Hyperhomocysteinemia in Renal Transplant Recipients
Abstract
Renal transplantation is a commonly performed curative procedure for end-stage renal disease. With the increase in renal allograft half-lives, attention is now being focused on cardiovascular morbidity and death in the renal transplant recipient (RTR) population. Among the more novel cardiovascular disease (CVD) risk factors for which this group is at risk is hyperhomocysteinemia. Hyperhomocysteinemia has been associated with an increased risk of CVD, although prospective randomized trials designed to prove causality are still ongoing. Since plasma total homocysteine levels are inversely related to renal function, RTRs have a greatly increased prevalence of hyperhomocysteinemia. Other determinants of homocysteine include B-vitamins, albumin, age, and genetic polymorphisms. Although RTRs are resistant to the typical B-vitamin doses used to correct hyperhomocysteinemia in the general population, they do respond to supraphysiologic dose therapy. In terms of prevalence, etiology, and treatment of hyperhomocysteinemia, RTRs are very similar to the much larger chronic renal insufficiency population. For this reason, RTRs have been chosen as an ideal study population in investigating the effect of reducing hyperhomocysteinemia on CVD outcomes.
Introduction
With the incidence of end-stage renal disease (ESRD) in the developed world climbing (1), the number of renal transplantations performed is steadily rising. As renal allograft half-lives progressively lengthen (1), attention is now being focused on long-term causes of morbidity and death in the renal transplant recipient (RTR) population, with cardiovascular disease (CVD) being foremost among them (1). In fact, the incidence of CVD in RTRs is approximately fivefold greater than predicted from Framingham Heart Study data in age- and gender-matched subjects (2). Given their exposure to a host of traditional cardiac risk factors, among them hypertension, diabetes mellitus, and dyslipidemia, as well as chronic immunosuppression, this is not necessarily surprising. However, as with the ESRD population (3), these risk factors may not be able to entirely explain this excess CVD risk (4). Other, more novel, cardiac risk factors, such as elevated total plasma levels of the putative atherothrombotic risk factor homocysteine, may also be playing a role. This review will focus on homocysteine metabolism and hyperhomocysteinemia as it relates to the RTR population, and conclude with homocysteine-lowering strategies and a discussion of the unique qualities of RTRs that may contribute to future homocysteine-related research.
Normal Homocysteine Metabolism
Homocysteine is a sulfur amino acid produced in all cells through the normal methylation process (see Figure 1). In healthy humans, approximately 75% of plasma total homocysteine (tHcy) is protein-bound, mostly to albumin. The vast majority of the remaining 25% is bound by a disulfide bond to other homocysteine or cysteine molecules (5). Less than 1% of tHcy is found in a ‘reduced’, or free form. As illustrated, homocysteine has two main degradative pathways. The remethylation pathway converts homocysteine to methionine using folate and vitamin B12 (cobalamin) as the major cofactors (6). An alternate remethylation pathway uses betaine as a methyl donor. In its first step, the transsulfuration pathway irreversibly degrades homocysteine to cystathionine, which is then hydrolyzed to cysteine and α-ketobutyrate. This pathway is facilitated by pyridoxal 5′-phosphate (PLP), the active form of vitamin B6 (6). Given these pathways, one would reasonably expect a correlation between tHcy levels and plasma B-vitamin (folate, vitamin B12, and vitamin B6) status. This relationship is, in fact, confirmed by population-based studies (7). Further supportive evidence originates from the reduction in both mean tHcy levels and the prevalence of hyperhomocysteinemia in the general US population since the mandatory folic acid enrichment of all cereal and grain products (140 µg/100 g flour) in 1996 (8).
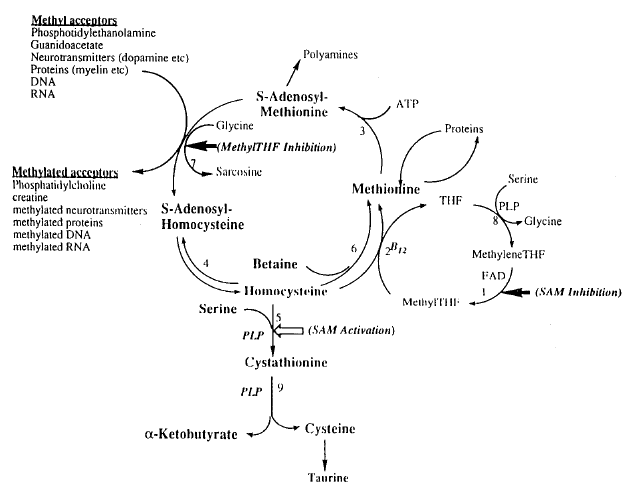
Homocysteine metabolism. Enzyme reactions that are regulated by S-adenosylmethionine (SAM) and 5-methyltetrahydrofolate (methylTHF) are indicated by large arrows. Open arrows indicate activation, closed arrows indicate inhibition. Enzymes: 1, 5,10-methylenetetrahydrofolate (MTHFR) reductase; 2, methionine synthase; 3, S-adenosylmethionine synthase; 4, S-adenosylhomocysteine hydrolase; 5, cystathionine β-synthase; 6, betaine:homocysteine methyltransferase; 7, glycine N-methyltransferase; 8, serine hydroxymethyltransferase; 9, cystathionase. Modified with permission from International Life Sciences Institute. PLP, pyridoxal 5-phosphate; SAH, S-adenosyl homocysteine.
Selhub and Miller (6) differentiate between two general forms of hyperhomocysteinemia. Impairment of the remethylation pathway, primarily from folate or vitamin B12 insufficiency, leads to hyperhomocysteinemia that typically manifests itself under fasting conditions. In contrast, impairment of transsulfuration from vitamin B6 deficiency is usually expressed after a methionine load, also referred to as post-methionine load hyperhomocysteinemia (PML).
In North America, tHcy levels up to 12 µmol/L are considered normative in the current era of folate-enriched grains, while hyperhomocysteinemia is arbitrarily defined as ‘mild’ between 13 and 30 µmol/L, ‘intermediate’ between 31 and 100 µmol/L, and ‘severe’ when > 100 µmol/L (9).
Prevalence of Hyperhomocysteinemia
A plethora of studies have documented the greatly increased prevalence of both fasting (10–15) and PML (16) mild-to-moderate hyperhomocysteinemia in RTRs compared with the general populace. Since the GFR (see below), and not the transplant process per se, is the major determinant of tHcy levels (17–19), and as renal dysfunction to some degree is fairly ubiquitous in this population, RTRs can, in a sense, be considered very similar to the chronic renal insufficiency (CRI) population in terms of plasma homocysteine levels. This is supported by a study that matched 86 stable RTRs with 238 CRI patients by serum creatinine levels (RTR, 0.6–4.2 mg/dL; CRI, 0.7–4.1 mg/dL, p = 0.798), and then compared plasma tHcy levels (20). tHcy levels did not differ between the two groups (RTR, 15.0 µmol/L; CRI, 14.9 µmol/L, p = 0.899) after adjusting for other major determinants of tHcy, including age, sex, B-vitamin levels, albumin, and creatinine.
Homocysteine as a CVD Risk Factor
Mudd et al. (21) first noted a link between severe hyperhomocysteinemia in children with cystathionine β-synthase deficiency and precocious arteriosclerosis. Subsequently, the literature on the relationship between hyperhomocysteinemia and CVD has grown dramatically. Case–control studies have almost uniformly supported the concept that hyperhomocysteinemia is an independent risk factor for CVD in the general population, while the prospective literature has been less consistent (22). However, a meta-analysis of all prospective studies through October 1999 found the estimated odds ratio for coronary heart disease for each 5 µmol/L increase in homocysteine to be 1.20 (95% CI = 1.14–1.25) (23).
Studies in the renal disease population also support a link between hyperhomocysteinemia and increased CVD risk. Pooled data from three prospective trials, one in CRI patients and two in ESRD dialysis-dependent patients, found the relative risk estimate for new or recurrent CVD events in those with mild-to-moderate fasting hyperhomocysteinemia to be 2.8 (95% CI = 1.6–5.0), when comparing the upper and lowest tertiles of tHcy (24). Another study of 207 stable RTRs followed for a mean of 21 months calculated the relative risk of CVD during follow-up using a Cox proportional-hazards analysis controlling for tHcy, previous CVD, time on dialysis, use of cyclosporine A, diabetes mellitus, gender, age, smoking, hypertension, dyslipidemia, and creatinine (15). Only tHcy (RR = 1.06; 95% CI = 1.04–1.09), age (RR = 1.55; 95% CI = 1.09–2.19), and creatinine (RR = 1.34; 95% CI = 1.08–1.66 for a 50 µmol/L increase) were found to be independent risk factors. This corresponded to an increased risk of 6% for each µmol/L increase in tHcy.
Ultimately, however, only ongoing well-powered randomized tHcy-lowering trials (see below) measuring CVD outcomes will definitively resolve this issue of causality.
Determinants/etiology of Hyperhomocysteinemia
There are other important determinants of plasma tHcy besides B-vitamins. Renal function, as measured by ‘gold standard’ glomerular filtration rate (GFR) tests, has been conclusively shown to be a primary determinant of tHcy (17–19). This is an inverse relationship, so as renal function declines, tHcy levels rise. Surrogate markers for GFR are also useful determinants of tHcy. Serum creatinine, the most commonly used marker, is consistently one of the most powerful determinants of tHcy in the general and renal disease (including RTR) population (11, 15, 25, 26). Besides being an indirect measurement of renal function, creatinine is also linked to tHcy through the production of creatine, its precursor molecule, by an S-adenosylmethionine-dependent methyl transfer reaction (see Figure 1). Cystatin C, an alternative endogenous filtration marker, has been shown to be a more accurate determinant than creatinine of fasting and PML tHcy levels in those with normal and elevated serum creatinine levels (27, 28).
The etiology of hyperhomocysteinemia in renal disease is not entirely understood (29). What is known is that the defect is primarily one of reduced plasma homocysteine clearance rather than increased production (30). Furthermore, homocysteine is minimally excreted in the urine regardless of kidney function (31). This suggests that the normal kidney may actively clear and metabolize plasma tHcy. Supportive evidence includes the existence of homocysteine-uptake mechanisms and metabolizing enzymes in the proximal renal tubule, the major role the kidney plays in clearing and metabolizing other amino acids, and rat arteriovenous measurements that show significant renal homocysteine extraction (32–35). In contrast, a study of renal arteriovenous homocysteine measurements in healthy humans found the mean renal tHcy difference to be nonsignificant (36). The authors subsequently concluded that hyperhomocysteinemia in renal disease may be due to ‘uremic toxin’ inhibition of extrarenal homocysteine metabolism rather than to a reduction in renal homocysteine metabolism. This study, however, was performed in the fasting state, which may affect homocysteine clearance, and did not exclude renal extraction of up to 30–40% of daily homocysteine production. Furthermore, their hypothesis is seriously challenged by the inverse relationship between tHcy and GFR that exists even throughout non-uremic ranges of GFR (17–19).
Other independent determinants of tHcy levels in the general and RTR population include albumin (37), which may be related to homocysteine–albumin plasma binding, and age (11), possibly because of age-related declines in GFR and B-vitamin status. An increased prevalence of hypothyroidism with age may itself be another determinant (38).
A relatively common genetic polymorphism inducing mild-to-moderate hyperhomocysteinemia under specific conditions is the methylenetetrahydrofolate reductase (MTHFR) mutation, which impairs the formation of 5-methyltetrahydrofolate (see Figure 1), thus limiting the conversion of homocysteine to methionine (39). Approximately 10–15% of the general and RTR population is homozygous for this mutation (40–42), though the prevalence varies somewhat with ethnicity, ranging from approximately 20% in Italian populations to only a few percent in black Americans (43). Only under conditions of folate depletion does this mutation tends to raise tHcy levels (44). For unclear reasons it is also associated with low folate levels (41). Although attention has recently focused on this mutation as an important cause of hyperhomocysteinemia in the RTR population, its effect is actually likely to be quite minor. One study of 108 stable RTRs found that after adjustment for renal function, the MTHFR polymorphism accounted for only 5% of tHcy variability (42). Its impact is likely to be attenuated even further in countries such as the USA, where folic acid-enriched food has significantly reduced the rates of folate deficiency (8). One uncontrolled study of 70 Italian RTRs treated for 2 months with folic acid 5 mg, vitamin B12 0.5 mg, and vitamin B6 50 mg/d found that tHcy levels dropped from 17 µmol/L pre-treatment to 7.5 µmol/L post-treatment (45). All of the RTRs, including those with the MTHFR genotype (29% of cohort), normalized their tHcy levels. Although these subjects were treated with supraphysiologic doses of B vitamins, these results confirm that even hyperhomocysteinemia in RTRs with the MTHFR defect responds promptly to vitamin supplementation.
Cyclosporine A, a mainstay of post-transplant immunosuppressive regimens, has been proposed as a determinant of tHcy that is particularly relevant to the RTR population. A cross-sectional study by Arnadottir et al. (14) found that RTRs on cyclosporine A-based regimens had significantly higher tHcy levels than other RTRs (19.5 ± 7.6 vs. 16.2 ± 4.8, p < 0.05). Although tHcy levels were not adjusted for differences in GFR, the authors concluded that cyclosporine A use was an independent risk factor for hyperhomocysteinemia in RTRs. However, numerous subsequent studies (11, 15, 20) adjusting for potential confounders have not confirmed this relationship between tHcy levels and cyclosporine A, or, for that matter, the other calcineurin inhibitor FK506 (46). One study (11) measured the determinants of fasting tHcy using a regression model that controlled for creatinine, creatinine clearance, folate, vitamins B6 and B12, age, sex, body weight, cyclosporine A use, and albumin levels. Of these, only creatinine, age, vitamin B6, folate, and vitamin B12 were independent predictors of tHcy. Furthermore, in vitro studies of human renal proximal tubule epithelial cells have found no evidence that cyclosporine A augments tHcy export from tubule cells (47).
Treatment of Hyperhomocysteinemia
Trials conducted in the general population have confirmed that physiologic doses of B-vitamins (e.g. folic acid 500 µg/d) can significantly reduce or normalize hyperhomocysteinemia (48). In contrast, for as yet unclear reasons, patients become increasingly refractory to physiologic B-vitamin treatment as renal function declines (29). Since renal insufficiency is extremely common in RTRs, they too are prone to this vitamin resistance. This was elegantly shown in a cross-sectional analysis of 86 RTRs and 175 coronary artery disease patients whose tHcy levels were measured after folic acid fortification of cereals/grains (10). Although both groups were equally exposed to the folate supplementation, the RTR group had 88% higher geometric mean fasting tHcy levels (15.6 vs. 8.3 µmol/L; p < 0.001), and a much greater prevalence of hyperhomocysteinemia (69.8 vs. 10.9%; p < 0.001).
Other studies have confirmed that RTRs require supraphysiologic B-vitamin supplementation to reduce hyperhomocysteinemia. One trial (49) randomized 60 stable RTRs by fasting tHcy levels to treatment for 12 weeks in one of three groups: (i) ‘supraphysiologic’ regimen containing folic acid 2.4 mg, vitamin B6 50 mg, and vitamin B12 0.4 mg/d; (ii) ‘standard’ multivitamin regimen containing folic acid 0.4 mg, vitamin B6 50 mg, and vitamin B12 0.4 mg/d; (iii) ‘placebo’ regimen containing folic acid 0 mg, vitamin B6 50 mg, and vitamin B12 0.4 mg/d. Group 1 had the largest reduction in mean tHcy levels, at 32.3 ± 2.4%, compared with group 2 (23.4 ± 2.3%) and group 3 (19.1 ± 2.3%). Furthermore, 50% of group 1 subjects normalized their tHcy levels, while only 9% of group 2 and 0% of group 3 subjects did. Another recent trial, this one uncontrolled, treated 35 stable RTRs with folic acid 10 mg/d for 3 months (50). In the 28 patients who completed the study, mean tHcy levels were reduced by approximately 25%, from 16.9 ± 6.4 to 12.8 ± 5.7 µmol/L (p < 0.0001).
The supraphysiologic vitamin requirement has been confirmed in both fasting and PML states in a study of 29 RTRs randomly assigned to four groups (51): (i) placebo; (ii) vitamin B6 50 mg/d; (iii) folic acid 5 mg and vitamin B12 0.4 mg/d; or (iv) folic acid 5 mg, vitamin B12 0.4 mg, and vitamin B6 50 mg/d. The vitamin B6 group had a 22.1% reduction in PML mean tHcy levels (p < 0.042), and the folic acid/vitamin B12 group had a 26.2% reduction in fasting mean tHcy levels (p < 0.027).
Although RTRs require greater B-vitamin supplementation to lower tHcy levels than those with normal renal function, they are more responsive than the ESRD dialysis-dependent population. This was shown in a prospective 12-week study of 10 RTRs and 39 chronic hemodialysis (HD) patients with equivalent baseline tHcy levels (RTRs, 14.2–23.6 µmol/L; HD, 14.4–24.9 µmol/L, p = 0.202) (52). RTRs received folic acid 2.4 mg, vitamin B12 0.4 mg, and vitamin B6 50 mg/d, while the HD subjects were given folic acid 15 mg, vitamin B12 1.0 mg, and vitamin B6 50 mg/d. Despite being treated with more than five times the amount of folic acid and twice the amount of vitamin B12, the HD subjects had only a 12.1% reduction in their tHcy levels compared with 28.1% in the RTRs. Furthermore, 50% of the RTRs normalized their tHcy levels compared with only 5.1% of the HD group.
RTRs as a Study Population
There are a number of ongoing large randomized clinical trials attempting to determine whether lowering tHcy levels with B-vitamins will reduce CVD outcomes. These include the Vitamin Intervention for Stroke Prevention (VISP), Women's Antioxidant Cardiovascular Disease (WACS), and the Heart Outcomes Prevention Evaluation (HOPE-2) studies. These studies are being conducted in the general population, and assume a 4–6 µmol/L reduction in plasma tHcy among those assigned to the treatment arm (53). However, the recent fortification of cereal grain products in the general population may cause these trials to be underpowered (53). In contrast, RTRs are an ideal study population to determine the effects of tHcy lowering on cardiovascular outcomes, for the following reasons:
- 1
They have unusually high rates of de novo and secondary cardiovascular events (1, 2, 4).
- 2
They continue to have a very high prevalence of mild-to-moderate hyperhomocysteinemia despite the fortification of cereal grain products in the USA (10).
- 3
Unlike ESRD dialysis-dependent patients, they are to a large degree able to normalize their hyperhomocysteinemic state with supraphysiologic B-vitamin therapy (49–52).
- 4
They are a highly motivated population (54) who have frequent physician follow-up, very often in large medical centers.
- 5
Results in the RTRs can be extrapolated to the much larger CRI population (20).
For these reasons, the NIDDK has recently funded a randomized, controlled tHcy-lowering trial (O1 DK61700-01) designed to measure CVD outcomes in 4000 RTRs over a 5-year period.
Conclusion
The RTR population is at high risk for primary and secondary CVD outcomes. Hyperhomocysteinemia, a novel, putative risk factor for arteriosclerosis and atherothrombosis, potentially increases this risk. Since renal function is a primary determinant of plasma tHcy levels, the prevalence of mild-to-moderate hyperhomocysteinemia is especially marked in this population. Although RTRs are relatively refractory to physiologic-range doses of B-vitamins in lowering plasma tHcy levels, they do respond to higher-dose supplements, in contrast to ESRD dialysis-dependent patients. For these and other reasons, RTRs are being used to study the effect of reducing hyperhomocysteinemia on CVD outcomes.
Acknowledgments
This material is based upon work supported by the US Department of Agriculture, under agreement no. 581950-9-001. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the US Department of Agriculture.




