The thymic medulla: who needs it?
The thymic medulla has long been considered an important microenvironment for the final maturation and negative selection of developing T cells. A new study from Bonito et al.1 challenges this view, reporting that selective ablation of nearly all medullary thymic epithelial cells (mTECs) does not appear to impair thymocyte differentiation or provoke widespread autoimmunity. In conjunction with other recently published papers, this study raises new questions about what the thymic medulla is really for.
Following their positive selection in the thymic cortex, thymocytes migrate to the medulla and reside there for several days prior to their export. During this sojourn, CD4 or CD8 single positive (SP) thymocytes are exposed to thousands of peripheral tissue antigens produced by mTEC expressing the transcriptional regulator, Aire. Deficiency in the expression of Aire or the ectopic antigens it regulates provokes organ-specific autoimmunity. Many studies have shown that this tolerogenic effect involves thymocyte deletion; however, it remains possible that an additional role for regulatory T cells (Treg) may also be important.2 Accordingly, disruption of genes required for mTEC differentiation, such as Relb (encoding a transcription factor) or Traf6 (encoding a E3 ubiquitin ligase protein), can also lead to autoimmunity in multiple organs.3 However, the loss of these genes in other cell types might also contribute to the tolerance defects observed in these mice.
To determine whether TRAF-6 deficiency in TEC alone was sufficient to provoke autoimmunity, Bonito et al.1 constructed a new mouse model. Mice bearing a Traf6 allele flanked by loxP sites were crossed with a knock-in mouse that expresses the Cre recombinase under the control of the gene, Foxn1 (encoding a transcription factor expressed almost exclusively by TEC). The resulting Foxn1CreTraf6fl/fl mice ablated TRAF-6 expression only in TEC. These mice had a normal thymic cortex and early thymocyte differentiation was untouched. By contrast, they had severe defects in the thymic medulla, with only 10% the normal number of mTEC (although all subtypes could still be detected, including some Aire+ mTEC). Surprisingly, despite the extensive loss of mTEC, Foxn1CreTraf6fl/fl mice had normal numbers of mature SP thymocytes and peripheral T cells, indicating that the thymic medulla is dispensable for thymocyte differentiation.
This outcome is reminiscent of findings in Ccr7−/− mice, which lack a chemokine receptor necessary for the migration of thymocytes to the medulla.4 These mice also have a normal complement of thymocytes and T cells, indicating that thymocyte differentiation and export can proceed independently of signals from the medulla. More recently, Cowan et al.5 showed that thymocytes at the earliest stages after positive selection can mature in Relb-deficient TEC grafts or when injected directly into the periphery, without input from mTEC. By contrast, when they assayed the development of Treg cells in Relb-deficient TEC grafts, they observed a five to eightfold reduction compared to control grafts, revealing an important role for the thymic medulla in Treg differentiation.5 Consistent with these findings, Foxn1CreTraf6fl/fl mice had diminished thymic Treg cells.1 Although normal Treg cell numbers were observed in the periphery, this restoration is likely due to the potent homeostatic mechanisms that normally restore Treg cell deficits.6
Collectively, these findings challenge the notion that conventional thymocyte maturation requires the thymic medulla, but what about immunological tolerance? Young Foxn1CreTraf6fl/fl mice initially appeared normal, but then lost weight and their health deteriorated after 6 months of age.1 Autoantibodies targeting a range of organs could be detected in their serum, indicating at least partial loss of tolerance. Despite the presence of autoantibodies, there was little leukocytic infiltration or tissue damage in the lung, kidney or gastrointestinal tract, suggesting that the loss of mTEC was not sufficient to cause substantial autoimmune disease in these organs. However, Foxn1CreTraf6fl/fl mice did exhibit many of the hallmark features of autoimmune hepatitis, including a range of lymphocytic infiltrates, the release of enzymes indicative of liver damage and autoantibody targeting of the soluble liver antigen. Importantly, the authors used in vitro stimulation assays and adoptive transfer experiments to provide evidence that defects in T-cell tolerance drive this disease, consistent with the idea that impaired thymic tolerance precipitates autoimmune hepatitis in Foxn1CreTraf6fl/fl mice. Therefore, the Foxn1CreTraf6fl/fl mouse model appears to be a new, spontaneous model of autoimmune hepatitis that recapitulates many of the features of the human disease.
Although the autoimmune hepatitis in these mice is striking, the mild effect on other organs of Foxn1CreTraf6fl/fl mice is in contrast with the widespread autoimmunity and early lethality observed in Traf6−/− or Relb−/− mice. These comparisons highlight the anti-inflammatory roles of TRAF-6 and Rel-B in the hematopoietic compartment and indicate that the autoimmune disease induced by germline deletion is the sum of defects in thymic tolerance1 and T-cell regulation.7
Another interesting comparison is the autoimmune phenotypes of Aire−/− and Foxn1CreTraf6fl/fl mice. In the former, the medullary microenvironment is largely intact but mTEC expression of peripheral tissue antigens is greatly reduced, whereas the latter lacks most mTEC altogether. On the C57BL/6 background, Aire−/− mice develop autoimmunity targeting the lung, eye, prostate and salivary glands, but little hepatitis,8 perhaps implying that Aire-independent hepatic antigens mediate thymic tolerance to the liver. Unfortunately, Bonito et al.1 did not present data on the eye, prostate or salivary glands, so conclusions regarding the relative roles of Aire and mTEC on autoimmunity in these organs await further study of the Foxn1CreTraf6fl/fl mouse model. The authors do note that a wider spectrum of autoimmunity was observed on a mixed (129/SvJ/C57BL/6) genetic background, a feature of many other models of autoimmune disease. For instance, Aire−/− mice on the autoimmune-prone NOD background succumb to widespread organ-specific autoimmunity that causes early lethality.8 In this regard, it might be illuminating to analyze the range of organ-specific autoimmunity observed in Foxn1CreTraf6fl/fl mouse on different genetic backgrounds to more comprehensively assay the importance of the thymic medulla for immunological tolerance.
So, who needs the thymic medulla? It seems that conventional SP thymocytes can do without it, but that Treg cells cannot (Figure 1). And although immunological tolerance is clearly impaired without an intact medulla, mechanisms that do not rely upon a full complement of mTEC can prevent autoimmune disease in organs such as the gut, lung and kidney. Future research will hopefully disentangle the relative importance of recessive tolerance mechanisms, such as SP thymocyte deletion, and dominant mechanisms like Treg cell selection in this unique microenvironment.
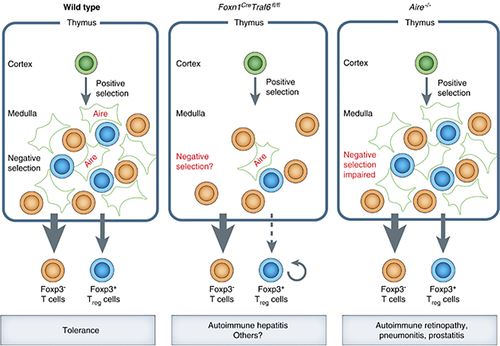
Schematic comparison of the consequences of medullary dysfunction. The medullary microenvironment in wild-type mice has many mTEC, some of which express Aire, which mediate negative selection and Treg cell differentiation to maintain immunological tolerance. In Foxn1CreTraf6fl/fl mice, severe mTEC deficiency reduces the numbers of thymic Treg cells, but not conventional T cells. Although the consequences of this defect on negative selection are not known, these mice develop autoimmune hepatitis. By comparison, Aire−/− mice have near-normal numbers of mTEC and Treg cells, but impaired negative selection leads to autoimmunity in several organs.





