The Association of Cytomegalovirus Sero-Pairing with Outcomes and Costs Following Cadaveric Renal Transplantation Prior to the Introduction of Oral Ganciclovir CMV Prophylaxis
Abstract
Cytomegalovirus (CMV) is an important cause of morbidity, mortality and cost in cadaveric renal transplantation. This study was designed to document the clinical and economic outcomes associated with donor and recipient CMV sero-pairing. Data were drawn from the United States Renal Data System (USRDS) on 17 001 cadaveric renal transplant recipients transplanted between 1995 and 1997 with recorded donor and recipient CMV sero-status. In multivariate analysis, CMV-seropositive recipients were associated with a significantly higher incidence of delayed graft function, a lower incidence of graft loss, and lower costs than CMV-seronegative recipients. CMV-seropositive compared to seronegative donors were associated with significantly higher incidence of CMV disease, graft loss, and higher costs when transplanted into CMV-seronegative recipients. However, CMV-seronegative donors into seropositive recipients had no significant association with outcome beyond a higher incidence of CMV disease compared to CMV-seronegative donor and recipient pairs. The outcomes associated with CMV-seropositive donors and seronegative recipients call for tailored management strategies which may include avoidance of such mismatching, antiviral therapy, immunization, or modified immunosuppression.
Introduction
Cytomegalovirus (CMV) is member of the herpes class of viruses which remain latent in host tissues after primary infection. After transplantation, CMV may be reactivated from the recipient's or donor's tissues due to the effects of immunosuppression (1). Primary infection is frequently more severe in a transplant recipient; however, secondary infection can be associated with serious adverse effects as well (2). Cytomegalovirus disease in a transplant recipient is most commonly associated with fever and leukopenia, but may include pneumonitis, colitis, esophagitis, hepatitis, pancreatitis, retinitis, and encephalopathy (3). There is also evidence of adverse effects of subclinical CMV activity in transplant recipients (4–6). Consequences of CMV infection and disease may include acute and chronic rejection, graft loss, and death (5–16).
Several factors motivated this study. While there exists a small amount of published information on the economic impact of CMV disease in transplantation, we know of no published report of the relationship between CMV sero-pairing and medical costs (17–19). The relationship between CMV sero-pairing on acute rejection is under debate, with reports from several single-center studies (4,5, 10–13). The relationship between CMV sero-pairing and delayed graft function is unknown. Finally, relationships between CMV sero-pairing and graft and patient survival and the relative incidence of CMV disease may have changed since earlier reports (14–16,20). This study was designed to address these issues using a national cohort of cadaveric renal transplant recipients drawn from the United States Renal Data System (USRDS) transplanted between 1995 and 1997, prior to the widespread adoption of ganciclovir prophylaxis for CMV disease in renal transplantation (21). This study provides baseline information from a large national cohort for further investigations of prophylaxis and management strategies for CMV in renal transplantation.
Materials and Methods
Data
The data used in this study were derived from the United States Renal Data System (22). The USRDS is a joint effort of the National Institute of Diabetes and Digestive and Kidney Diseases, and the Center for Medicare and Medicaid Services (CMS). It was designed to collect, analyze, and distribute data describing end-stage renal disease (ESRD) in the United States, including: prevalence, treatment modality, survival, and cost of care. For the purposes of the proposed study, the USRDS provides information for all transplants performed in the United States recorded in the United Network for Organ Sharing (UNOS) renal transplant registry (23).
Patient characteristics
Characteristics of the donor and recipient were drawn from the UNOS registry as supplied by the USRDS, unless otherwise indicated. These included the following attributes: in the donor– body mass index, age, age less than 5 or greater than 55, race, gender, terminal serum creatinine, terminal serum creatinine greater than 2.5 mg/dL, blood urea nitrogen greater than 50 mg/dL, cause of death, HLA mismatches, positive B- or T-cell cross-match, antibody induction therapy, ABO incompatibility, Rh incompatibility, pretreatments, infection, histories of: cigarette use, alcohol use, illegal drug use, hypertension, or diabetes, non-heart-beating donor, CMV serology, cold ischemia time, cold ischemia time greater than 24 h, warm ischemia time, warm ischemia time greater than 60 min, and pulsatile perfusion; in the recipient– peak panel reactive antibody (PRA) percentage, peak PRA greater than 10% and greater than 80%, body mass index, age, age greater than 60, race, gender, diabetes, insulin dependence, peripheral vascular disease, chronic obstructive pulmonary disease, CMV serology, physical limitations, employment limitations, pretransplant transfusions, pretransplant dialysis, type of dialysis, characteristics of immunosuppression including tacrolimus, cyclosporine or neither, mycophenolate mofetil, azathioprine or neither, and antilymphocyte induction, previous pregnancies, previous kidney and/or other transplants, multi-organ recipient, kidney-pancreas recipient, and information concerning the recipient's area of residence by the zip code drawn from the UNOS registry merged to data from the 1990 US census, including: the percent of adult residents who have graduated from high school, the per cent of adult residents who have received bachelor's degrees, and median income (24).
Many responses in the UNOS registry are left blank or marked as unknown despite the option of indicating a negative, or not present, response. We believe the large majority of these cases are actual negatives either intentionally left blank as an indication of negative, or marked unknown by a transplant coordinator or other data-entry person because clear evidence of negative was not provided to the coordinator by the medical team. Therefore, characteristics with discrete values, such as race, were coded to measure the recorded presence of the factor, with blank and unknown information included with negative responses. Relationships with these characteristics are relationships with known presence, which may differ from actual presence. Missing or unknown values of continuous characteristics, such as age, are more difficult to manage. Therefore, missing or unknown values of continuous covariates were excluded from univariate analyses of associations with CMV serology. However, in multivariate analysis, excluding any patient with one or more missing or unknown continuous covariate value leads to considerable sample size reductions due to the large number of continuous variables used in the analysis. Therefore, missing or unknown values of continuous covariates were replaced with the mean known value in multivariate analysis in order to preserve sample size. This will tend to increase the variance of estimated effects, reducing significance, but in general, does not bias results (25).
Outcome measures
A patient in whom it was indicated that dialysis was required during the week following transplantation was considered to have delayed graft function (26–28). An indication of an acute rejection either by transplant discharge, at a 3-month post-transplant follow-up, or at a 3-month post-transplant follow-up was considered an acute rejection within 6 months post transplant. Graft loss was determined by date of indicated graft loss, return to dialysis, or death, and was censored on the longest available follow-up date or date of lost to follow-up status.
Costs were calculated from the perspective of Medicare for patients believed to have used Medicare as the primary payer for their transplant. Primary Medicare insurance cannot be directly determined from the database. Therefore, the following methods have been developed by the USRDS (22) to identify patients using Medicare as their primary insurer. We excluded patients from economic analysis if: (i) no Medicare payment for their transplant hospitalization was listed, or (ii) a Medicare payment for their transplant hospitalization was less than $15 000. Reported costs are Medicare payments for all services provided to a patient. Medicare payments for organ acquisition are unavailable and therefore excluded. All costs are reported in US dollars adjusted for inflation, using the medical component portion of the consumer price index and 2000 as the base year (29).
The time to first incidence of CMV disease was determined by the date of the first appearing International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis of CMV disease or Medicare billing record of intravenous ganciclovir treatment within the Medicare billing records of the USRDS (30–32). Prophylaxis data were not available in the database. Patients were excluded if it was determined that they did not use Medicare as their primary insurer for their transplant. Observations were censored at death, graft loss and the last recorded date the patient received Medicare-covered immunosuppression. A renal transplant recipient who does not use the Medicare immunosuppression benefit must have some alternative source of coverage for medications, and perhaps other medical needs. Therefore, we cannot be certain that Medicare will have all medical records for a patient if that patient has stopped, or never started, using the Medicare immunosuppression benefit.
Statistics
Differences in the characteristics of patients by CMV serology were tested with Student's t-test for continuous variables, Fisher's exact test for binary categorical variables, chi-square for multicategorical variables, and the Mantel-Haenszel chi-square for ordered multicategorical variables. Differences in the incidence of graft loss and death were calculated using survival analysis techniques. Univariate survival analysis was performed with the Kaplan–Meier methodology. Multivariate survival analysis was performed with Cox regression. Covariates were drawn from the lists of donor and recipient characteristics noted above. Numerous covariates were insignificant. The inclusion of large lists of insignificant variables may produce ‘misspecification’ bias (25). To analyze this large set of covariates, given the possible problems of misspecification bias, we used stepwise variable selection. All statistical tests were two-tailed. p-values were required to be less than 0.05 for significance in univariate comparisons of outcomes and cost. p-values less than 0.01 were required for significance in comparisons of patient characteristics and multivariate models because of the large number of covariates considered.
Results
Patients
Patients included in the study were drawn from all recipients of first kidney alone transplants between January 1, 1995 and December 31, 1997 from a cadaveric donor who were recorded in the UNOS Scientific Renal Transplant Registry (n = 19 329). After exclusions for missing data concerning CMV serostatus, acute rejection, cost and CMV disease diagnosis, 17 001 patients were included in analyses of characteristics, delayed graft function, and survival outcomes; 14 437 patients were included in analyses of acute rejection incidence by 6 months post transplant; 11 358 patients were included in analyses of Medicare costs of transplant and care through 1 year post transplant, and 8941 for 2-year costs; and 7825 patients were included in analyses of CMV disease diagnosis incidence.
Characteristics associated with CMV serology
The donor was significantly more likely to be CMV sero-positive if the donor had a higher body mass index, more HLA mismatches, shorter cold and warm ischemia times, a history of hypertension, a history of cigarette use, blood urea nitrogen greater than 50 mg/dL, died from a cerebrovascular accident but not anoxia, pulsatile perfusion was not used, was older, over the age of 5 or 55 years, female, African American, or heart beating. Older recipient age was also significantly related to donor CMV sero-positive. The recipient was significantly more likely to be CMV sero-positive in univariate analysis if the donor had a warm ischemia time less than 60 min, more HLA mismatches, older age, positive B-cell cross-match, hypertension, was African American, or Rh incompatible, pretreatments were used, or pulsatile perfusion was not used. The associations provide further evidence of donor and recipient pairing associated directly or indirectly with CMV serology. The recipient was significantly more likely to be CMV sero-positive in univariate analysis if the recipient had a higher body mass index, higher peak PRA, peak PRA greater than 10% and 80%, pregnancy prior to transplant, pretransplant blood transfusions, diabetes, pretransplant dialysis, employment disability at transplant, no peritoneal dialysis, lived in a zip code with a lower percentage of high-school and college graduates, and a lower median income, was older, over the age of 20 and 60, female, or African American. It is important to note that these observed associations may be spurious, caused by random variation and not true relationships with CMV serology.
Donor and recipient CMV sero-pairing
The prevalence of CMV-seropositive status was similar in donors (D, 60.4%) and recipients (R, 63.4%). The distribution of CMV sero-pairings was 39.1% D +/R +, 21.3% D +/R–, 24.2% D –/R +, and 15.3% D –/R –. This is evidence of a small but statistically significant nonrandom distribution of organs, with 2.0% more seronegative organs transplanted in seronegative and 1.9% more seropositive organs transplanted in seropositive recipients than would be expected from a random distribution as determined by Fisher's exact test (p < 0.001). However, more direct evidence of distribution of organs by CMV sero-status would require a formal analysis of the distribution of CMV sero-status on the waiting list and the effect of sero-status on waiting times.
Clinical outcomes
Delayed graft function, acute rejection, and CMV disease: Outcomes associated with recipient CMV sero-status are presented in Table 1. There was a 19.3% increased relative risk of delayed graft function in CMV-seropositive compared to seronegative recipients (26.0% vs. 21.8%, p < 0.001). Recipient CMV serology was not significantly related to acute rejection incidence by 6 months post transplant. There was a 36.1% higher incidence of CMV disease by 1 year post transplant in seronegative compared to seropositive recipients (20.8% vs. 13.3%, p < 0.001). This is due to a much larger effect of donor sero-status on CMV disease incidence in CMV-seronegative compared to seropositive recipients (Figure 1). These effects were confirmed by multivariate analysis.
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Clinical outcomes | n,% | CMV+ | CMV– | ΔR+/R– | p-value | ΔR+/R– | p-value |
| Delayed graft functiona | 17001, 100% | 26.0% | 21.8% | +19.3% | <0.001 | +13.6% | 0.002 |
| Rejection by 6months | 14437, 84.9% | 24.2% | 25.2% | −4.0% | NS | −1.6% | NS |
| CMV disease by 1year | 7825, 46.0% | 13.3% | 20.8% | −36.1% | <0.001 | −39.0% | <0.001 |
| Survival at 2years | |||||||
| Risk of graft loss | 17001, 100% | 20.2% | 20.2% | +0.3% | NS | −9.3% | 0.018 |
| Risk of death | 17001, 100% | 5.5% | 4.9% | +12.2% | NS | −11.3% | NS |
| Cost (Medicare payments) | |||||||
| Transplant hospital | 11358, 66.8% | $31085 | $31037 | +0.2% | NS | −0.2% | NS |
| Post-TX to 1year | 11358, 66.8% | $29536 | $30756 | −4.0% | NS | −8.7% | <0.001 |
| 1year total | 11358, 66.8% | $60572 | $61750 | −1.9% | NS | −4.5% | <0.001 |
| 1year to 2years | 8941, 52.6% | $14632 | $14135 | +3.5% | NS | −1.8% | NS |
| 2years total | 8941, 52.6% | $76001 | $77436 | −1.9% | NS | −4.7% | 0.006 |
- a Delayed graft function as determined by need for dialysis within the first week post transplant.
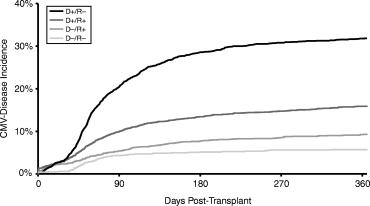
The incidence of diagnosed CMV disease and treatment through 1 year post transplant as estimated with univariate Kaplan–Meier methods was 5.7% for D –/R –, 9.3% for D –/R +, 15.9% for D +/R +, and 31.8% for D +/R – CMV sero-pairings (p < 0.001). The increased risk of CMV disease associated with a CMV-seropositive compared to seronegative donor was relatively large in CMV-seronegative recipients (+ 445%, p < 0.001) compared to seropositive recipients (+ 72.8%, p < 0.001). These patterns were confirmed by multivariate analysis.
Notably, in multivariate analysis, the use of mycophenolate mofetil compared to azathioprine, or neither, was associated with a 25% (p = 0.006) increase in the incidence of CMV disease in seropositive recipients and a 27% (p = 0.005) increase in the incidence of CMV disease in CMV-seronegative recipients. The use of antilymphocyte agents for induction immunosuppression was associated with a 32% (p < 0.001) increase in the incidence of CMV disease for CMV-seropositive recipients. However, there was no association between antilymphocyte agents and CMV disease in CMV-seronegative recipients. Tacrolimus and cyclosporine were not associated with different CMV disease incidence in either CMV-seropositive or CMV-seronegative recipients.
Outcomes associated with donor sero-status separated by recipient sero-status are presented in Table 2. There was 11.9% greater risk of delayed graft function in seropositive recipients from seropositive donors, perhaps due to age and other factors (24.7% vs. 27.6%, p < 0.001). However, this result was not confirmed by multivariate analysis which was adjusted for age and other factors. There was no significant relationship between donor sero-status and delayed graft function in seronegative recipients. No significant relationship between donor sero-status and acute rejection was observed in either seropositive or seronegative recipients. The risk of coded CMV disease by 1 year post transplant in seropositive recipients was 72.8% higher with seropositive donors (9.3% vs. 15.9%, p < 0.001), which was confirmed in multivariate analysis at 73.1% (p < 0.001) higher (Figure 1). The effect of donor seropositive status was remarkably higher in seronegative recipients, with a 445% increase in incidence in univariate analysis (5.7% vs. 31.8%, p < 0.001) and a 630% (p < 0.001) increase in incidence in multivariate analysis.
| Recipient CMV+ | Recipient CMV– | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||
| Clinical outcomes | n,% | ΔD+/D– | p-value | ΔD+/D– | p-value | ΔD+/D– | p-value | ΔD+/D– | p-value |
| Delayed graft functiona | 17001, 100% | +11.9% | <0.001 | +9.4% | NS | +5.9% | NS | −0.3% | NS |
| Rejection by 6months | 14437, 84.9% | +5.1% | NS | +0.3% | NS | +6.0% | NS | +1.5% | NS |
| CMV disease by 1year | 7825, 46.0% | +72.8% | <0.001 | +73.1% | <0.001 | +445% | <0.001 | +630% | <0.001 |
| Survival at 2years | |||||||||
| Risk of graft loss | 17001, 100% | +6.8% | NS | +3.4% | NS | +33.6% | <0.001 | +21.4% | <0.001 |
| Risk of death | 17001, 100% | +6.1% | NS | +12.6% | NS | +41.2% | 0.004 | +27.0% | NS |
| Cost (Medicare payments) | |||||||||
| Transplant hospital | 11358, 66.8% | +0.2% | NS | +0.0% | NS | +0.3% | NS | −0.5% | NS |
| Post-TX to 1year | 11358, 66.8% | +5.3% | 0.042 | +4.0% | NS | +24.0% | <0.001 | +19.1% | <0.001 |
| 1year total | 11358, 66.8% | +2.6% | NS | +2.2% | NS | +11.2% | <0.001 | +8.2% | <0.001 |
| 1year to 2years | 8941, 52.6% | +8.0% | NS | +4.0% | NS | +12.1% | NS | +5.6% | NS |
| 2years total | 8941, 52.6% | +3.0% | NS | +2.9% | NS | +11.3% | <0.001 | +7.1% | 0.022 |
- a Delayed graft function as determined by need for dialysis within the first week post transplant.
Patient and graft survival: The effect of a CMV-seropositive donor was small in CMV-seropositive recipients and large in CMV-seronegative recipients, which when averaged together produced nearly identical graft loss rates in CMV-seropositive and seronegative recipients overall (Figure 2). However, in multivariate analysis the risk of graft loss was significantly lower by 9.3% in seropositive recipients (p = 0.018). There was no significant relationship between recipient sero-status and patient survival.
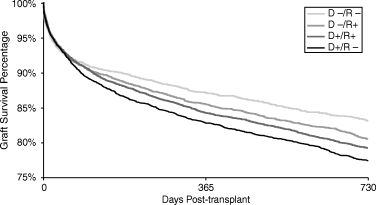
Graft loss through 2 years post transplant as estimated with univariate Kaplan–Meier methods was 16.9% for D –/R –, 19.4% for D –/R +, 20.8% for D +/R +, and 22.6% for D +/R – CMV sero-pairings (p < 0.001). There was no significant association between graft loss and donor CMV sero-status in CMV-seropositive recipients. However, there was a significantly higher incidence of graft loss associated with CMV-seropositive compared to seronegative donors in CMV-seronegative recipients (+ 33.6%, p < 0.001). These patterns were confirmed by multivariate analysis.
There were no significant relationships among donor serology and graft or patient survival in seropositive recipients. However, there were considerable effects of donor serology when the recipient was seronegative (Figure 2). The risk of graft loss in seronegative recipients was 33.6% higher with a seropositive compared to a seronegative donor (16.9% vs. 22.6%, p < 0.001), which was confirmed with multivariate analysis (21.4%, p < 0.001). The risk of death in seronegative recipients was 41.2% higher with a seropositive donor (4.3% vs. 6.1%, p = 0.004). However, the risk of death in seronegative recipients was not significantly associated with donor sero-status in multivariate analysis. While the estimated effect was large at 27.0%, significance is difficult to achieve with event rates as low as they are for death, even with the large samples used in this study.
Cost: There was limited evidence of a relationship between donor serostatus and Medicare payments in CMV-seropositive recipients. However, there were several significant associations between donor sero-status and Medicare payments in seronegative recipients. In univariate analysis, seropositive donors in seronegative recipients were associated with Medicare payments 24.0% higher from transplant discharge through 1 year post transplant ($27 686 vs. $34 330, p < 0.001), 11.2% higher from transplant through 1 year post transplant ($59 841 vs. $66 543, p < 0.001), and 11.3% higher from transplant discharge through 2 years post transplant ($75 658 vs. $84 207, p < 0.001). Although reduced in size, these effects all remained significant in multivariate analysis with seropositive donors in seronegative recipients associated with higher Medicare payments from transplant discharge through 1 year post transplant (19.1%, p < 0.001), from transplant through 1 year post transplant (8.2%, p < 0.001), and from transplant discharge through 2 years post transplant (7.1%, p = 0.022). Multivariate adjusted costs during the first year post transplant after transplant discharge are presented in Figure 3.

Medicare cost during the first year post transplant after transplant discharge adjusted with multivariate regression using CMV D +/R + as the reference group was $24 269 for D –/R – and $28 904 for D +/R – (p < 0.001) and $28 823 for D –/R + and $29 977 for D +/R + (p = NS). While similar patterns were observed during different time intervals, the differences associated with CMV sero-status were most pronounced during this period from transplant discharge through 1 year post transplant (see Tables 1 and 2).
Discussion
Our findings document the importance of CMV on outcomes during the era just prior to the widespread use of ganciclovir prophylaxis in cadaveric renal transplantation. We have shown that donor serology has a larger impact than recipient serology in general, and that the donor effects are most pronounced in CMV-seronegative recipients. CMV-seropositive recipients were associated with a higher incidence of delayed graft function, but lower rates of CMV disease, graft loss and Medicare costs. Donor CMV-seropositive status in seropositive recipients was associated with an increased risk of delayed graft function and CMV disease, but had little effect on other outcomes.
There were important limitations of this study. Patients were not prospectively enrolled in the study, but instead drawn retrospectively from the UNOS registry. While this limits the design of the study to information contained in the registry, it does provide samples representing the entire United States renal transplant population. In addition, a prospective study of the impact of CMV on renal transplant outcomes with samples of the size used in this study is unlikely to be performed, and no large-scale investigation of the outcomes associated with CMV sero-status beyond patient and graft survival has been published to our knowledge (14–17). Further, information on CMV prophylaxis was unavailable because the study population was drawn from a registry without this information. In part because of this limitation, the study was designed to examine outcomes associated with CMV sero-status during a time period when there was effective treatment available but very little use of prophylaxis in renal transplantation. Today, CMV prophylaxis is widespread in renal transplantation, but not universal, and with considerable variation in practice (3,21,33–37). The results presented here provide baseline information for studies of the efficacy and cost-effectiveness of prophylaxis strategies.
Univariate associations between CMV-seropositive recipients and increased risk of delayed graft function and decreased risk of CMV disease were observed and supported by multivariate analysis. Delayed graft function was significantly more prevalent in seropositive recipients if the donor was seropositive compared to seronegative, but this relationship was not significant in multivariate analysis. It is interesting that we did observe evidence of some relationships between CMV sero-status and delayed graft function when, to our knowledge, such a relationship has not been reported previously. However, no significant relationship between CMV sero-status and acute rejection was observed, while there have been several publications suggesting a relationship between CMV and acute rejection (4,9–13). It is possible that the published relationships between CMV sero-status and acute rejection are center specific or due to statistical errors caused by small sample sizes. It is also possible that uncontrolled patterns in this registry analysis have masked this relationship. Further, although we have shown a strong relationship between CMV sero-pairing and CMV disease, CMV sero-pairing itself is not a direct measure of the activity of CMV within a transplant recipient, perhaps limiting CMV sero-pairing as a measure of the relationship between CMV and acute rejection.
CMV-seronegative recipients were at significantly higher risk of CMV disease in both univariate and multivariate analysis. Further, CMV-seropositive donors were associated with significantly greater incidence of CMV disease in both univariate and multivariate analyses for seropositive and seronegative recipients. Not unexpectedly, the magnitude of the effect of a CMV-seropositive donor is much larger in seronegative compared to seropositive recipients, explaining in part the relative incidence in seronegative and seropositive recipients (1–3). Interestingly, we were able to confirm previous research suggesting associations between CMV disease and the use of mycophenolate mofetil and antilymphocyte agents (38,39). However, no differences were found between tacrolimus and cyclosporine.
Caution should be used when interpreting the CMV disease results presented here. It should be noted that while this methodology for determining incidence of disease has been validated for other diagnoses, it has not been validated for CMV disease (32). The use of i.v. ganciclovir may have been for prophylaxis and not treatment of disease, which may cause an overstatement of the incidence of CMV disease. Further, no information on the specifics of attempted prophylaxis was available. Therefore, we cannot be certain that the figures presented here represent estimates of the exact difference in CMV disease incidence associated with CMV sero-pairing. However, we do expect that our CMV disease results reflect the relative frequency of CMV disease incidence by CMV sero-pairing.
Cost patterns were similar to the graft loss patterns. This is not surprising, given that one of the most expensive events that can occur post transplant is return to dialysis. Costs were similar between CMV-seropositive and seronegative recipients in univariate analyses, but were significantly lower at 1 and 2 years post transplant in multivariate analysis. There was little evidence of an association between donor CMV sero-status and cost for seropositive recipients. However, costs were significantly higher at 1 and 2 years post transplant for CMV-seropositive compared to seronegative donors in CMV-seronegative recipients. Through 1 year post transplant from multivariate estimates, costs were approximately $5000 higher in CMV-seronegative recipients if they received a CMV-seropositive organ.
We have shown evidence of clinical and economic effects associated with CMV sero-status in cadaveric renal transplantation. This is most evident in CMV-seronegative recipients of CMV-seropositive donor organs. There is clear clinical and economic motivation for management strategies designed to reduce the impact of the CMV-seropositive donor in the CMV-seronegative recipient, including antiviral treatment and prophylaxis strategies, and immunization. Further, CMV-seropositive recipients of seronegative organs appear not to be at increased risk of rejection, while they are at impressively increased risk of CMV disease. This may call for strategies with reduced immunosuppression in these patients, balancing the harm caused by acute rejection with the harm caused by CMV disease. We see little justification for different management strategies for CMV-seropositive compared to seronegative donors in CMV-seropositive recipients. However, this may suggest a role for allocation. While the management strategies discussed above may reduce the impact of CMV-seropositive donors in the seronegative recipient, allocation may provide equivalent or superior benefits without the associated expenses and risks. However, further study including simulation modeling and clinical trials will be required to verify this conjecture.
Acknowledgments
Supported in part by a grants from the National Institute of Diabetes, Digestive, and Kidney, Diseases 1R01-DK-47801–01, Thomas C. Bailey, M. D., P. I., and K25-DK-02916–01, Mark A. Schnitzler, Ph. D., P. I.
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.




