Which Obesity Indicators Are Better Predictors of Metabolic Risk?: Healthy Twin Study
Abstract
No consensus exists as to the most sensitive and specific obesity indicator associated with metabolic risk factors. We aimed to validate anthropometry as the predictor for obesity-related metabolic risk factors through comparison with direct body composition measures in Korean adults. A total of 995 Korean women and 577 Korean men who participated in the Healthy Twin study were the subjects. Anthropometric measurements included BMI, waist circumference (WC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHTR). Direct body composition measures included the percentage of body fat (%BF) measured using dual-energy X-ray absorptiometry scanners and bioelectrical impedance analyzer (BIA). The following criteria were used to define abnormal metabolic risk factors: blood pressure ≥ 130/85 mm Hg, fasting glucose (≥ 100 mg/dl), insulin (≥ 25 μU/ml), homeostasis model assessment (HOMA) (≥ 2.61), high-density lipoprotein (HDL) (<40 mg/dl for men or <50 mg/dl for women), triacylglycerol (≥ 150 mg/dl), uric acid (>7 mg/dl for men or >6 mg/dl for women), high-sensitivity C-reactive protein (hs-CRP) (≥ 2.11 mg/l). In multiple regression analyses (adjusted for age, education, smoking, alcohol, exercise and past/current medical history, and treated families as a random effect), WC, WHTR, and BMI were consistently associated with all metabolic risk factors regardless of the subject's gender. Some of the areas under the receiver-operating characteristic curves regarding abnormal metabolic risk factors were significantly higher for the three indicators of central obesity than for %BF. Our study validates the usefulness of anthropometry over direct body fat measures to predict metabolic risks.
Introduction
Effective obesity indicators for predicting obesity-related health risks are very important for public health. Several studies have been used to assess the validity of obesity indicators as the predictors of metabolic risk factors, cardiovascular risk factors, and even mortality (1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18). Simple anthropometric parameters (BMI, waist circumference (WC), waist-to-hip ratio (WHR), and waist-to-height ratio (WHTR)) and percentage of body fat (%BF) mass measured using dual-energy X-ray absorptiometry (DXA) and bioelectrical impedance analysis (BIA) were evaluated with respect to those relationships.
WC and WHR have been used to estimate abdominal fat accumulation as components of the metabolic syndrome (19,20,21,22), and WHTR has also been reported as a measurement strongly associated with cardiovascular risk factors (15,16,17,18, 23,24,25,26).
Even though the simple anthropometric measurements are practical at the population level, a DXA scanner has been recommended as a reference method of %BF estimation (27) and BIA as an instrument in population-based studies because of its low cost and easy application (28).
However, no consensus exists about the most sensitive and specific obesity indicator associated with metabolic risk factors. Some studies presented that simple anthropometric parameters were more strongly related to those risks compared to the measurement of body composition (4,5,6,7,8), whereas other studies showed that %BF was a better predictor of risks compared to anthropometry (1,2,3). Ideally, to find the best obesity indicator for obesity-related metabolic risk factors, all indicators should be compared in the same subjects. However, in previous studies, %BF was measured by either DXA or BIA.
We recently reported a nationwide Korean twin family study, “Healthy Twin,” which recruits adult same-sex twins and their adult family members. This study sought to elucidate the genetic variations responsible for common complex diseases/traits and MS-related phenotypes (29). Here, we aimed to validate simple anthropometric parameters as the predictors for obesity-related metabolic risk factors through comparison with direct body composition measures in the Korean adults registered in Healthy Twin (29).
Methods and procedures
Subjects and design
The Healthy Twin study recruits adult same-sex twins aged ≥30 years and their first-degree family members. The overall methodology of this multicenter cross-sectional survey conducted in three Korean cities (Seoul, Busan, and Cheonan) has been previously described (29). Healthy Twin was advertised in a nationwide newspaper and through posters in ∼300 hospitals and health-related governmental agencies. Approximately 12,500 individual twins whose addresses were available were contacted through mail. The individual twins and their families who were willing to participate in Healthy Twin answered a questionnaire and visited one of the centers to undergo health examination, clinical tests, biochemical tests, and physical measurements. Efforts were made to standardize the survey method among the three centers in terms of the development of a standard protocol and the training of research coordinators and research assistants.
This study originally proposed to recruit as many as 3,000–4,000 individuals of twins and their families to assist the gene discovery study of the most common diseases. A total of 1,572 individuals (995 women and 577 men) finished the full survey from April 2005 to January 2007. The third year of the survey is currently under way.
Written informed consent was obtained from all participants. The study protocol was approved by the ethics committee of the Samsung Medical Center and Busan Paik Hospital.
Background characteristics
The questionnaire consisted of more than 400 questions regarding demographics, past/current medical history, family history of disease, smoking and alcohol habits, physical activity, lifetime weight change, food frequency, eating behaviors, and psychiatric status. For this study, we selected questions regarding smoking, alcohol use, regularity of exercise, education level, and past/current medical history of cardiovascular disease (cerebrovascular disease or ischemic heart disease), hypertension, dyslipidemia, and diabetes mellitus. The participants were classified according to the following lifestyle characteristics: current smoker and ≥400 cigarettes/lifetime, ex-smoker and ≥400 cigarettes/lifetime, and <400 cigarettes/lifetime according to smoking status (30); non-alcohol user, ex-alcohol user, and current alcohol user according to alcohol consumption; regular and irregular exercise according to the regularity of moderate–intensity exercise; and education level ≤9, 10–14, and ≥15 years.
Obesity indicators
Trained research coordinators and assistants measured all participants' body weight (kg) and height (cm) with light clothing, but without shoes, using a digital balance (Tanita, Seoul, Korea) and stadiometer (Samwha, Seoul, Korea). Weight and height were measured to the nearest 0.1 kg and 0.1 cm. The BMI was calculated by dividing the weight in kilograms by the square of the height in meters. Those with 25 kg/m2 ≤ BMI <30 kg/m2 and BMI ≥30 kg/m2 were classified as being overweight and obesity, respectively (31). The WC was measured at a level midway between the lowest lateral border of the ribs and the uppermost lateral iliac crest, and hip circumference was measured as the widest over the femoral greater trochanters while the participants were standing. The circumferences were measured to the nearest 0.1 cm. WHR was calculated by dividing WC to hip circumference and WHTR by dividing WC to height. A WC ≥90 cm in men and ≥80 cm in women were used to indicate central obesity according to the Asian-specific WC cutoff points of International Diabetes Federation criteria (22). A WHR >0.9 in men and >0.85 in women were used to classify central obesity according to the World Health Organization criteria (19). All anthropometric measurements were performed twice, and the mean value of the two measurements was chosen for analyses.
DXA scans were conducted to measure the estimates of fat mass with three DXA devices used in each of three hospitals (Lunar DPX-L, DPX-MD; Lunar, Madison, WI; Delphi A; Hologic, Bedford, MA). The participants were examined in a supine position with arms by the side. The %BF was also measured with BIAs (InBody 2.0, InBody 3.0; Biospace, Seoul, South Korea) using multifrequency segmental bioelectrical methods. Participants should be on fasting for at least 8 h and should not drink water and exercise at least 4 h before measurement with BIAs. They stood on a footplate, which had two electrodes per foot, and held a handgrip with two electrodes per hand.
Metabolic risk factors
The metabolic risk factors included in this study were blood pressure (BP), glucose, insulin, homeostasis model assessment (HOMA), high-density lipoprotein (HDL) cholesterol, triacylglycerol (TG), high-sensitivity C-reactive protein (hs-CRP), and uric acid. BP measurements were performed twice with a standard manual sphygmomanometer while participants were in a sitting position. Blood samples were collected from three centers and shipped to the central laboratory within 24 h. The participants had to be on fasting for at least 8 h before blood samples were obtained. Blood samples were drawn from an antecubital vein. Plasma glucose was assayed by a hexokinase enzymatic method and insulin by a radioimmunoassay method. HOMA was calculated as fasting insulin (μU/ml) multiplied by fasting glucose (mmol/l) divided by 22.5 (ref. 32). Serum HDL and TG were measured using homogeneous and enzymatic methods, respectively. hs-CRP was measured using latex immune complex turbidimetry. Uric acid was measured by an uricase enzymatic colorimetric method. The following criteria were used to define abnormal metabolic risk factors: BP ≥130/85 mm Hg (20,21,22), fasting glucose (≥100 mg/dl) (21,22), insulin (≥25 μU/ml), HOMA (≥2.61) (ref. 32), HDL (<40 mg/dl for men or <50 mg/dl for women), TG (≥150 mg/dl) (20,21,22), hs-CRP (≥2.11 mg/l) (33), uric acid (>7 mg/dl for men or >6 mg/dl for women) (34). The number of abnormal metabolic factors was used to classify the subjects as either low (less than three abnormal metabolic factors) or high (three to eight abnormal metabolic factors) metabolic risk factor group.
Statistical analyses
All analyses were performed separately by sex. Age-adjusted means and 95% confidence intervals of BMI, WC, BP, fasting glucose, TG, and HDL in twins and their families were calculated separately to compare with those values in the population data (35). All biochemical tests were logarithmically transformed to be normally distributed, because P value of Shapiro–Wilk tests for normality regarding the biochemical tests were <0.05. Student t-tests or chi-square tests were used to compare continuous variables or categorical variables between the sexes, respectively. Random effect model was used to model the association between the metabolic risk factors and the obesity indicators adjusted for age, education, smoking, alcohol, exercise, and past/current medical history. In this model, families were treated as a random effect to consider their dependency, and the obesity indicators were standardized by Z-transformation. Before separately analyzed by sex, an interaction term between sex and individual Z-transformed obesity indicator was included in each model. The interaction terms between sex and obesity indicators were significant (P < 0.01) except for the terms between sex and three obesity indicators (WHTR, BMI, and DXA-measured %BF) regarding the model for uric acid. The area under the receiver-operating characteristics curves (AUCs) was calculated for each index of obesity and abnormal metabolic risk factors using receiver-operating characteristics (ROC) curves. ROC curves calculated the sensitivity and specificity of the WC, WHR, and BMI for cutoff values of central obesity and overweight for identifying a high metabolic risk factor profile. Pairwise comparisons between the AUCs of two indices of obesity were conducted and P value was adjusted using Bonferroni correction (P < 0.0033). Otherwise, statistical significance was based on P < 0.05. All statistical analyses were conducted with the software package SPSS 12.0K for Windows (Release 12.0.1; SPSS, Chicago, IL) and MedCalc version 9.3.0.0 (MedCalc Software, Mariakerke, Belgium).
Results
Characteristics of participants
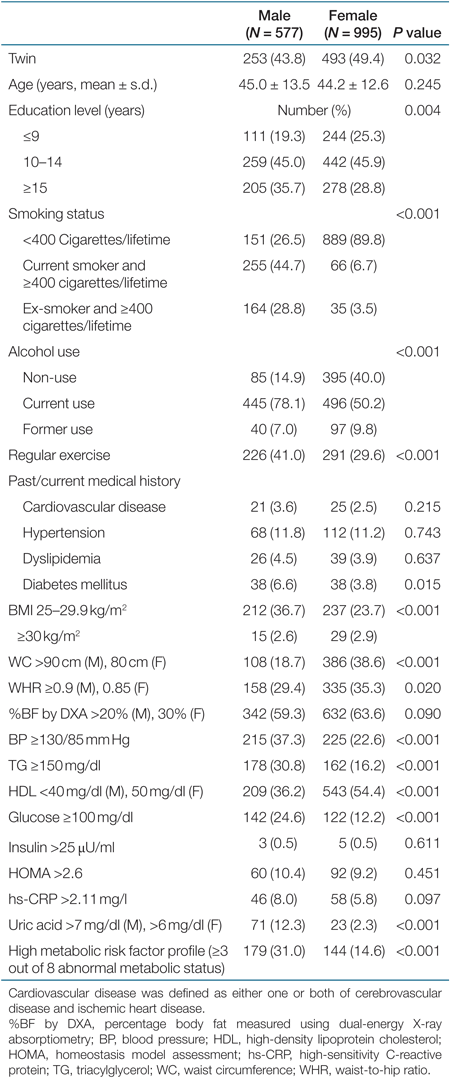
Table 1 shows the characteristics of 577 Korean men and 995 Korean women who participated in the Healthy Twin study. Out of 1,572 subjects, 746 (47.4%) were same-sex twins. Women were more likely to be twins compared to men. Men were more likely to be highly educated, smoke, use alcohol, and exercise regularly compared to women. There were no significant differences in the prevalence of previous medical history between men and women. Even though the prevalence of overweight was higher in men than in women, the prevalence of central obesity was higher in women than in men. For metabolic risk factors, men were more likely to have high BP, abnormal levels of TG, fasting glucose, uric acid, and a high metabolic risk factor profile compared to women (Table 1). There were no significant differences in the age-adjusted means of BMI, WC, BP, fasting glucose, TG, and HDL between male twins and their male families, whereas the age-adjusted means of WC and BMI in female twins were lower compared with their female families (P < 0.05). Twins and their families were more likely to have low age-adjusted means of metabolic risk factors except for HDL compared with the population of the National Health and Nutrition Survey of Korea 2001 (Table 2).
 |
 |
Relationships between obesity indicators and metabolic risk factors
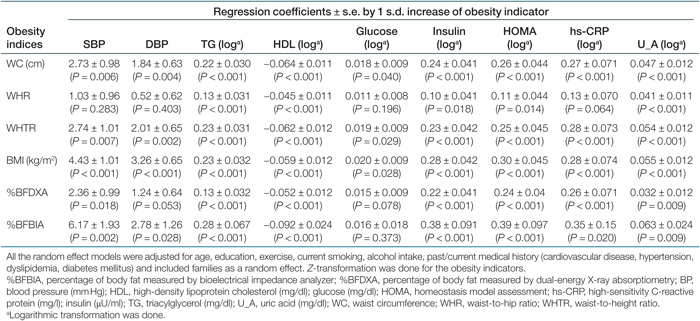
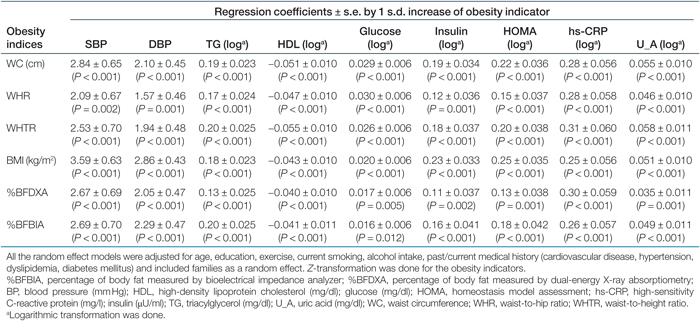
The regression coefficients and 95% confidence intervals by 1 s.d. increase of the obesity indicators regarding metabolic risk factors for men and women are shown in Tables 3 and 4, respectively. After being adjusted for age, lifestyle, and past/current medical history, and treated families as a random effect, all obesity indicators had statistically significant associations with nine metabolic risk factors in women. By contrast, for men, WHR was not significantly associated with BP, glucose, and hs-CRP. Likewise, DXA-measured body fat was not associated with diastolic BP and glucose in men. The increase of the obesity indicators predicted the increase of systolic BP, diastolic BP, TG, insulin, glucose, HOMA, hs-CRP, and uric acid, whereas the increase of these indicators predicted the decrease of HDL (Tables 3 and 4).
 |
 |
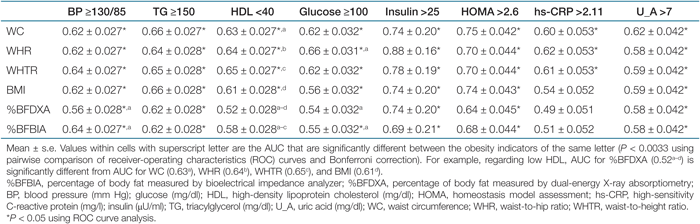
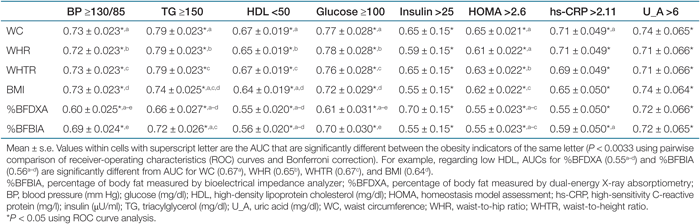
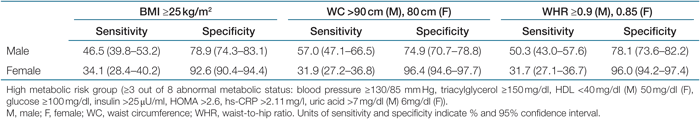
Tables 5 and 6 show the AUC values of the ROC analyses regarding abnormal metabolic risk factors in men and women. For men, the AUC values for the indicators of central obesity (WC, WHR, or WHTR) in detecting abnormal metabolic risk factors were consistently significant (P < 0.05), while the AUC value for DXA-measured %BF regarding high glucose, low HDL, and high hs-CRP were not significant. For low HDL, the AUC values of simple anthropometric measurements were significantly higher than for the DXA-measured %BF by pairwise comparisons (P < 0.0033). There were no significant difference between DXA-measured %BF and BIA-measured %BF in detecting abnormal metabolic risk factors, except for high BP (P < 0.0033). No significant differences were found for the comparison of the AUC values among simple anthropometric measurements in men (Table 5). For women, all AUC values for the obesity indicators were significant in detecting eight abnormal metabolic risk factors (P < 0.05), However, the AUC values for DXA-measured %BF in detecting high BP and high glucose were significantly lower than for other obesity indicators (P < 0.0033). The AUC values of three indicators of central obesity in detecting abnormal metabolic risk factors were not significantly different from each other, whereas WC was better than was BMI in detecting high TG and low HDL (Table 6). The sensitivity and specificity of the BMI, WC, and WHR cutoff values for overweight and central obesity for identifying a high metabolic risk group are presented in Table 7. The cutoff values had low sensitivity (31.7–57%) and high specificity (74.9–97.7%). The BMI cutoff for overweight and WC cutoff and WHR cutoff for central obesity had similar sensitivities and specificities.
 |
 |
 |
Discussion
It is important to determine valid obesity indicators as the assessment tools for predicting obesity-related metabolic risk factors in routine clinical evaluation. Here we demonstrated that six obesity indicators were associated with the metabolic risk factors. Additionally, we confirmed that simple anthropometric measurements were more likely to be consistent for predicting abnormal metabolic status than direct body fat measurements.
These observations add to the findings of previous studies showing that %BF has no advantage over BMI and WC in the prediction of obesity-related complications and metabolic risk factors (4,5,6,7,8). However, other studies showed a higher prediction of direct body fat measures for those associations (1,2,3). Of those comparative studies, body composition was examined using BIA or DXA. The inconsistency of previous observations may be related to different equipment accuracies in estimating body composition (5). In this study, our subjects exhibited moderate correlations between the two %BF measured by DXA and BIA (correlation coefficient, 0.77), and the AUC values with respect to some of the abnormal metabolic risk factors for DXA-measured %BF were significantly different from BIA-measured %BF. In the ROC analysis, we found that three indicators of central obesity (WC, WHR, and WHTR) were comparable indicators for predicting abnormal metabolic risk factors. The AUC values regarding those risk factors for the indicators of central obesity were 0.6–0.75. The AUC is a measure of the degree of accuracy. Thus, values of 0.6–0.75 for the AUC mean that, 60–75% of the time, a random selection from the subjects having abnormal metabolic risk factors will have a score greater than a random selection from the subjects having normal metabolic risk factors. Our results agree with those of Bosy-Westphal et al. (5) and Schneider et al. (15,16), which showed closer associations of WC and WHTR with metabolic risk factors compared to the indices of general adiposity. In this study, the correlation coefficients between the indices of central obesity were high: 0.89 between WC and WHTR, 0.83 between WC and WHR, and 0.79 between WHR and WHTR. Taken together, these indicators may be equivalent indicators for predicting obesity-related metabolic risk factors. The sensitivity and specificity of BMI cutoff value for overweight and WC/WHR cutoff values for central obesity also have similarity for identifying a high metabolic risk group. However, low sensitivity and high specificity mean that the measures miss a large number of subjects who are in the high-risk group, but the measures do not incorrectly classify many subjects as being at high risk when they are not.
Both pros and cons exist regarding the application of anthropometry. While WC has been the best obesity indicator that predicts visceral fat mass by Magnetic resonance imaging/Computed tomography and the simplest index of central obesity to measure (9,36), different WC cutoff values are recommended for different ethnicities (37). WHR would mask the status of central obesity by an increase in hip circumference, and would be affected by the measurement errors of WC and hip circumference. Particularly, for men in this study, WHR was less likely to be predictive for metabolic risk factors compared to other anthropometry. Incidentally, WHTR (WC adjusted for stature) is likely to be applicable in the populations with a wide range of heights (17,26). For BMI, it has better reproducibility compared to indices of central obesity, but it has been argued that the relationship between BMI and %BF differs according to ethnicity (36). In this study, the correlation coefficient between %BF measured by DXA and BMI was only 0.313.
The age-adjusted means of BMI, WC, and metabolic risk factors in twins were similar to their families, whereas those means in some factors in current subjects tend to be healthier compared with those values in Korean population. Then, twining effect does not seem to explain those differences; however, healthier volunteers seem to participate in this study. Although this means current subjects may not be a representative sample of Korean population, it does not likely to cause a bias to find the associations between obesity indicators and metabolic risk factors. Some limitations need to be considered. As our study was conducted by a cross-sectional design, the statistically significant associations between obesity indicator and metabolic risk factors do not prove causality. Also, we did not adjust for a potential bias in the measurement of %BF of three different DXA scans. Finally, the results of this study may not be generalized to other populations. Therefore, further studies are needed to see whether the same results will be found in other populations.
In conclusion, our study validates the usefulness of simple anthropometric measurements over direct body fat measures to predict metabolic risks. Therefore, we recommend measuring WC, WHR, WHTR, or BMI in obesity-related health evaluation in population studies.
DISCLOSURE
The authors declared no conflict of interest.
Acknowledgments
We participated in study design and conducting the study. K.L. and J.S. worked for the data interpretation. K.L. and Y.S. contributed to editing the manuscript. This study was supported by a grant of the Korea Centers for Disease Control and Prevention (Serial number 2005-347-2400-2440-215, 2006-347-2400-2440-215) and National Cancer Center grant (0610552-2).





