ACE Inhibition and AT1 Receptor Blockade Prevent Fatty Liver and Fibrosis in Obese Zucker Rats
Abstract
Objective: Non-alcoholic steatohepatitis (NASH), which is a common liver disease in industrialized countries, is associated with obesity, hypertension, and type-2 diabetes (metabolic syndrome). Since angiotensin II (ANG II) has been suggested to play an important role in liver inflammation and fibrosis, the purpose of this study was to investigate whether therapy against renin-angiotensin system (RAS) may provide some beneficial effect in liver of an animal model of metabolic syndrome.
Methods and Procedures: For 6 months, obese Zucker rats (OZRs) were treated as follows: OZR-group, OZR + Perindopril (P) group, OZR + Irbesartan (IRB) group, OZR + Amlodipine (AML) group, and lean Zucker rats (LZRs) group as a control. Livers were evaluated by immunohistochemistry techniques using corresponding antibodies.
Results: All treated groups showed a similar reduction in blood pressure compared to untreated OZR. Therapy either with IRB or P improves insulin sensitivity and reduces hepatic enzyme level with respect to untreated OZR. Conversely, AML failed to modify both parameters. Untreated OZR displayed higher hepatic ANG II levels and steatosis together with a marked increase in tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and transforming growth factor-β1 (TGF-β1) level compared to LZR. Following RAS inhibition either by P or IRB, a significant reduction (P < 0.01) in the immunostaining of TNF-α, IL-6 and TGF-β1 compared to untreated OZR was observed.
Discussion: These results indicate that ANG II expression is increased in the liver of these animals with steatohepatitis. Furthermore, RAS control by either angiotensin-converting enzyme inhibition or AT1 receptor blockade seems to provide a beneficial modulation concerning the inflammatory response to liver injury in this model. Consequently, blockade of RAS could be a new approach to prevent or to treat patients with NASH.
Introduction
Non-alcoholic steatohepatitis (NASH) is commonly associated with obesity, type 2 diabetes, dyslipidemia, and high blood pressure (1,2,3). Nevertheless, numerous studies have evaluated the pathogenesis of NASH employing non-diabetic animal models. Therefore, the significance of metabolic syndrome, which includes obesity, type 2 diabetes, dyslipidemia, and arterial hypertension in the development of NASH, has not been clearly elucidated.
Recently, NASH has been recognized as a major cause of liver fibrosis and it is included in a disease spectrum ranging from a simple steatosis to advanced fibrosis and cirrhosis (2,3). Although the pathogenesis of NASH is not well understood, the most important theory proposed to explain the development of this disease is the “two-hit hypothesis” (4). Consistent with this theory, the first “hit” is the fat accumulation within the liver; this is associated with an “insulin resistance state.” Then, the hepatic steatosis development into NASH is related with injury caused by oxidative stress and inflammatory cytokines (the second hit) (2). As a result, abnormal cytokine production within the liver may be playing an essential role in the pathogenesis of NASH.
There is accumulating evidence that renin-angiotensin system (RAS) does not only play an important role in the regulation of systemic hemodynamic but is also involved in hepatic inflammation and fibrogenesis (3,5,6,7). Angiotensin II (ANG II) is recognized to induce hepatic inflammation and to stimulate a range of fibrogenic action, including cell migration, cell proliferation, secretion of proinflammatory cytokines, and collagen synthesis predominantly throughout AT1 receptor (6). Furthermore, several reports indicate that ANG II is able to be generated by different cells in a variety of tissues, and its production is activated in certain pathological states associated with tissue repair (8). The mechanism postulated for fibrotic actions of ANG II seems to involve an important mediator of fibrous tissue formation such as transforming growth factor-β1 (TGF-β1) (3,9). In different experimental models of fibrotic process, our group as well as others have showed that the inhibition of RAS significantly attenuates TGF-β1 expression and fibrosis in heart (10), kidney (8), and liver (5).
Recently, we have demonstrated that obese Zucker rat (OZR) show excessive fat accumulation in the liver together with an increased expression of ANG II (11), suggesting that local ANG II generation may contribute to the pathogenesis of NASH. Therefore, due to its biological properties, RAS is an important target to prevent fibrosis in chronic inflammatory states.
Consequently, the aim of this study was to evaluate whether therapy against RAS, either by angiotensin-converting enzyme (ACE) inhibition or by AT1 receptor blockade, would provide some beneficial effect in liver of OZRs, a well-characterized model of metabolic syndrome.
Methods and procedures
All the experiments were approved by the Hospital Alemán Ethic Committee and the Teaching and Research Committee and followed the National Institute of Health Guide for the Care and Use of Laboratory Animals. Ten-week-old male Zucker rats, obese (fa/fa) (OZR) and lean (Fa/fa) (LZR), (Charles River Laboratories, Wilmington, MA) were housed in individual cages at 21 ± 2 °C and a 12 h. light/darkness cycle (7 am to 7 am). Rats were divided into five groups: OZR group (G1, n = 10), OZR + OZR + Perindopril (P) group (G2, n = 10), OZR + Irbesartan (IRB) group (G3, n = 10), OZR + Amlodipine (AML) group (G4, n = 10) and LZR group (G5, n = 10). All the animals were allowed to drink tap water, and fed standard rat chow (16–18% protein, Cargill-Argentine) “ad libitum.”
During 6 months, G2 received a daily dose of 3 mg/kg of P by gavage between 11.00 am and 11.30 am; while G3 received IRB 50 mg/kg and G4 AML 3 mg/kg, G1 and G5 received an equal volume of vehicle (normal saline solution) throughout the experiment. In order to obtain equivalent control of blood pressure, the dose of antihypertensive agents was chosen based on previous studies (10,11,12,13,14,15). Dose treatment was adjusted each week based on the calculation of the body weight for each animal. After 6 months of treatment, all rats were killed by subtotal exsanguination under anesthesia (sodium thiopental 40 mg/kg, intraperitoneal), according to institutional guidelines for animal care and use.
The liver was perfused with isotonic saline solution through the abdominal aorta until the blood was washed out and the parenchyma presented a pale appearance. Then the liver was rapidly excised and harvested for LM and immunohistochemical studies.
Blood pressure measurement
At baseline and throughout the experiment, systolic blood pressure (SBP) was measured monthly by tail-cuff plethysmography. Measurements were obtained with the rats restrained in a plastic chamber without anesthesia. A pneumatic pulse transducer positioned on the ventral surface of the tail distal to the occlusion cuff detected the return of the pulse following a slow deflation of the cuff. Cuff pressure was determined by a Pneumatic Pulse Transducer, using a Programmed electro-sphygmomanometer PE-300 (Narco Bio-Systems, Austin, TX), and pulses were recorded on a Physiograph MK-IIIS (Narco Bio-Systems, Austin, TX). A minimum of three such determinations were taken at each session, and the SBP registered was the average of the three readings.
Biochemical procedures
After 14-h fasting, rat blood samples were collected from the tail vein in capillary tubes at baseline and at the end of the experiment from the inferior cava vein before the rats were killed. Plasma glucose levels were measured by the glucose oxidase method with an Automatic Analyzer (Hitachi 911, Tokyo). Aliquots of sera were assayed for cholesterol and triglycerides according to standard methods. Serum insulin was determined by a solid phase two-site immunoassay with monoclonal antibodies (DRG Instruments GmbH, Marburg, Germany). Serum samples were stored before testing. The detection limit of the test was 0.07 μg/l, calculated as two s.d. above the zero standard. Total coefficients of variation% (CV%) of the assay was 4%.
Tissue processing and examination
Decapsulated liver was fixed in phosphate-buffered 10% formaldehyde (pH 7.2) and embedded in paraffin. Three-micron sections were cut and stained with hematoxylin-eosin, periodic acid–Schiff reagent and Masson's trichrome. In order to detect fat deposits in liver tissue, frozen sections from the livers were processed by Oil red O (Sigma-Aldrich, Milwaukee, WI). Fatty liver was assessed on 10 consecutive microscopic fields per section, as well as examined at a magnification of ×100 and expressed as a percentage of hepatocytes, with large (macrovesicular) or small (microvesicular) empty vacuoles in their cytoplasm, per microscopic field.
Immunohistochemical staining and morphological analysis
Paraffin sections were cut at 3 μm, deparaffined, and rehydrated. Endogenous peroxidase activity was blocked by treating tissue sections with 0.5% H2O2 in methanol for 30 min. Local ANG II was detected using an anti-human ANG II antibody (Peninsula, CA) at a dilution 1:500 as previously described (11,16).
Immunostaining was carried out using a commercially modified avidin–biotin–peroxidase complex technique, Vectastain ABC kit (Universal Elite, Vector Laboratories, CA) and counterstained with hematoxilin (17). Four liver sections from each experimental animal were stained. On each section, 10 consecutive microscopic fields (×100 and ×400 magnification) were analyzed to evaluate Oil red O and ANG II immunostaining density in liver, respectively. The staining was expressed as density per millimeter square.
Monoclonal antibody against TGF-β1 (Santa Cruz Biotechnology, Santa Cruz, CA) was used at a dilution 1:100. Antibody against rat tumor necrosis factor-α (TNF-α) (R&D Systems, Minneapolis, MN) was used at a dilution 1:50 and interleukin-6 (IL-6) (Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution 1:100.
In all cases, two independent observers performed a blinded fashion evaluation, and the mean percentage value was then calculated for each rat. Histological sections were studied in each animal using a light microscope Nikon E400 (Nikon Instrument Group, Melville, NY). All measurements were carried out using an image analyzer Image-Pro Plus 4.5 for Windows (Media Cybernetics, LP Silver Spring, MD).
Statistical method
Values were expressed as mean ± s.d. All the statistical analyses were performed using absolute values and processed through GraphPad Prism, version 2.0 (GraphPad Software, San Diego, CA). Assumption test to determine the Gaussian distribution was performed by the Kolmogorov and Smirnov method. For parameters with Gaussian distribution, all the comparisons between groups were carried out using ANOVA. The difference of mean values between groups was assessed by Tukey–Kramer multiple comparisons test. Statistical analysis for those parameters such as histological data with non-Gaussian distribution was performed by Kruskal–Wallis test (Non-parametric ANOVA) and Dunn's multiple comparison test. A value of P < 0.05 was considered significant.
Results
Animal characteristics
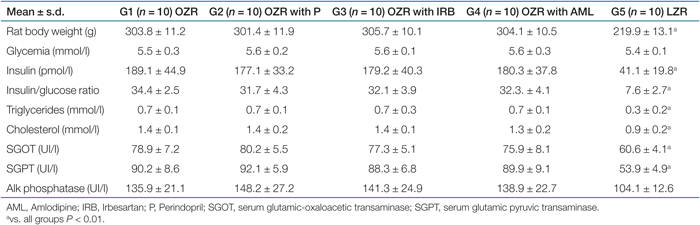
As expected, OZR (compared to LZR) were significantly heavier throughout the experiment, at the beginning as well as at the end of the study, as illustrated in Tables 1 and 2. At baseline, OZR displayed significantly higher serum insulin, insulin/glucose ratio, cholesterol, and triglycerides (Table 1). Moreover, we observed an important increase in hepatic enzymes in all OZR groups compared to LZR.
 |
 |
Obese and lean rats did not present differences in plasma glucose at the beginning of the study (Table 1). In accordance with our previous studies (11,14,18), at the end of the study, all OZR groups showed significantly higher blood glucose levels compared with LZR.
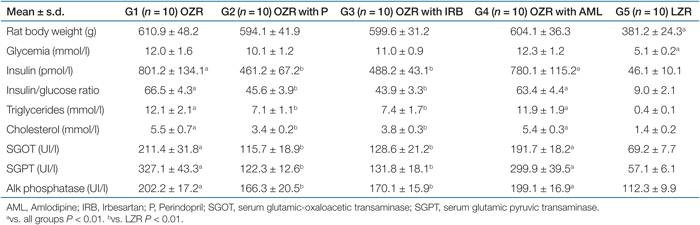
After 6 months of treatment with P or IRB, OZR presented lower insulin/glucose ratio, together with a significant reduction in both serum triglycerides and cholesterol with respect to the untreated OZR and OZR with AML. In addition, hepatic enzymes were significantly lower in the groups that received therapy with P or IRB compared to untreated OZR and OZR with AML, as indicated in Table 2.
SBP
Whereas OZR presented a significant (P < 0.01) increase in SBP in comparison with LZR throughout the study, OZR with P, IRB, and AML displayed a significant reduction in their SBP with similar values to the control LZR, as illustrated in 1.
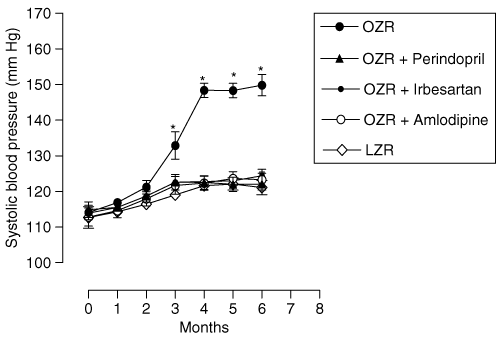
: Monthly evolution of systolic blood pressure obtained by tail-cuff method. Values are expressed as (mean ± s.d.). LZR, lean Zucker rat; OZR, obese Zucker rat. *vs. other groups P < 0.01.
OZRs displayed an increased expression of ANG II in liver
In the immunohistochemical study, the liver from untreated OZR, OZR with IRB, and OZR with AML presented a remarkable (P < 0.01) higher immunostained area for ANG II (15.3 ± 3.0%, 14.2 ± 2.3%, and 15.2 ± 2.7%, respectively) when compared to LZR (3.2 ± 1.3%), as shown in 2 (upper panel). On the other hand, OZR with P showed a significant (P < 0.01) small area stained by ANG II, with values which were not different from those observed in LZR (4.0 ± 1.1% and 3.2 ± 1.3%, respectively). In addition, microvesicular steatosis (cytoplasm vacuolization) in hepatocytes was significantly diminished in this group, as illustrated in 2 (lower panel). Interestingly, although no differences were observed between untreated OZR and OZR with IRB and with AML concerning the percentage of immunostaining for ANG II in liver, OZR with IRB presented a lower proportion of macro and microvesicular steatosis in hepatocytes, in accordance with our previous study (11). In contrast, OZR with IRB at similar percentage of immunostaining for ANG II in liver presented a significant reduction in cytoplasm vacuolization of liver cells.
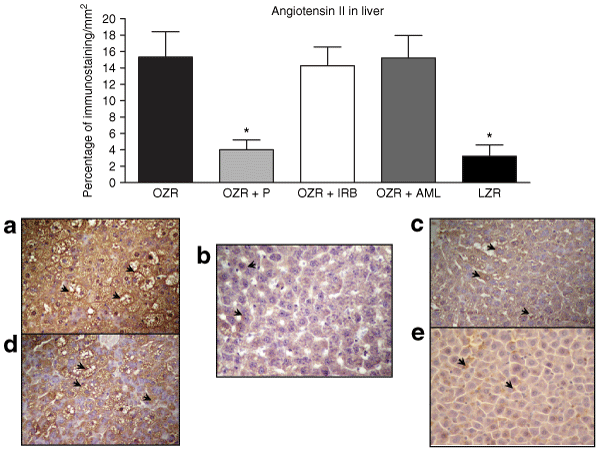
: At the top, bars represent the percentage of immunostaining for angiotensin II in liver in all groups (mean ± s.d.). Lower panel shows immunostaining for angiotensin II in liver (original magnification ×400). (a) Untreated obese Zucker rat (OZR). Hepatic tissue with an extended area of positive staining for angiotensin II indicated by arrows. Note marked steatosis in numerous hepatocytes. (b) OZR with Perindopril (P). Reduction in the area of positive staining for angiotensin II (arrows) and hepatocytes without cytoplasm vacuolization. (c) OZR with Irbesartan (IRB). Similar area of positive staining for angiotensin II (arrows) compared with untreated OZR. However, there is a scanty amount of hepatocytes with cytoplasm vacuolization. (d) OZR with Amlodipine (AML). No different area of positive staining for angiotensin II (arrows) compared to untreated OZR with a similar number of hepatocytes with steatosis. (e) Lean Zucker rat (LZR). Small area of positive staining for angiotensin II (arrows). *vs. OZR, OZR + Irbesartan, and OZR + Amlodipine P < 0.01.
Reversal of hepatic steatosis in OZR by both inhibition of ANG II production and AT1 receptor blockade
A large area of Oil red O positive staining was observed in the liver of untreated OZR compared to LZR, P < 0.01 (3). Conversely, OZR presented a significant (P < 0.01) reduced area of Oil red O positive staining after long-term treatment either with P or with IRB (3). Nevertheless, OZR treated with AML did not improve hepatic steatosis (5). These findings suggest that interaction against RAS may interfere with lipid accumulation in hepatic tissue.
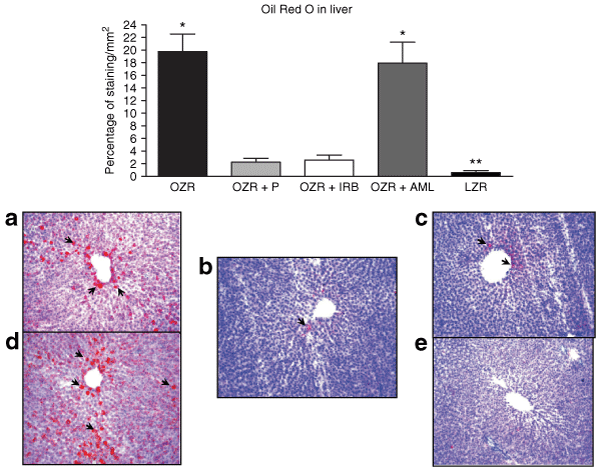
: Bars represent the percentage of staining with Oil red O in liver in all groups (upper panel). In the lower panel, representative images from liver showing lipid distribution in hepatocytes. Oil red O (original magnification ×100). (a) Untreated obese Zucker rat (OZR), note lipid deposits (arrows) in hepatocytes. (b) OZR with Perindopril (P) with scanty lipid deposit (arrows) near the central hepatic vein. (c) OZR treated with Irbesartan (IRB). Small area of positive staining for Oil red O. (d) OZR with Amlodipine (AML) with similar lipid distribution to untreated OZR. (e) Lean Zucker rat (LZR) with isolated lipid deposits. *vs. OZR + Perindopril, OZR + Irbesartan, and LZR P < 0.01; **vs. all groups P < 0.01.
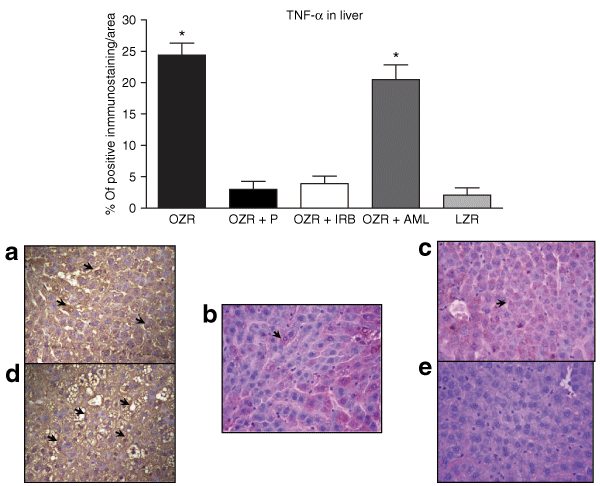
: Bars represent the percentage of immunostaining for tumor necrosis factor-α (TNF-α) in liver of each group (upper panel). (a) Hepatic section from obese Zucker rat (OZR) showing a large area of immunostaining for TNF-α. (b) Perindopril (P)-treated, and (c) Irbesartan (IRB)-treated groups, both showing weaker immunostaining intensity for TNF-α compared to untreated OZR. (d) Amlodipine (AML)-treated OZR showing similar expression of TNF-α to untreated OZR. (e) Control lean Zucker rat (LZR) without staining for TNF-α (original magnification ×400). *P < 0.01 vs. OZR + P; OZR + IRB, and LZR.
Effect of RAS inhibitors on IL-6 and TNF-α expression in the liver of OZR
The evaluation of local proinflammatory cytokine production in the liver by immunostaining showed that both IL-6 and TNF-α were increased (P < 0.01) in hepatic tissue in OZR compared to LZR (4 and 5, respectively). Notably, the administration of either P or IRB during 6 months substantially ameliorates the expression of these cytokines. However, AML treatment did not decrease the expression of IL-6 or TNF-α compared to untreated OZR (4 and 5, respectively). It is well known that both IL-6 and TNF-α are inflammatory cytokines that play a crucial role in the regulation of inflammatory responses. On this basis, the anti-inflammatory effects of anti-RAS therapy may be associated with a reduction in the local expression of these cytokines.
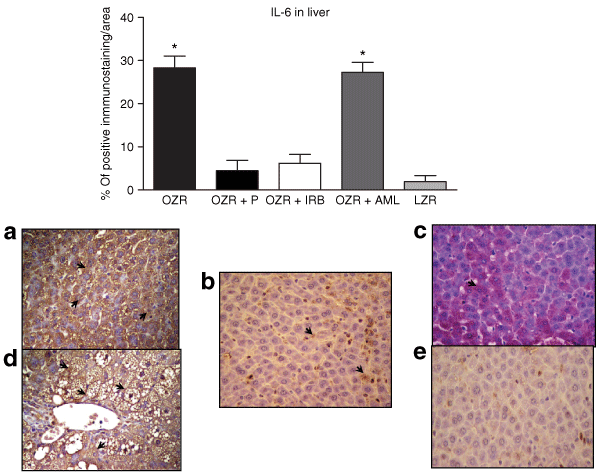
: Bars represent the percentage of staining for interleukin-6 (IL-6) in each group (upper panel). (a) Hepatic section from obese Zucker rat (OZR) showing a large area with positive IL-6 immunostaining. (b) Perindopril-treated group and (c) Irbesartan-treated group, both showing small area of positive immunostaining for IL-6. (d) OZR with Amlodipine (AML) showing positive staining for IL-6, with similar expression to untreated OZR. (e) Control lean Zucker rat (original magnification ×400). *P < 0.01 vs. OZR + P; OZR + IRB, and LZR.
Effects of ACE inhibition or AT1 receptor blockade treatment on TGF-β1 expression and fibrosis in liver of OZR
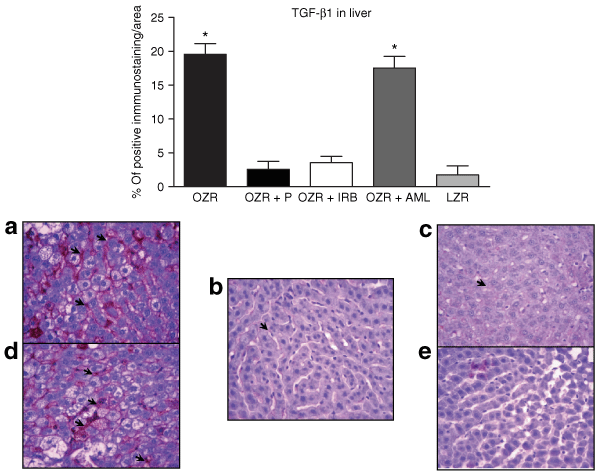
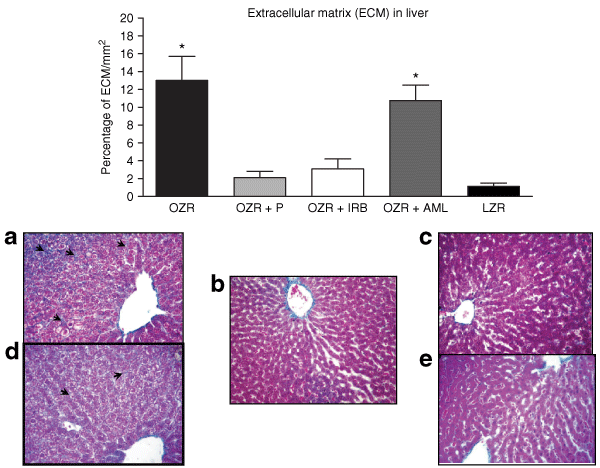
While both untreated OZR and OZR with AML therapy presented a large area with positive immunostaining for TGF-β1 in liver, in comparison to the control LZR, those OZR that had received either ACE inhibition or AT1 receptor blockade showed a significant lower expression of this fibrotic cytokine, as represented in 6. In accordance with this finding, evaluation of extracellular matrix by Masson's Trichrome staining revealed a substantial expansion of extracellular matrix, along with a variable degree of hepatic fibrosis in untreated OZR and OZR with AML groups, compared to control LZR (P < 0.01), as illustrated in 7. Remarkably, after chronic administration of anti-RAS therapy, animals receiving either P or IRB presented no different extracellular matrix dimension when compared to control LZR. These observations suggest that ANG II control provides an actual modulation in the fibrosis process in liver.
: Bars represent the percentage of staining with Masson's trichrome in liver in each group (upper panel). (a) Hepatic fibrosis in untreated obese Zucker rat (OZR) (arrows). Images illustrate hepatic sections from OZR treated with Perindopril (P) (b), Irbesartan (IRB) (c). Note evident benefit with both treatments. (d) OZR with Amlodipine (AML) with similar fibrosis (arrows) to untreated OZR. (e) Control lean Zucker rat (LZR) (Masson's trichrome, original magnification ×100). TGF-β1, transforming growth factor-β1. *P < 0.01 vs. OZR + P; OZR + IRB, and LZR.
: Bars represent the percentage of staining for transforming growth factor-β1 (TGF-β1) in each group (upper panel). (a) Liver from untreated obese Zucker rat (OZR). Note hepatic tissue presenting an extended area with positive TGF-β1 immunostaining (arrows). (b) OZR with Perindopril (P) with scanty TGF-β1 expression (arrows). (c) OZR treated with Irbesartan (IRB). Small area of positive immunostaining for TGF-β1. (d) OZR with Amlodipine (AML) with similar TGF-β1 staining to untreated OZR. (e) Control lean Zucker rat (LZR) (original magnification ×400). *vs. OZR + P, OZR + IRB, and LZR.
Discussion
There is recent evidence indicating that RAS is involved in hepatic fibrogenesis (3,6,9). In accordance with this, in a previous study, we demonstrated that OZR, an animal model of steatohepatitis, presented an increased expression of hepatic ANG II (11). Although the pathogenesis of NASH remains unclear, numerous factors such as insulin resistance, oxidative stress and proinflammatory cytokines have been proposed (2). In this study, we have showed that RAS blockade either by ACE inhibition or AT1 receptor blockade preserves liver biochemical parameters together with a substantial reduction in the liver injury in OZR. These findings are in agreement with previous reports showing that ANG II plays a major role in liver fibrogenesis (3,6,19).
The association of obesity, diabetes, arterial hypertension, and hyperlipidemia significantly increases the risk of severe stages of non-alcoholic liver disease (1,2,3,20). There is a large body of information showing that interaction against RAS does not only reduce blood pressure but also improves insulin sensitivity (11,21,22). In a previous study, we demonstrated that the administration of IRB improved insulin resistance and led to a significant reduction of lipid accumulation in the hepatocytes of OZR (11). In the current study, we have observed that an ACE inhibitor such as P causes a reduction in circulating levels of insulin, cholesterol, triglycerides, as well as a reduction in SBP and hepatic enzymes in OZR.
Present results reinforce previous information indicating that specific RAS inhibition enhances whole-body insulin sensitivity in several models of insulin resistance (11,21,22). In this experiment, we have also investigated the effect of a calcium antagonist, AML, on the hepatic fibrosis of NASH. Calcium channel blockers are well known and highly efficient drugs for lowering blood pressure. However, the effects of these antihypertensive agents on the lipid profile and insulin sensitivity are controversial (23). In agreement with previous studies, we have observed that chronic treatment (6 months) with AML caused a reduction in blood pressure but did not modify circulating levels of insulin, cholesterol, and triglycerides (15).
The “two-hit hypothesis” has turned into the most important theory to explain the pathogenesis of NAFLD (4). The first “hit” is the fat accumulation within the liver, a progression associated with insulin resistance. The second “hit” is related to injury caused by oxidative stress and inflammatory cytokines. Thus, abnormal cytokine production within the liver may be playing an important role in the pathogenesis of NASH. Moreover, it has been reported that ANG II induces hepatic inflammation (24,25), suggesting an association between ANG II signaling and hepatic inflammation. In addition, several studies have proposed that TNF-α plays a pathogenic role in insulin resistance and NAFLD (26,27). Other inflammatory mediators such as IL-6 have been demonstrated to cause insulin resistance in several experimental models directly (28,29). Nevertheless, the role of this factor in hepatic fibrogenesis is controversial (30).
In the current study, we have demonstrated that hepatic steatosis was associated with increased hepatic TNF-α expression and inflammatory liver injury and fibrosis. In addition, TNF-α can exert a fibrogenetic effect which is released by hepatocytes and Kupffer's cells and induces hepatic stellate cells activation in a paracrine way (2,31). Furthermore, activation of RAS is thought to be one of the factors that upregulates local production of TNF-α. Therefore, it is not surprising that anti-RAS therapy either with P or IRB has reduced the generation of this cytokine in the liver of OZR, suggesting that interaction against local RAS modulates liver injury and prevents the progression to steatohepatitis. Although differing results have been reported, we have also observed high levels of IL-6 in liver of OZR. After chronic inhibition of RAS, there was a significant reduction in hepatic immunoreactivity to IL-6 in OZR. This result suggests that such TNF-α, IL-6 may lead to steatosis and inflammation. On this basis, the beneficial anti-inflammatory effects of P and IRB may be partly related to reductions in the expression levels of these cytokines. Interestingly, compared to antihypertensive drugs that interact against RAS, AML (calcium antagonist) did not exert anti-inflammatory properties in liver of OZR. Therefore, our experimental data suggest that therapeutic intervention against RAS is associated with an improvement of the chronic inflammatory states related to obesity and insulin resistance.
Undoubtedly, TGF-β1 is one of the most important cytokines in the fibrotic process. In previous experiments, we demonstrated that OZR showed high levels of TGF-β1 in different tissues, including myocardium (10) and kidney (14). In this study, we have observed that OZR presented a remarkable high hepatic expression of TGF-β1. In the liver of OZR, RAS inhibition favorably modulated the TGF-β1 expression and the expansion of extracellular matrix, impeding fibrosis progression. These results are in agreement with previous laboratory experiments, as well as experiments of other groups (5,7,19,32). Consequently, data shown in the current study reinforce the concept that ANG II plays a major role in liver fibrogenesis in a model with steatohepatitis associated with obesity and insulin resistance.
Several reports have postulated that leptin, an adipocyte-secreted hormone, presents profibrotic effects by acting synergistically with ANG II through stimulating inflammation and TGF-β1 expression (33). In addition, recently, leptin has been shown to be of importance in the development of hepatic fibrosis (34,35). However, in this study, we have observed that OZR, a model characterized by lack of functional leptin receptor, showed an elevated expression of TGF-β1 with hepatic fibrosis. Most of the research addressing the mechanism by which obesity, insulin resistance, and diabetes are associated with hepatic fibrosis has been performed in young rodents of obese genetic models such as ob/ob mice, fa/fa rats and high-fat diet models. Nevertheless, in opposition to previous works (35,36) in our study, we have observed that OZR develop steatohepatitis, possibly due to the age of the animals used, 34 weeks old (since we began the treatment with 10-week-old animals and they reached 34 weeks at the end of the study). It has already been described that ob/ob mice also develop mild steatohepatitis with age and that age-related steatohepatitis is generated by feeding them with high-fat diets (31).
In conclusion, we have shown here that the inhibition of RAS either by ACE inhibition or AT1 receptor blockade leads to favorably modulate the inflammatory response and liver fibrosis together with an improvement in the insulin sensitivity state in OZR. Furthermore, it is possible that RAS inhibition might regulate the progress of liver fibrosis throughout a suppression of TGF-β1. These data, therefore, suggest that the blockade of RAS could be taken into account as a potential new approach to prevent or to treat patients with NASH.
DISCLOSURE
The authors declared no conflict of interest.





