Evolution of Endpoints for Renal Transplant Outcome
Abstract
Progressive improvement in short-term kidney transplant survival and reduction in acute rejection rates have restricted our ability to assess newer therapy. Past and present conventional endpoints, such as short-term graft survival and acute rejection rates, are no longer practical. This has prompted investigators to search for alternative endpoints. Long-term graft survival is an ideal endpoint. However, this requires a large cohort of patients with longer follow-up. A simpler approach would be to identify short-term markers, which can predict long-term survival. Short-term potential markers that can predict long-term survival are: clinical (renal function), histological (renal pathological markers) and immunological (anti-donor antibody, blood and urine cytokines). Post-transplant renal function estimated by serum creatinine, cystatin C and creatinine clearance within 1 year, and histological indices, as the Banff chronicity score, have the potential to predict long-term graft survival. Serum creatinine is limited as a marker by its variability based on recipient age, body weight, race and sex. Histological indices are limited, due to the invasive nature of evaluation. Post-transplant renal function and histological indices can be used potentially as a composite endpoint, in combination with conventional endpoints, such as graft loss, death and acute rejection. A practical approach for assessing newer therapies in future studies is to use composite endpoints, which combine conventional endpoints (graft loss, death, acute rejection) with newer endpoints (renal function, histological indices).
Introduction
Significant improvements in short- and long-term kidney transplantation survival rates and declining incidents of acute rejection present a paradox for researchers who are attempting to assess the efficacy of newer immunosuppressive agents. The conventional endpoints of patient and graft survival and acute rejection, which have been customarily used to measure renal outcome, are not practical due to fewer such events as newer therapies have yielded greater success.
Over the past two decades, the improving results allowed investigators to shift the focus of outcome assessments from patient and graft survival endpoint to acute rejection endpoint. From the use of prednisone in the early 1950s and azathioprine (Aza) in 1960s to cyclosporine A (CsA) in the 1980s (1–3), the introduction of the next generation of immunosuppressive agents in the 1990s, such as mycophenolate mofetil (MMF), tacrolimus (TAC) and sirolimus (SR) (4–9) and molecularly engineered humanized antibodies (10,11), have resulted in a steady decline in acute rejection rates and substantial improvements in short-term renal transplant survival. At the same time, long-term kidney graft survival has also substantially improved (12). Investigators are innovatively approaching the progress paradox by actively evaluating the use of alternative surrogate markers, such as renal function, histological findings and immunological markers, to assess immunosuppressive advances that will continue to improve long- and short-term outcomes.
This article briefly overviews the conventional endpoints that have been traditionally used to measure renal transplant outcome over the past 20 years, and discusses the advantages and disadvantages of using alternative short-term endpoints of renal function, histological findings and immunological markers. The discussion also includes the use of a composite endpoint, which is a combination of conventional endpoints (acute rejection, graft loss, death), and alternative newer endpoints (renal function, renal histology).
Conventional Endpoints
Conventional endpoints discussed here are short- and long-term graft survival and acute rejection. The advantages and disadvantages of these endpoints are shown in Table 1.
| Endpoints | Advantages | Disadvantages |
|---|---|---|
| Acute rejection | ||
| Biopsy proven Clinical acute rejection | Definitive marker Correlates with late failure | Vanishing endpoint, cost and time |
| Silent acute rejection | ||
| Short-term failure | ||
| Graft/patient loss | Gold standard | Vanishing endpoint |
| Long-term failure | ||
| Five-year graft/patient survival Half-life (years) | Definitive endpoint | Longer follow-up needed,cost, time and large cohort size needed |
| Renal function | ||
| Serum creatinine | Simple, reproducible | Dependant on age, sex, race and body weight |
| Creatinine clearance | More accurate | Cost, time and dependant on accurate collection |
| Cystatin C | Better than creatinine | Cost |
| Nuclear clearance | Accurate | Operator dependant, radioactivity, cost |
| Patient morbidity | ||
| Immunosuppressive related | Clinically relevant | Large cohort size required |
| (infections, tumors) | ||
| Non-immunosuppressive related (cardiovascular risk) | Related to age, renal disease, diabetes and obesity | |
| Renal histology | ||
| Interstitial fibrosis (CADI, BCI) | Accurate | Invasive, Cost and may be related to donor disease |
| Immunological markers | ||
| Anti-donor antibodies blood and urine cytokines | Immune specific | Detect only immunological graft failure, cost, availability |
| Composite endpoint | ||
| Treatment failure | Clinically relevant | Related to donor factors |
| 1) Acute rejection+ | ||
| graft loss+ death+ | ||
| elevated creatinine or | ||
| delta creatinine | ||
| 2) Acute rejection+ | Clinically relevant | Invasive, cost |
| graft loss+ death+ histological indices, delta histology score | ||
Short-term graft survival
Table 2 illustrates the immunosuppressive trials with endpoints and outcome used in prospective de novo renal transplant studies during the past two decades. Historically, graft failure at 1 year was used as a definitive endpoint (Figure 1). In a multi-centre European trial, a total of 232 cadaveric recipients were evaluated with CsA (n = 117) therapy and compared with Aza and prednisone (n = 115) treatment (1). After a short follow-up of 11 months, graft survival was found to be superior with CsA therapy (73% vs. 53%). Similarly, short-term graft survival benefit was seen in a Canadian trial with CsA, Aza and prednisone vs. Aza and prednisone therapy (80.4% vs. 64%), p = 0.003 (2). Thus, substantial short-term survival benefit with CsA therapy was detected in a small cohort of patients.
| Reference no. | Study | n | Publication year | Follow-up months | Bx proven AR (%) | Clinical AR (%) | Graft survival(%) | Death (%) | Rx failure (%) Death, graft loss, acute rejection | Renal function (mean) mg/dL | Short-term endpoints |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | CsA/P | 209 | 1983 | 12 | 80.4 | 2.9 | 2.6 | Patient/graft loss | |||
| Aza/P | 64.0 | 10.4 | 2.0 | ||||||||
| p | 0.003 | <0.03 | 0.003 | ||||||||
| 4 | MMF | 499 | 1995 | 6 | 19.80 | 26.3 | 94.5 | 3.6 | 31.7* | 1.91 | Treatment failure |
| 2 mg | |||||||||||
| MMF | 17.50 | 23.5 | 91.5 | 5.5 | 31.3** | 1.91 | |||||
| 3 mg | |||||||||||
| Aza | 38.00 | 45.8 | 88.6 | 5 | 47.6 | 2.37 | |||||
| p | *0.0015, | ||||||||||
| **0.0021 | |||||||||||
| 5 | MMF | 503 | 1996 | 6 | 19.70 | 31.8 | 95.4 | 0.5 | 38.2* | 2 | Treatment failure |
| 2 mg | |||||||||||
| MMF | 15.90 | 26.8 | 96.3 | 1.4 | 34.8** | 1.66 | |||||
| 3 mg | |||||||||||
| Aza | 35.50 | 48.2 | 95.8 | 1.4 | 50 | 1.99 | |||||
| p | *0.0045, | ||||||||||
| **<0.028 | |||||||||||
| 6 | MMF | 491 | 1995 | 6 | 17.0 | 93.3 | 30.3* | Treatment failure | |||
| 2 mg | |||||||||||
| MMF | 13.8 | 91.2 | 38.8** | ||||||||
| 3 mg | |||||||||||
| Placebo | 46.4 | 89.8 | 56 | ||||||||
| p | *<0.001, | ||||||||||
| **=0.001 | |||||||||||
| 7 | FK | 412 | 1997 | 12 | 30.7# | 91.2 | 4.4 | 1.66 | Acute rejection | ||
| CsA | 46.4 | 87.9 | 3.4 | 1.44 | |||||||
| p | 0.001 | ||||||||||
| 8 | SR | 576 | 2001 | 6 | 24.7* | 33.0* | 93.0 | 2 | 41.4 | 1.82 | Acute rejection |
| 2 mg | |||||||||||
| SR | 19.2** | 27.4** | 93.0 | 4 | 46.1* | 1.90 | |||||
| 5 mg | |||||||||||
| Placebo | 41.5 | 51.5 | 88.0 | 5 | 60.1** | 1.75 | |||||
| p | *0.003 | *= 0.004 | *=0.002 | ||||||||
| **<0.001 | **<0.001 | **=0.013 | |||||||||
| 9 | SR | 719 | 2000 | 6 | 16.9* | 91.9 | 5.3 | 18.7* | 1.81 | Acute rejection | |
| 2 mg | |||||||||||
| SR | 12.0** | 88.7 | 7.3 | 16.8** | 1.85 | ||||||
| 5 mg | |||||||||||
| Aza | 29.0 | 92.1 | 6.2 | 32.3 | 1.52 | ||||||
| p | *=0.002 | *=0.002 | |||||||||
| *<0.001 | **<0.001 |
- AR: acute rejection; Bx : biopsy, treatment failure: death, graft loss and acute rejection; Aza: azathioprine; CsA: cyclosporine; MMF: mycophenolate mofetil; FK: tacrolimus; P: prednisone; SR: sirolimus; Rx: treatment.
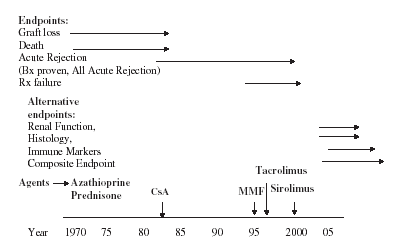
Timeline and potential future endpoints in renal transplantation.
Acute rejection
The benefit of CsA therapy was detected in improvements in 1-year graft survival. For subsequent immunosuppressive therapies, there was a shift from graft failure to acute rejection as an endpoint for measuring transplant results (Figure 1). Acute rejection is an immunological endpoint, which represents under immunosuppression, and it has been shown to adversely influence long-term graft survival (12). During the 1990s, immunosuppressive agents such as MMF, TAC, SR and IL-2 (Interleukin 2) receptor blockers were prospectively evaluated to measure acute rejection rates within 1 year post-transplantation as shown in Table 2 (4–11).
A total of 499 recipients were studied either with MMF 2 or 3 g daily or with Aza therapy (4). Reduced acute rejection rates at 6 months were observed with MMF 2 g daily (19.8%) and 3 g daily (17.5%), compared with Aza (38%) treatment (Table 2). This study used short-term treatment failures (graft loss, death and acute rejection) as an endpoint, rather than acute rejection rates alone, to assess the efficacy of MMF. Treatment failures were substantially lower with MMF (31.1% and 31.3%) compared with Aza therapy (47.6%), p = 0.0015, 0.0021, respectively (4). Similar results were obtained with MMF therapy in the European and Tri-continental trials, and treatment failure was considered as an endpoint to assess the impact of MMF over Aza (5,6). However, pooled analysis of these three studies (n = 1493) revealed significant reductions in short-term acute rejection rates with MMF therapy (13).
Additional trials have used biopsy proven acute rejection as an endpoint. In a randomized trial, TAC (n = 205) was compared with CsA (n = 207) when concomitantly treated with Aza and prednisone. TAC therapy was associated with lower acute rejection rates (30.7%) compared with CsA treatment (46.4%), p = 0.001 (7). In a recent trial, SR 2 and 5 mg daily (n = 284, 274 respectively) were compared with Aza therapy (n = 161) (8). The frequency of biopsy proven acute rejection at 6 months post-transplant was lower with SR 2 mg daily (18.7%) and 5 mg daily (16.8%) therapy compared with Aza (32.3%), p = 0.002, <0.001 (8). Similar results were obtained with SR use in an international study (9). In clinical trials with SR therapy, treatment failures (graft loss, patient loss and acute rejection) were also substantially lower with the trial agent. In the above three trials (FK vs. CsA and SR 2 mg or 5 mg vs. Aza), biopsy proven acute rejection was used as an endpoint. These trials were designed to address short-term acute rejection rates, but not graft survival.
Long-term survival prediction in those who develop acute rejection is dependant on the reversibility of acute rejection. Non-reversibility to baseline results in poor long-term graft survival. A progressive decline in acute rejection rates and absence of perfect correlation with long-term graft failure has limited its usefulness as an endpoint for future trials.
Long-term survival
It would be ideal to consider long-term survival as an endpoint for future studies. The current 5-year renal graft survival rate after cadaveric transplantation is approximately 65%. Functional graft survival (after censoring for death) at 5 years is approximately 75%. Approximately 50% of long-term graft failure during this period is due to death with a functioning kidney, and the remaining 50% due to chronic rejection and recurrent disease (14). However, Table 3 shows the sample size that would have to be studied in each group to detect various differences in 5-year functional survival with various levels of power. Detecting survival improvements by 3% or 5% would require 4195 or 1462 patients in each group.
| Power | 80% | 90% | 95% |
|---|---|---|---|
| Improvement in 5-year graft survival from 75% | |||
| 78% | 3133 | 4195 | 7334 |
| 80% | 1092 | 1462 | 2556 |
| 82% | 538 | 720 | 1258 |
| Improvement in renal function at 1 year from 1.6 mg/dL | |||
| 1.52 mg/dL (5% decline) | 1477 | 1977 | 2444 |
| 1.44 mg/dL (10% decline) | 370 | 494 | 611 |
| 1.36 mg/dL (15% decline) | 164 | 219 | 272 |
Patients enrolled in a CsA trial were followed for 4 years (3); those in MMF trials (US, European and Tricontinental) were followed for 3 years (15–17); and those in a TAC trial (TAC vs. CsA) were followed for 5 years (18). The long-term follow-up of the recipients enrolled in these short-term studies has not revealed any survival benefits. Interpretation of intent-to-treat results from a long-term follow-up remains an analytical problem. Measuring projected kidney half-life is an alternative method to evaluate long-term results. However, this approach also requires a large cohort of patients with a minimum follow-up of 3 years. Thus despite its definitiveness as an endpoint, the long follow-up and large cohort size needed for clinical trials preclude long-term survival as a gold standard endpoint. Evaluating alternative short-term endpoints, which can predict long-term survival, offers a more practical solution.
Short-term endpoints predicting long-term survival
Identifying markers within a few months after transplantation that can be used to predict long-term survival is a valuable method for early identification of patients with potentially poorer long-term outcome. Such endpoints can be classified as clinical, histological and immunological types. Potential advantages and disadvantages are shown in Table 1.
Clinical Endpoints
Serum creatinine
Post-transplant renal function within a few days after transplantation (19) or at the time of discharge from the hospital (20) has been correlated with long-term survival. Renal function may be estimated by the serum creatinine level. Serum creatinine at 6 and 12 months after renal transplantation has been shown to correlate with long-term graft survival. This has been illustrated in a large database, as well as a single center analysis (21,22). Specifically, analysis of UNOS/OPTN data on 105,742 adult renal transplant recipients has clearly shown that 6-month and 1-year creatinine >1.5 mg/dL correlates with lower long-term graft survival (21). The projected median graft half-life of a cadaveric kidney transplant with creatinine ≤ and >1.5 mg/dL was 15.8 and 8.3 years, respectively (N. Siddiqi, March 2003, unpublished data). For each elevation of serum creatinine by 1.0 mg/dL at 1 year without a change in serum creatinine from 6 months, the risk of graft failure increases by 63%, p < 0.0001. However, when a serum creatinine value increases by 0.5 mg/dL from 6 months to 12 months, the risk of graft failure is increased by 126%, p < 0.0001. The delta creatinine (change in creatinine values from 6 to 12 months) is even more sensitive for its correlation with long-term survival. In the same analysis, when acute rejection and serum creatinine were both included in the model, it was renal function that impacted graft survival, not acute rejection episodes. Thus, renal function served as an important marker, by identifying a group of recipients with elevated serum creatinine, caused by poor response to acute rejection treatment or to other reasons.
To differentiate the efficacy of newer therapy, it is conceivable to use short-term renal function as an endpoint. Table 3 shows the number of patients required for such a study that can evaluate the difference in renal function between the control and the trial group. This sample size is adjusted for 7% graft failure during the first year and assumes an alpha level of 0.05. For example, for 90% power, to detect a 5% and 10% decline in serum creatinine, one will need to evaluate 1977 and 494 patients in each group. Such an evaluation is valid provided that acute rejection rates are similar between groups.
Creatinine clearance
Calculated clearance [(Cockcroft–Gault method, MDRD method) used in clinical practice] or clearance studies, such as 24-h urine collection or nuclear studies (used in clinical trials) have been shown to improve the accuracy of short-term renal function as an endpoint for predicting long-term outcome. In a recent retrospective study, the calculated clearance at 6-months and 1-year has been shown to correlate with long-term survival (23). This justifies the use of serum creatinine as a marker, due to its simplicity over creatinine clearance. Renal function measured by serum creatinine and clearance as an endpoint should be validated in future prospective studies.
Change in 1/creatinine has been evaluated to predict transplant outcome (24). Kasiske et al. evaluated 101 transplant recipients and detected changes in 1/creatinine and creatinine clearance as strong predictors of transplant failure. A decline in 1/creatinine of 40% increases risks of graft failure by 491%, p < 0.0001. However, the authors did not find the slope of 1/creatinine to correlate with transplant failure. It should be noted that these studies were done in patients who were not receiving CsA therapy.
Cystatin C
Estimation of cystatin C is an alternative marker for evaluating renal function. Cystatin C has been compared with serum creatinine and creatinine clearance in renal transplant recipients (25). Plasma cystatin C correlates with plasma creatinine (r = 0.741, p < 0.0001) and creatinine clearance (r = 0.882, p < 0.001). The diagnostic accuracy of cystatin C is slightly better than serum creatinine; however, it is not different from creatinine clearance or β2 microglobulin and does not appear to have any advantage over creatinine clearance. Although it has a marginal benefit over serum creatinine, its cost limits its potential use (26). The use of cystatin C is also dependant on its free availability and validation in large clinical trials.
Elevated serum creatinine is related to immunological events such as acute rejection, which is derived from degree of HLA mismatches, elevated PRA, prior transplantation and prolonged cold ischemia time, and to nonimmunological recipient and donor demographics variables, such as recipient's race (black) and sex (male), and donor age (increasing), race (black) and sex (female). Because these variables influence renal function, short-term renal function is limited as a stand-alone endpoint, in predicting long-term graft survival. In addition, use of noncalcinuerine inhibitor regimen may result in lower serum creatinine, but may result in higher acute rejection rates. Both acute rejection and elevated creatinine are known to impact long-term survival. Thus, a balance between acute rejection rates (which signifies lower immunosuppression) and elevated creatinine (which represents poor long-term survival) is an important measure in evaluating transplant success.
Renal Histology
Renal histological findings pre-dates renal dysfunction and hence become an attractive short-term endpoint.
Histological acute rejection
Until recently, renal histological evaluation, a gold standard in evaluating transplant renal dysfunction, was reserved for patients who experienced renal dysfunction. Rush and co-workers have now performed renal biopsies in transplant recipients with stable renal function (27). The diagnosis of subclinical acute rejection has resulted in preservation of renal function without impacting graft success on long-term follow-up (27). However, a recent study revealed a low prevalence of silent acute rejection with MMF and TAC combination therapy (28). Although the additional costs associated with detecting silent acute rejection and the ability to ensure a 100% biopsy rate are a challenge to logistics, future studies should include protocol biopsies to detect silent rejection as an alternative endpoint.
Chronic allograft nephropathy
Chronic allograft nephropathy (CAN) is an important cause of late graft failure that has not been evaluated in clinical trials, mainly because a large patient pool is required to evaluate the difference between protocols with prolonged follow-up (Table 3). Instead, focus should be directed to diagnosing clinical or subclinical histological chronic changes.
Renal scarring has been quantified using chronic indices such as the Banff Score Index and the Chronic Allograft Disease Index (CADI) score, and has been correlated with late graft failure (29,30). The CADI score from protocol renal biopsies at 2 years correlates with graft function at 6 years (r = 0.717, p = 0.0001) with a strong prediction to development of CAN (29). Histological findings, such as macrophages in the renal graft (peritubular capillary lesions detected by electron microscopic evaluation within 1-year post-transplant) also have been correlated with histological chronic rejection (31,32). A computerized histomorphometric assessment of protocol renal biopsies also has correlated with lower glomerular filtration rates (33). The fraction of collagen III immunostain > 40% at 6-months post-transplant correlates with GFR at 2 years. A simple histological evaluation using CAN with and without transplant vasculopathy has been shown to correlate with long-term survival (34). The data suggest that the early histological evaluation after transplantation or perhaps quantifying the change in degree of scarring from transplantation to 3 or 6 months post-transplantation are potential endpoints worth validating in clinical trials. However, the invasive nature of biopsies and associated time, cost and morbidity are the key limiting factors. The ability to assess renal histological parameters also depends on the availability of this information on all patients enrolled in a clinical trial.
Increasing donor age may adversely affect graft survival (35). Differentiating the histological features of donor disease from the early events after transplantation (DGF, acute rejection and drug toxicities) remains a huge challenge. Nevertheless, these histological endpoints such as the Banff chronicity scores are important and should be included in future clinical trials. With limited organ availability and possible future secondary interventional trials, which may delay graft failure, early detection of chronic histological findings is an important marker to be considered for transplant evaluation.
Currently used endpoint such as acute rejection and proposed endpoints such as renal function and scarring have a good correlation, but without satisfactory positive and negative predictive values. Post-transplant renal function predicting long-term survival is also dependant on duration of follow-up and progression of renal disease. Immunosuppressive therapy without a calcinuerine inhibitor and management of hypertension with angiotensin converting enzyme inhibitor or angiotensin receptor blocker can alter the progression of renal failure. Identifying an appropriate surrogate marker with a high positive and negative predictive value will remain a challenge.
Immunological Markers
A better understanding of immunology and the introduction of potent immunosuppressive agents aimed at blocking specific target pathways have yielded substantial short-term success; therefore, identification of specific immunological markers, which can be detected before the development of renal dysfunction, should be assessed.
Anti-donor antibodies
The degree of HLA mismatching has had a substantial effect on short- and long-term transplant outcome (36). The detection of anti-donor antibodies early after transplantation has been correlated with immunological injury and renal dysfunction (37). Prospective clinical studies are warranted to evaluate the use of anti-donor antibodies as a marker for measuring transplant outcome.
Tissue, blood and urine immune markers
The immunological acceptance of a graft can be evaluated using blood, tissue and urine cytokines. Transforming growth factor-β (TGF-β) has been used as a marker for acute rejection, and has also been correlated with calcineurin inhibitor toxicity and nonspecific fibrosis (38). Markers such as granzyme B and perforin have more specificity for acute rejection. Li et al. have demonstrated that a measurement of perforin and granzyme from urinary cells from transplant recipients is correlated with acute rejection (39). However, these require confirmation and lack very good sensitivity and specificity. Although the reproducibility and availability of such markers in clinical trials and practice will remain a challenge, they are attractive future endpoints, due to their noninvasive nature.
Morbidity and mortality
The risk of death after renal transplantation is higher during the first 100 days post-transplant, when compared with patients who are maintained on dialysis treatment (40). However, after 100 days, the overall risk of death falls to less than that of wait-listed dialysis patients (40). Still, the most important cause of long-term graft failure is death with a functioning graft (41). Mortality after transplantation is a component of composite endpoint; however, it cannot be used as a solitary endpoint, as few deaths occur during the first year.
Immunosuppression cannot be achieved without immunosuppressive side-effects. Overt over- immunosuppressive side-effects seen after transplantation are infections (CMV, EBV, pnuemocystis carinii, polyoma virus) and tumors (lymphomas, skin cancers), which should be included in transplant outcome measurement. Similarly, nonimmunosuppressive-related morbidities, such as cardiovascular risk factors (hypertension, diabetes and hyperlipidemia) are important events, which adversely influence long-term graft and patient survival. As the rates of acute rejection and late graft failure continue to decline, morbidity is becoming increasingly relevant in clinical practice. Measuring morbidity endpoint is not only vital to enhance long-term patient and graft survival, but also provides an opportunity to identify their correlation with newer therapies. The number and percent of patients experiencing efficacy failures (acute rejection, immunological graft loss) and mortality/morbidity endpoints (death, nonimmunological graft loss, infections and tumors) should be measured in future clinical trials. Similarly, morbidities that affect long-term survival (e.g. cardiovascular risk factors – hypertension, hyperlipidemia and diabetes) should be measured separately.
Composite Endpoint
Conventional endpoints, such as acute rejection, graft loss and death are important, but can no longer stand alone for future clinical trials. Although limited as single endpoints, the potential candidates discussed above may be suitable as combination endpoints for future clinical trials. Such potential combinations should include conventional endpoints (graft loss, death, acute rejection), and newer endpoints (renal function, renal histological indices). These are discussed below: the composite endpoint incorporating renal function and acute rejection should either include:
- 1
acute rejection, graft loss, death and serum creatinine >1.5 or 2.0 mg/dL at 1 year post-transplant, or
- 2
acute rejection, graft loss or death at 1 year post-transplant and delta creatinine >0.3 mg/dL from 6 months to 1 year.
A change in serum creatinine (delta) from 6 months to 1 year reveals a dynamic status of renal function and correlates better than 6 months or 1 years serum creatinine values alone for long-term graft failure (21). Incorporating such a composite endpoint is simple, economical and critical for future success. Such an approach will evaluate the immunological injury (acute rejection) and nonimmunological injury (nephrotoxicity), which are known to impact long-term survival. This also evaluates the balances between under immunosuppression (acute rejection, immunological graft loss) and overimmunosuppression (death, nephrotoxicity and nonimmunological graft loss) with immunosuppressive therapies.
Histological renal injury results from immunological events (silent acute rejection) and nonimmunological injury (nephrotoxicity, donor disease). The composite endpoint incorporating renal histology should either include:
- 1
acute rejection, graft loss, death at 1 year post-transplant and renal histological findings at 3 or 6 months (silent acute rejection), or
- 2
acute rejection, graft loss, death at 1 year post-transplant and renal histological findings at 3 or 6 months (chronicity index).
The above approach will aid in identifying a group of transplant recipients who have suboptimal graft survival, resulting from ongoing renal damage due to either rejection or toxicity despite of stable renal function. Immunosuppressive therapy without a calcineurin inhibitor has been shown to preserve graft function; however, this is associated with higher rejection rates. The composite endpoints presented here include conventional endpoints (death and graft loss), with either an immunological event (silent acute rejection) or a nonimmunological marker (chronicity index), as a tool to assess transplant outcome. This distinguishes a subset of recipients who either receive less or more immunosuppressive therapy, which adversely influences graft success.
The potential composite endpoints described above can be measured as an event or as a weighted score. For example, assign a hypothetical score of 1.0 for death, 0.5 for graft loss, and 0.25 for acute rejection, serum creatinine >1.5 mg/dL or chronicity score. For 100 cadaveric transplants at 1 year, events can be measured as a cumulative number: death – 3%, graft loss – 8% and acute rejection – 16%, and graft dysfunction – 40%. The cumulative score for these events is 67; the weighted score is 21. The goal of interventional immunosuppressive trials is to decrease the weighted score (by creating a balance between acute rejection and renal dysfunction) without altering mortality or graft failure.
The declining rate of mortality, graft failure and acute rejection has made current endpoints impractical for assessing transplantation results. The use of composite endpoint accounts for events such as renal dysfunction or renal scarring, and thus, increases the probability of correlating these events with poor long-term transplant outcome. Such an approach also opens a new avenue to use immunosuppressive agents, which can preserve renal function despite increasing acute rejection rates.
Composite endpoints described above should be evaluated in a retrospective fashion using large database such as UNOS. By this investigators should evaluate the impact of transplant year or the efficacy of one immunosuppressive agent over another after correcting for all key variables. This will open an avenue towards using a composite endpoint for future immunosuppressive trials.
Conclusion
In conclusion, improvements in standard outcomes of graft survival and declining rates of acute rejection are making it necessary to adopt newer endpoints for assessing transplant outcome. Short-term markers, which can predict long-term survival, should be incorporated in evaluating transplant results. Such markers, including post-transplant renal function, as it correlates with long-term outcome, should be implemented in clinical practice as well as in clinical trials. Other markers such as Banff scores obtained from renal histological evaluation should be included in future clinical trials. Immunological markers, such as anti-donor antibodies and measurement of blood, tissue and urine cytokines, remain a challenge and will change our approach to measure and alter transplant outcome. Measuring morbidity and mortality as separate endpoints will alter our approach in assessing newer combination immunosuppressive agents. However, these newer endpoints will not stand by themselves in the current era. The most practical approach is to consider composite endpoints in which newer endpoints such as renal function and/or histological indices should be combined with conventional endpoints such as acute rejection, graft loss and death. As current transplant outcome is equally or perhaps more influenced by nonimmunological factors, it is mandatory to include renal function and or chronicity scores, which are derived from both immune and nonimmune events influencing transplant outcome. The ultimate test for a new endpoint is that it measures or strongly predicts an important aspect of the integrity of the graft and/or the health of the recipient. In addition, if it is to be used in a trial, there must be a strong expectation that it can be modified significantly by the agent being evaluated. Only then will it be convincing to the transplant community and acceptable to the regulatory bodies.




