Interaction Between ICOS-B7RP1 and B7-CD28 Costimulatory Pathways in Alloimmune Responses In Vivo
Abstract
The B7-CD28 pathway is one of the foremost costimulatory pathways involved in T-cell activation. Recently, a number of additional costimulatory pathways have been described and preliminary data suggest that they play important roles in alloimmunity. However, the interactions between these different pathways are not well understood. We studied the effect of targeting ICOS ligand, B7RP1, in a rat cardiac transplant model, with and without concomitant blockade of the B7 pathway using CTLA4Ig. In a fully mismatched WF to LEW vascularized cardiac allograft model, without therapy, grafts were acutely rejected (MST 10.8 ± 1.6 days). Early (day of transplant) B7RP1 blockade with ICOSIg alone had little effect on graft survival and rather than being additive with B7 blockade, ICOSIg abrogated the prolonged graft survival induced by CTLA4Ig treatment. By contrast, delayed (day 2 post-transplant) blockade of B7RP1 did not have such an effect. These findings were not related to cytokine deviation but may be in part related to the pattern of down-regulation of B7.2 expression following early B7RP1 blockade. This is the first report describing the complex interactions between ICOS-B7RP1 and B7-CD28 costimulatory pathways in alloimmunity in vivo.
Introduction
It is now well established that complete T-cell activation requires both an antigen-dependent signal through the T-cell receptor and a second antigen-independent signal provided by engagement of costimulatory molecules, the prototypic and most significant of which is the B7-CD28 signalling pathway (1). Naïve T cells are crucially dependent on the engagement of B7 with the stimulatory CD28 molecule. However, the capacity of T cells from CD28-deficient animals to initiate an immune response and the relative B7-independence of recall T-cell responses suggests that alternative costimulatory pathways operate under certain circumstances (2,3). This is demonstrated in allotransplantation models by the failure of B7-CD28 pathway blockade to reproducibly induce transplant tolerance or prevent graft loss as a result of chronic rejection, especially in stringent transplant models (4–6). Recently a number of novel receptor-ligand pairs have been described that act as efficient T-cell costimulatory molecules (7,8). These include members of the B7-CD28 family such as inducible costimulator (ICOS) and its ligand B7RP1, as well as members of the TNF superfamily (8,9). The large number of such costimulatory molecules results in a complex network of potential interactions and interdependence, of which we still understand relatively little, but which may have profound effects on how we attempt to utilise these pathways therapeutically.
The ICOS-B7RP1 pathway has been shown to play a role in both alloimmune and autoimmune responses (10–16). In fully MHC-mismatched murine and rat allotransplantation models, its inhibition results in a modest prolongation of cardiac and liver allograft survival (10,13). Furthermore, it contributes towards chronic graft rejection, as its inhibition synergizes with CD154 blockade and abrogates the development of transplant arteriosclerosis (10). Interestingly, in murine experimental autoimmune encephalomyelitis (EAE), ICOS pathway blockade may ameliorate or exacerbate disease depending on the timing of its administration. The reasons for these divergent effects are not completely clear, although differences were found in T-cell IFN-γ production during the priming vs. efferent phases of the immune response (11).
We now report for the first time on the interactions between the ICOS-B7RP1 pathway with the B7-CD28 pathway in a fully MHC-mismatched rat cardiac transplantation model.
Materials and Methods
Animals
WF (MHC-RT1u), and LEW (MHC-RT1l) rats were purchased from Charles River (Wilmington, MA). All animals were housed in accordance with institutional and National Institutes of Health guidelines.
Heterotopic heart transplantation
Vascularized heart grafts were transplanted using microsurgical techniques as previously described (17). Briefly, the harvested donor heart was placed in 4 °C saline until transplantation. The recipient rat was anaesthetized by intraperitoneal injection of xylazine and ketamine. The donor aorta was sutured to the recipient aorta and the donor pulmonary artery to the recipient inferior vena cava end-to-side using 10–0 suture. Transplant function was evaluated by daily abdominal palpation. Rejection was defined as complete cessation of a palpable cardiac beat and was confirmed by direct visualization. Loss of graft function within 48 h of transplantation was considered a technical failure (<5% overall), and these animals were omitted from further analysis. Graft survival was subjected to the log-rank test for statistical comparison.
Fusion proteins, immunoglobulins and treatment protocols
All antibodies and fusion proteins were administered intraperitoneally. ICOSIg, consisting of the murine-ICOS molecule joined to human IgG-Fc, was a kind gift from Dr Toshimitsu Uede (Division of Molecular Immunology, Institute for Genetic Medicine, Hokkaido University School of Medicine, Sapporo, Japan) and was purified from an adenoviral transfection system by BioExpress Inc. (West Lebanon, NH). Early treatment consisted of a dose of 500 µg on the day of transplantation, followed by three doses of 250 µg on days 2, 4 and 6. Late treatment consisted of the same protocol but starting 2 days post-transplantation. Humanised CTLA4Ig was a kind gift of Dr Robert Peach (Bristol Myers Squibb, Princeton, NJ) and was given as a single injection of 500 µg on day 2 post-transplantation. Control human IgG (Sigma, St Louis, MO) was given in an identical manner to the ICOSIg. In some recipients, donor-specific transfusion consisting of 30 million WF splenocytes were injected intravenously on the day of transplantation.
Flow cytometry
Single-cell suspensions of splenocytes from transplanted animals were produced and red cells lysed using ACKLysing buffer (Biowhittaker, Walkersville, MD). Cells (1 × 106) were resuspended in PBS containing 1% fetal calf serum and stained with PE-labelled anti-CD80, or anti-CD86 and counterstained with FITC-labelled anti-CD3 mAb (all PharMingen, San Diego, CA) or anti-ED1 (Peninsula Laboratories, San Carlos, CA). Stained cells were analyzed on a FACSCalibur (Becton Dickinson, San Jose, CA) using Cellquest software (Becton Dickinson). Results were expressed as the percentage of total lymph node cells, or ED1-positive cells expressing CD80 or CD86.
Mixed lymphocyte reactions
Single-cell suspensions of splenocytes were produced and red cells lysed using ACKlysing buffer (Biowhittaker). Responder LEW splenocytes (5 × 105 cells) were incubated in a round-bottom 96-well plate with irradiated (3000 rads) stimulator WF cells at a 1 : 1 ratio. To some of the wells doubling concentrations of the fusion proteins or control human IgG were added. Cells were incubated for 4 days, at which time supernatants were collected. Wells were pulsed with 1 µCi of cH-thymidine/well (PerkinElmer Life Sciences, Boston, MA) for the last 16 h of incubation. Plates were harvested onto glass fibre filters (PerkinElmer Life Sciences), using an automated harvester (Tomtec, Hamden, CT) and thymidine incorporation measured using an automated β counter (PerkinElmer Life Sciences). Results were expressed as counts per minute (c.p.m.) for triplicate wells. IFN-γ production was assessed by ELISA performed on the culture supernatants using an IFN-γ Cytoscreen kit (Biosource International, Camarillo, CA) according to manufacturer's instructions.
ELISPOT analysis
Single-cell suspensions of splenocytes were produced and red cells lysed using ACKLysing buffer (Biowhittaker). Viable cells were enumerated using Trypan blue (Sigma) exclusion. The ELISPOT assay has previously been described (18,19) and was adapted to measure IL-4 and IFN-γ secreting cells. ELISASpot plates (Cellular Technology Limited, Cleveland, OH) were coated with capture antibodies against IL-4 (clone OX-81) or IFNγ (OPTEIA IFN set) (both PharMingen) in phosphate-buffered saline (PBS) and left overnight at 4 °C. The plates were blocked with PBS-BSA 1% for 1 h and then washed with PBS. A total of 5 × 105 splenocytes were added to each well in 100 µL of complete RPMI 1640 medium containing 10% fetal calf serum (Sigma), 2 mM of L-Glutamine, 100 U/mL of Penicillin/Streptomycin (BioWhittaker) and 50 mM of 2-mercaptoethanol (Sigma). Control wells contained responder splenocytes plus medium alone. Cells were incubated with irradiated (3000 rads) donor splenocytes at a ratio of 1 : 1. After 36–48 h, the plates were washed thoroughly, biotinylated detection anti-IL-4 (clone B11-3) and IFN-γ (OPTEIA IFN set) (Pharmingen) antibodies added and the plates were left for a further overnight incubation at 4 °C. After further washing, Horseradish peroxidase-conjugate (Dako, Carpinteria, CA) was added for 2 h at room temperature. Development was with 200 µL per well of AEC (Pierre pharmaceuticals, Rockland, IL). The resulting spots were counted on a computer-assisted ELISASpot Image Analyser (Cellular Technology Ltd). The results were then calculated as cytokine-producing cells per million splenocytes (20,21).
Histopathology
Transplanted allogeneic hearts were removed 7 days following transplantation and fixed in 10% buffered formalin (Sigma) and embedded in paraffin. Coronal sections were taken, stained with Hemotoxylin and Eosin using standard procedures and assessed in a blinded fashion using light microscopy.
Results
Effect of ICOSIg with or without CTLA4Ig on cardiac allograft survival
Untreated recipients of major mismatched cardiac allografts promptly rejected their grafts (MST: 10.8 ± 1.6 days, n = 10) (Figure 1). Treatment with single injection of CTLA4Ig on day 2 post-transplantation significantly prolonged cardiac allograft survival (21 ± 2.1 days, n = 5, p = 0.0038) (Figure 1), as previously reported (17,22). Therapy with ICOSIg (500 µg on the day of transplantation, 250 µg on days 2, 4 and 6) alone had no effect on graft survival (10.5 ± 1.3 days, n = 6) (Figure 1). The addition of ICOSIg to CTLA4Ig resulted in accelerated rejection of cardiac allografts when compared with CTLA4Ig alone (12 ± 1.8 days, n = 6, p = 0.0085 compared with CTLA4Ig treatment alone) (Figure 1). Indeed, allograft survival in recipients treated with ICOSIg plus CTLA4Ig was comparable to that of untreated controls (Figure 1). Addition of control human IgG to CTLA4Ig had no significant effect on graft survival (18.5 ± 0.96, n = 4, p = NS vs. CTLA4Ig alone).
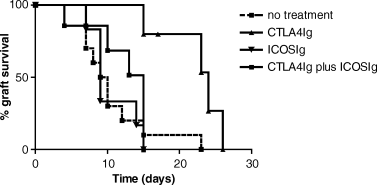
Kaplein–Meyer survival graph of cardiac allografts in control animals and those treated with early ICOSIg, CTLA4Ig, or a combination of early ICOSIg and CTLA4Ig. Untreated control animals acutely rejected their grafts (mean survival time 10.8 ± 1.6 days, n = 10). ICOSIg alone has no effect on allograft survival (10.5 ± 1.3 days, n = 6), whereas CTLA4Ig significantly prolonged allograft survival compared with untreated control animals (21 ± 2.1 days, n = 5, p = 0.0038). Combined ICOSIg and CTLA4Ig treatment resulted in abrogation of the beneficial effect of CTLA4Ig alone (12 ± 1.8 days, n = 6, p = 0.0085 compared with CTLA4Ig treatment alone) and resulted in similar survival to control animals.
Histological analysis of the transplanted cardiac allografts at day 7 post-transplantation reflected the survival data. The control allografts demonstrated the most severe pathology, with extensive, diffuse transmural parenchymal necrosis, followed in order by the ICOSIg-treated animals, the combined ICOSIg and CTLA4Ig-treated animals and finally the most preserved tissue was seen in the CTLA4Ig-only treated animals.
It has been previously demonstrated that administration of donor splenocytes (DST) on the day of transplantation followed by delayed blockade of B7 by CTLA4Ig results in long-term cardiac allograft survival with donor-specific tolerance (22) and protection from chronic allograft vasculopathy (23). Therefore, we tested the effect of ICOSIg on the combined DST plus CTLA4Ig protocol. Figure 2 demonstrates that DST in combination with CTLA4Ig resulted in long-term graft survival in all animals (>200 days, n = 6). The addition of ICOSIg to this protocol significantly accelerated allograft rejection (n = 7, p = 0.02 against DST and CTLA4Ig); >70% of the grafts were rejected within 30 days (Figure 2).
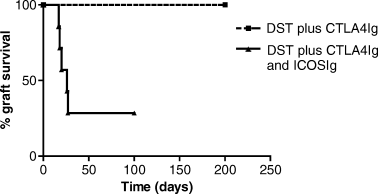
Kaplein–Meyer survival graph of cardiac allografts in animals treated with donor-specific transfusion (DST) and CTLA4Ig or DST combined with CTLA4Ig and ICOSIg. The addition of ICOSIg abrogates the long-term graft survival (>200 days) induced by the DST and CTLA4Ig in 71% of the animals (n = 7, p = 0.02 against DST and CTLA4Ig).
One potential explanation for the observed effects of ICOSIg therapy on allograft survival is that ICOSIg may be a stimulatory molecule. In order to examine this possibility we set up a mixed lymphocyte reaction (MLR) assay using irradiated WF (stimulator) and LEW (responders) splenocytes in the presence of increasing concentrations of fusion proteins, control human IgG or medium alone. The addition of ICOSIg had little effect on cell proliferation, similar to control human Ig, indicating that it is not a stimulatory molecule in vitro. Only the addition of CTLA4Ig significantly inhibited the MLR in a dose–dependent manner with complete inhibition at concentrations of 10–50 µg/mL. The addition of ICOSIg had no effect on the inhibitory effect of CTLA4Ig alone in vitro (data not shown) with respect to proliferation or IFN-γ production.
Combined ICOSIg and CTLA4Ig does not induce a cytokine deviation
It was first suggested that ICOS signalling may be important in Th2 cell differentiation (14,24). Therefore, ICOSIg therapy may be blocking generation and/or function of regulatory Th2 cells induced by B7 blockade (25). In order to examine this possibility we set up an ELISPOT assay to measure the frequency of Th1 (IFN-γ) and Th2 (IL-4) alloreactive T cells in recipients treated with CTLA4Ig with or without ICOSIg. One week following transplantation splenocytes were isolated from recipients treated with CTLA4Ig alone, ICOSIg alone, combined treatment or no treatment and ELISPOT analysis carried out. A marked decline (ranging from 67 to 89%) in the frequency of IFN-γ producing alloreactive cells was demonstrated in the CTLA4Ig treatment group (Figure 3). While in the ICOSIg alone treated animals there was an increase in the frequency of IFN-γ producing cells (up to 70%), and in the combined ICOSIg and CTLA4Ig-treated group there was a decline in the IFN-γ frequency of similar magnitude to the CTLA4Ig alone (70–95%). Moreover, no difference in the IL-4-producing frequencies was observed between the CTLA4Ig-treated group and the combined CTLA4Ig plus ICOSIg-treated group.
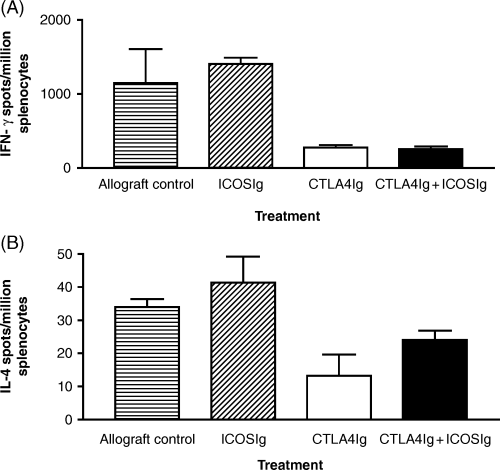
Graph demonstrating the frequency of cytokine-producing alloreactive splenocytes 7 days following transplantation, from control animals and those treated with ICOSIg, CTLA4Ig or the combination of CTLA4Ig and ICOSIg. In comparison with the untreated allograft control animals, there is a modest increase in frequency of IFN-γ-producing cells and no change in the frequency of IL-4-producing cells, in animals treated with ICOSIg. In the CTLA4Ig-treated animals there is a decrease in frequency of both IFN-γ- and IL-4-producing cells. However, there is no difference in the pattern of cytokine production between the CTLA4Ig- and the combined CTLA4Ig and ICOSIg-treated animals. Results are shown as the mean and standard error of the mean for triplicate wells and are representative of four experiments with one animal per experimental group.
ICOSIg down-regulates expression of B7.1 and B7.2 in vivo
Recent data have indicated the intimate relationship between ICOS expression and CD28/CTLA4 ligation. ICOS up-regulation is inhibited in the absence of B7, but is restored following CD28 ligation (26), while CTLA4 ligation blocks ICOS costimulation by preventing cell-surface expression and downstream second signals (27). We therefore wanted to examine the effect of B7RP1 ligation on the B7-CD28 pathway by using ICOSIg therapy and assessing expression of B7.1 and B7.2 in vivo. Two days following cardiac transplantation (time point when CTLA4Ig is administered) in the untreated control animals or those treated with ICOSIg, the spleen and draining lymph node cells were removed and cells stained for CD3, ED-1, B7.1 and B7.2. In comparison to the untreated transplanted controls, the ICOSIg-treated animals demonstrated a decrease in the level of expression of both B7.1 and B7.2 on lymph node cells including ED1 + monocytes/macrophages (Figure 4), and to a lesser degree on CD3+ T cells (data not shown), although only the change in B7.2 was statistically significant (p = 0.028 by Mann–Whitney U-test). In addition similar changes were demonstrated in cells taken from the spleen (data not shown). In order to conclusively show that the down-regulation of B7 expression is mechanistically linked to the abrogation of the beneficial effect of CTLA4Ig by ICOSIg, we delayed the administration of ICOSIg to day 2 post-transplantation, thus allowing B7 up-regulation to occur and administering it concomitantly with CTLA4Ig. This protocol did not result in acceleration of graft rejection, but rather produced similar graft survival to the CTLA4Ig alone (24.3 ± 3.5 days, n = 4, p = NS compared to CTLA4Ig alone) (Figure 5). Finally, we investigated whether ICOSIg has a direct effect on antigen-presenting cells (APCs) resulting in diminished B7 expression in vitro. Using T-cell depleted (adherence and CD3 magnetic bead depletion) splenocytes we could not demonstrate such an affect on APCs, indicating that under the conditions tested the fusion protein has no direct effect on APCs and that its effect appears to be related to targeting ICOS–B7RP1 interactions.
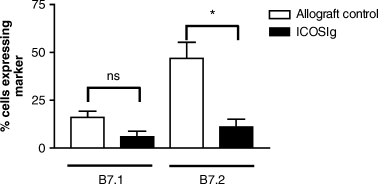
Graph showing the percentage of draining lymph node cells expressing B7.1 and B7.2, taken 2 days following transplantation, from animals treated with or without ICOSIg on the day of transplantation. Following ICOSIg treatment there is marked down-regulation of both B7.l and B7.2 expression, although only the latter is statistically significant (p = 0.028 by two tailed Mann–Whitney U-test). Similar patterns of B7 down-regulation were obtained for ED1+ cells as well as CD3+ T cells although to a lesser extent (not shown). Results are combined from four separate experiments with two animals per experiment.
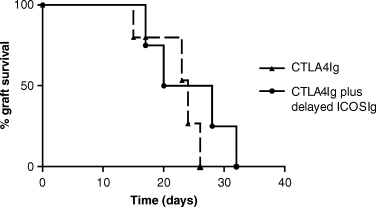
Kaplein–Meyer survival graph of cardiac allografts in animals treated with CTLA4Ig with or without delayed ICOSIg. There was no difference in allograft survival between animals treated with CTLA4Ig alone or CTLA4Ig and delayed ICOSIg.
Discussion
The potential of costimulatory blockade as a therapeutic modality for clinical transplantation has not yet been fulfilled (28,29). In part this is because of the failure of costimulatory blockade (targeting B7 and CD40 pathways) to completely abrogate alloreactive T-cell activation, especially in stringent transplant models (4–6,30). Recently, it has been appreciated that a number of alternative costimulatory pathways, such as the ICOS-B7RP1 pathway, exist and that these may serve to fully activate the alloreactive T cells (7,31–33). Individually targeting these pathways may not be sufficient to completely prevent alloreactive T cells from becoming activated, and so combination therapy may be more attractive as a therapeutic tolerogenic strategy. However, the interactions between the different pathways and their interdependence on each other are only now becoming clear. This is especially true for the ICOS-B7RP1, B7-CD28/CTLA4 and CD40-CD154 pathways (26,27,34). For example, T-cell ICOS up-regulation is reduced in the absence of B7.1 and B7.2, but is restored following CD28 stimulation (26), whereas CTLA4 ligation blocks ICOS costimulation by preventing cell-surface expression and downstream second signals (27). Moreover, ICOS signalling, which is essential for immunoglobulin class switching, is dependent on the CD40-CD154 pathway for this effect (34). These data demonstrate that these pathways are intimately linked. Furthermore, important clinical consequences arise from these interactions, such as ICOS pathway blockade synergizing with CD154 blockade to prevent chronic allograft rejection, while either strategy alone has only limited effect (10). We therefore set out to investigate how the B7RP1-ICOS and B7-CD28/CTLA4 pathways interact in allotransplantation and how they may be exploited therapeutically.
Our data clearly demonstrate that certain regimens of combined ICOS-B7RP1 and B7-CD28 pathway blockade may abrogate the beneficial effects of single pathway B7-CD28 blockade. This effect is critically dependent on the timing of administration of the biological agents. Early ICOS pathway blockade results in the loss of the beneficial effect of CTLA4Ig. This is not because of T-cell signalling by the ICOSIg nor is it because of an altered Th1/Th2 cytokine profile, consistent with more recent data demonstrating ICOS regulation of both Th1 and Th2 cytokine production (15,35). Rather, it relates to the diminution of B7 expression on APCs following B7RP1 ligation by ICOSIg. We only found a statistical reduction in B7.2, but this is consistent with previous data demonstrating that blockade of B7.2 alone was sufficient to induce long-term graft survival (36). We believe that this represents the first description of a direct effect of B7RP1 ligation and ICOS pathway blockade on B7 expression. Moreover, this is in keeping with a recent report demonstrating that CTLA4Ig ligation of B7 molecules on the APC results in signal transduction in the APCs and subsequent changes in APC phenotype and function (37). However, to date we have been unable to demonstrate that ICOSIg diminishes B7.2 on isolated APCs in the absence of T cells, indicating that the observed effect in vivo appears to be related to targeting ICOS–B7RP1 interactions.
We have previously demonstrated that an intact CTLA4 signalling pathway is required for the prolongation of allograft survival induced by CTLA4Ig (36). Thus, diminished cell-surface expression of B7.1 and B7.2 would result in a decrease in the number of target molecules available for CTLA4Ig and CTLA4 binding, both responsible for inhibiting effector T-cell function. Interestingly and supporting this notion, if B7 up-regulation following transplantation is allowed to occur unhindered then ICOSIg treatment (on day 2) in combination with CTLA4Ig did not have a negative effect. We believe this is as a result of allowing sufficient ligation of B7 molecules by CTLA4 expressed on the activated T cells. Divergent effects following ICOS blockade have also been reported in an autoimmune model of murine EAE, and in murine cardiac transplantation (the authors, unpublished data, 2002). In these studies disease outcome being dependent on the timing of ICOS blockade; in EAE, early blockade exacerbating disease and late blockade ameliorating it (11). However, in EAE differences were observed in the IFN-γ production following the two regimens, while differences in B7 expression were not reported.
We did not find prolongation of graft survival with ICOSIg in our rat cardiac transplant model, while other reports using different models and reagents have suggested a modest prolongation with ICOS blockade (10,13). The discrepancy between these results may relate to the biological agents used or to the animal models studied. Comparison of different reagents is underway in rat and mouse transplant models to more fully understand the underlying mechanisms in vivo.
Our data, along with previous reports, demonstrate that a two-way interaction exists between ICOS-B7RP1 and B7-CD28 T-cell costimulatory pathways in alloimmune responses in vivo. These data have important implications for novel therapeutic strategies in organ transplantation. The optimal timing of ICOS blockade in association with other costimulatory blocking protocols remains to be defined, and requires a fuller understanding of the mechanisms of pathway interdependence defined using transplant models in vivo. Future data should provide the basis for more efficacious therapeutic manipulations of these pathways in tolerogenic transplantation strategies.
Acknowledgments
This work is supported by NIH grant RO1 AI-34965 (M.H.S.). A.D.S. is the recipient of a Travelling Fellowship from the Peel Medical research Trust and the British Renal Association.




