americans compared to Hispanics? This scenario is a viable hypothesis to explain, for example, the ethnic disparity in prostate cancer, long been known to be higher in African Americans (Table 1).
Visceral fat and ectopic fat
Visceral fat (adipose tissue inside the abdominal cavity) has long been hypothesized to be one of the major factors linking increased obesity to increased disease risks (22). However this hypothesis is not universally accepted (23), and our own studies in children have led to mixed results. Cross-sectional (7) and longitudinal studies in white and African-American children fail to show a significant independent effect of visceral fat on insulin sensitivity (24,25), whereas studies in Hispanic children showed a small, but significant and independent effect of visceral fat on insulin sensitivity (26). Paradoxically, it has also been shown that African Americans, who are at increased risk for obesity-related diseases, especially cardiovascular disease, have lower visceral fat, and this is already evident before puberty, both cross-sectionally (27), and longitudinally, with a lower growth-related increase in visceral fat in African Americans compared to whites (28). More recent studies have also shown that ectopic fat, spillover of triglycerides into peripheral tissues and organs, such as muscle and liver is also an important factor linking obesity to increased disease risk (29). Other studies have similarly shown that ectopic fat deposition, in muscle and liver, also vary by ethnicity in the same way as visceral fat. The largest study of hepatic fat accumulation measured hepatic fat fraction by nuclear magnetic resonance spectroscopy in 2,287 participants in the Dallas Heart Study (30). This study found that fatty liver disease was highest in Hispanics (45%) and lowest in African Americans (24%). Obesity, insulin resistance, or alcohol intake did not explain this striking ethnic difference. This ethnic difference is also seen in the pediatric population where a study of liver autopsy data from 742 children aged 2–19 years showed that 13% of all subjects had fatty liver disease (defined as ≥5% of hepatocytes with macrovesicular fat), with the prevalence increasing with age and obesity status (31). However, reflecting the adult data, the prevalence (after adjusting for age and obesity) was highest in Hispanics (11.8%) and lowest in African Americans (1.5%). Moreover, one other recent study in adolescents found undetectable levels of hepatic fat and lower muscle fat accumulation in obese African American compared to whites and Hispanics (32). Collectively, these studies show a consistent pattern of lower visceral and ectopic fat accumulation in African Americans compared to Hispanic subjects.
Adipose tissue biology
Increasing evidence is beginning to suggest that differences in body fat pattern and accumulation may result from fundamental differences in adipose tissue biology. The increase in body fat content with obesity can occur by either an increase in adipocyte cell size or number or by the spillover of triglycerides to ectopic tissues as described above. When adipocyte cell size increases with progressing obesity, it is an indication of the inability of adipocytes to expand in number to accommodate the extra triglyceride accumulation (29). Increased adipocyte cell size is also related to greater insulin resistance and predicts the development of type 2 diabetes independent of total adiposity (33). Larger adipocytes have also been shown to be associated with more lipid deposition in visceral and hepatic tissues (not muscle), and this may contribute to insulin resistance (34). Furthermore, it is now also evident that adipose tissue is infiltrated with macrophages (35). One animal study has shown that accumulation of excess body fat in response to excess caloric intake, leads to increasing fat cell size and then to adipocyte death, with the excess fat instead deposited in the liver (36).
Despite the important role that adipose tissue biology appears to play in the link between obesity and related disease risk, there is a lack of studies examining potential ethnic differences. Some studies have compared adipocyte cell size in African American and whites, but have not shown any difference in subcutaneous abdominal or gluteal adipocytes from obese women (37). There are no data in the literature comparing ethnic differences in adipose tissue biology in Hispanics, and the potential relationship between adipocyte cell size and spillover of triglycerides to other ectopic storage depots like liver and pancreas. One possible mechanism is that Hispanics may have larger fat cells than African Americans that are more likely to die due to greater macrophage infiltration, thus leading to the greater likelihood of ectopic fat accumulation in Hispanics. Another possible hypothesis is that the higher obesity-related IGF-1 in African Americans may contribute to a greater likelihood for adipocyte proliferation during energy surplus (38), leading to less likelihood for spillover of fat into ectopic depots, with the opposite scenario in Hispanics (lower obesity-related IGF-1 profile). Thus, differences in the obesity-IGF pathway and adipose differentiation/growth factor pathways may also elucidate mechanisms explaining ethnic differences in obesity-related disease profile. These hypotheses might explain the disparity of lower fatty liver disease in African Americans compared to Hispanics and merit further investigation.
Summary and conclusions
There are distinct obesity-related phenotypic differences between African Americans and Hispanics that suggest distinct profiles and possible pathways to obesity-related disease outcome. These differences and hypothetical pathways are depicted on 1. Obese Hispanics seem more prone to an ectopic fat pattern, and this might be driven by greater fat cell size, greater likelihood of adipocyte macrophage infiltration and cell death, and decreased capacity for fat cells to differentiate, possibly due to the lower obesity-related IGF-1 profile. These observations would explain the higher prevalence of ectopic fat-related obesity diseases in Hispanics such as increased fatty liver disease, and perhaps type 2 diabetes through ectopic deposition of fat in the pancreas contributing to β-cell failure. On the other hand, obese African Americans seem more prone to some forms of obesity and insulin-related cancers compared to Hispanics (39), and less likelihood of ectopic fat conditions such as fatty liver disease. These differences could be driven by the much higher obesity-related hyperinsulinemia (especially in response to glucose) and IGF-1 profile in African Americans. These suggestions and pathways are summarized in 1, and are offered in an attempt to unify the many findings and differences that we, and others, have observed to help explain the interesting paradox in obesity-related disease outcomes between Hispanics and African Americans. Although we have focused, for the purposes of this review, on the interesting paradox between Hispanics and African Americans, it seems just as likely that there will be just as much interindividual variation in the pathways explaining the causes and consequences of obesity. Gender is also likely to be a contributing factor. This seems especially important because there does seem to be ethnicity by gender differences in obesity prevalence. For example, in both children and adults, the prevalence of obesity/overweight is lower in African American males (compared to both white and Hispanic males), and higher in African American females (40). The causes and consequences of obesity are clearly different in different people, and much more work is needed to establish the specific mechanisms linking obesity to the various disease outcomes. These mechanistic issues are fundamental to understanding the basic pathophysiology of why increased body fat is related to poor health outcome, and ultimately will have widespread implications for the application of more individualized prevention and treatment approaches.
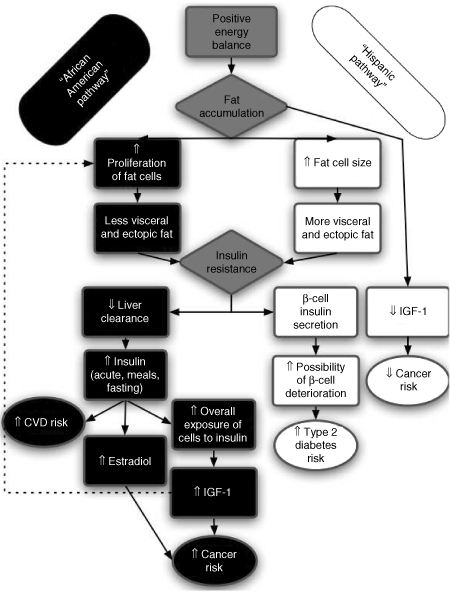
Hypothesized pathways linking obesity-to-disease outcome showing differences in African Americans and Hispanics.
Acknowledgment
This work was supported by the USC Center on Transdisciplinary Research on Energetics and Cancer (U54 CA 116848), RO1 DK 59211, RO1 HD 33064, and the Dr Robert C and Veronica Atkins Foundation.
Disclosure
The author declared no conflict of interest.





