Co-Culture of Mouse Epidermal Cells for Studies of Pigmentation
Abstract
Interactions between melanocytes and keratinocytes in the skin suggest bi-directional interchanges between these two cell types. Thus, melanocytes cultured alone may not accurately reflect the physiology of the skin and the effects of physiological regulators in vivo, because they do not consider possible interactions with keratinocytes. As more and more pigment genes are identified and cloned, the characterization of their functions becomes more of a challenge, particularly with respect to their roles in the processing and transport of melanosomes and their transfer to keratinocytes. Immortalized melanocytes mutant at these loci are now being routinely generated from mice, but interestingly, successful co-culture of murine melanocytes and keratinocytes is very difficult compared with their human counterparts. Thus, we have now optimized co-culture conditions for murine melanocytes and keratinocytes so that pigmentation and the effects of specific mutations can be studied in a more physiologically relevant context.
Abbreviations –
-
- bFGF
-
- basic fibroblast growth factor
-
- EGF
-
- epidermal growth factor
-
- FCS
-
- fetal calf serum
-
- MCM
-
- melanocyte complete medium
-
- PMA
-
- phorbol 12-myristate 13-acetate
-
- SKM
-
- standard keratinocyte medium
-
- UV
-
- ultraviolet
Introduction
The major source of skin color in humans is melanin, which is produced in highly specialized cells (melanocytes) located in the basal layer of the epidermis [for reviews, cf. (1)]. Melanocytes in the skin are highly dendritic cells that are in close contact with the neighboring keratinocytes, and together they form the epidermal melanin unit, the functional unit that produces and distributes melanin. A typical epidermal melanin unit in human skin is composed of one melanocyte in contact with ∼35–40 neighboring keratinocytes. The availability of immortalized mouse keratinocyte cell lines (such as SP-1) and immortalized mouse melanocyte cell lines (such as melan-a), which can be induced to differentiate, suggest readily available in vitro models that could be used to study the effects of pigment genes and interactions regulating pigmentation. It is worth noting that most melanocytes in the skin of adult mice are localized in hair follicles (except in the ears and tail), but melan-a melanocytes were derived from 18-day embryo mice, at which time the melanocytes still reside in the epidermis. In a preliminary study (2), an SP-1 keratinocyte:melan-a melanocyte co-culture was used to study pigmentation, based on conditions developed for human melanocyte:keratinocyte co-cultures, but without optimizing those conditions for the murine lines which are significantly more difficult to grow than are their human counterparts. In fact, a search of the PubMed literature database currently retrieves 12 articles, all of them dealing with co-cultures of human melanocytes and keratinocytes (3–14).
More than 100 pigment genes have now been identified in mice and about 40% of those have been cloned to date. The availability of coat color mutants of mice that harbor specific mutations in each of these loci is an incredible resource, and immortalized melanocytes derived from these are now being quickly and routinely generated (http://www.sghms.ac.uk/departs/anatomy/pages/WTFGCB.htm). To take advantage of these mutant melanocytes and to characterize the functions of these pigment genes, especially those involved with the processing and transport of melanosomes and their transfer to keratinocytes, it is essential that conditions are established to allow long-term co-cultures of murine melanocytes and keratinocytes to study these functions.
Thus, the aim of the present study was to investigate optimal conditions for co-culturing melanocytes and keratinocytes, and to establish a model culture system which uses immortalized murine cell lines. The advantages in using mouse cell lines in place of human cells is that they are significantly more responsive to physiological factors, such as α-melanocyte stimulating hormone (MSH), ultraviolet (UV) radiation, etc. and in addition, a number of mutant melanocyte cell lines are available as noted above which allow the effects of various pigment genes on melanocyte–keratinocyte interactions to be assessed.
Methods
Cell Lines and Cell Culture
Murine melan-a melanocytes were a kind gift from Prof. Dorothy C. Bennett (St George's Hospital, London, UK) (15). They were originally derived from C57BL/6J (black, a/a) mice and are routinely passaged in melanocyte complete medium (MCM), which is RPMI 1640 medium with 5% heat-inactivated fetal calf serum (FCS), 50 U/ml penicillin, 50 μg/ml streptomycin, 100 μM α-mercaptoethanol, 2 mM l-glutamine, and 200 nM phorbol 12-myristate 13-acetate (PMA). Murine SP-1 keratinocytes were derived from SENCAR mice and were generously provided by Dr Stuart H. Yuspa (Laboratory of Cellular Carcinogenesis and Tumor Promotion, NCI, NIH). They were originally developed from papillomas generated by a standard initiation-promotion protocol with 7,12-dimethylbenz[a]-anthracene as the initiator and PMA as the promoter (16). SP-1 keratinocytes are cultured in standard keratinocyte medium (SKM) consisting of Eagle's minimal essential medium with 8% Chelex-treated FCS, 0.2% penicillin/streptomycin, and low Ca2+ (0.05 mM) to maintain a basal cell-like population of undifferentiated cells. Under these conditions, the doubling time for melanocytes and keratinocytes is about 2 and 3 days, respectively.
Co-Culture and Assays
Melan-a melanocytes were harvested by treatment with trypsin/ethylenediaminetetraacetic acid (EDTA) and were resuspended in RPMI 1640 medium. Viable cells, determined by trypan blue exclusion, were counted in a hemocytometer and were seeded at 1.2 × 104 cells/well (∼1% confluence in the well) in six-well plates in MCM. Two days later, the plates were rinsed with phosphate buffered saline (PBS) twice to eliminate traces of PMA. SP-1 keratinocytes were collected by trypsin/EDTA and 2.4 × 105 cells/well (20% confluence in the well) were added in SKM (with 0.05 mM Ca2+) to each well containing the melanocytes, which were about 2% confluent at that time. The initial seeding ratio of keratinocytes to melanocytes was thus 10:1 and co-cultures of melanocytes and keratinocytes were maintained thereafter in SKM (with 0.05 mM Ca2+). Melanocytes (5% confluent, 6 × 104 cells/well) and keratinocytes (20% confluent, 2.4 × 105 cells/well) were cultured separately in the medium described above (without PMA) to serve as controls.
Two, 4 and 5 days later, fresh SKM (with 0.05 mM Ca2+) containing 100 nM αMSH (Sigma Chemical Co, St Louis, MO, USA) or 0.2 mg/ml arbutin (Maruzen Pharmaceuticals, Hiroshima, Japan) or no additives (as a control) was added; one day after that, the cultures were photographed and were then harvested with trypsin/EDTA. After the cells were dislodged with occasional agitation, 2 ml SKM was added to inactivate the trypsin, and 100 μl aliquots were then seeded into flat-bottom 96-well tissue culture plates for the MTT(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) viability/proliferation assay (Roche, Indianapolis, IN, USA). The remainder of each cell suspension was centrifuged for 5 min at 1500 g, washed with cold PBS, and then solubilized in 300 μl extraction buffer (1% Nonidet P-40, 0.01% SDS, 0.1 M Tris-HCl, pH 7.2, and protease inhibitor cocktail from Roche). Extracts were solubilized at 4°C for 1 h and various assays were then conducted on each sample, at least in duplicate. Tyrosinase activity and melanin contents were determined as described previously (17, 18); synthetic melanin (Sigma) was used to generate a standard curve.
Results and discussion
Since the pioneering work of Rheinwald and Green (19), many systems for the co-culture of human epidermal cells have been developed to mimic the in vivo situation of normal human skin. Such models are increasingly employed as in vitro test systems in pharmaco-toxicological studies (20). In contrast with human epidermal cells, co-cultures of mouse epidermal cells have not yet been optimized. By establishing such a co-culture system, advantage could be taken of the many mutant melanocyte cell lines already in existence and those that become available (http://www.sghms.ac.uk/departs/anatomy/pages/cell bank holdings.htm) that could quickly allow the effect(s) of genes on melanocyte–keratinocyte interactions to be assessed. Further, in primary human cell cultures, the material is usually limited and standardization of conditions is usually poor because of the wide individual biologic variations. Working with murine cell lines is also advantageous in this regard because of their independence from donor variation and their availability in unlimited quantity. The major disadvantages of immortalized cell lines are possible alterations in cell proliferation and in their cellular metabolism compared with primary cells. However, immortalized mouse melan-a melanocytes have been used widely in the field as a model for normal melanocyte behavior and, in general, they respond to the environment as do melanocytes in vivo (15, 21). Thus, melan-a melanocytes have been shown to be a suitable substitute for normal primary mouse melanocytes. In addition, melan-a cells are free of contaminating keratinocytes often found in primary cell cultures. The SP-1 keratinocyte cell line is one of the rare murine cell lines that can grow in the absence of epidermal growth factor (EGF) or other growth factors, and can thus readily be used for co-culture with melanocytes. Both types of cells can be induced to differentiate melan-a melanocytes by MSH or by UV, and SP-1 keratinocytes by calcium (2, 22).
For these reasons, melan-a melanocytes and SP-1 keratinocytes were chosen as models for the cell populations present in mouse skin. Melanocytes co-cultured with keratinocytes grow well in SKM, whereas in monoculture, they require a specific culture medium with various growth additives such as PMA. In preliminary experiments (not shown), it was observed that melan-a melanocytes proliferated well in the absence of PMA when in the presence of keratinocytes and they retained their normal dendritic morphology in the low-calcium (0.05 mM) medium which is optimal for the growth of SP-1 keratinocytes. These findings thus support the view that keratinocyte-derived factors enhance melanocyte proliferation, dendricity, and pigmentation (4, 23, 24). For the first time, optimal conditions for co-culturing murine melanocytes and keratinocytes are reported in this paper.
In a number of preliminary experiments (not shown), we have tried various initial seeding densities and times of incubation, as well as different ratios of melanocytes and keratinocytes. Cells were seeded in various ratios (e.g. melanocyte:keratinocyte ratios of 1:6, 1:8, 1:10 and 1:12), in various medium formulations, and with varying levels of calcium (a critical factor in regulating keratinocyte differentiation). Although melanocyte–keratinocyte co-cultures would grow reasonably well in the various conditions tested, we found that an initial seeding ratio of 1:10 in SKM with low calcium was ideal for co-cultures that could be maintained for six or more days. The results presented herein (and summarized in Fig. 1) were the optimal parameters found consistently throughout these studies.
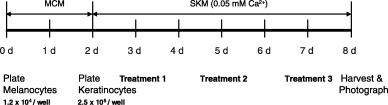
A schematic representation of the co-culture schedule.
An initial seeding of melanocytes and keratinocytes at a ratio of 1:10 in SKM with low calcium, which reflects the actual ratio of melanocytes and keratinocytes present in the basal layer of the epidermis, resulted in a homogeneous distribution of dendritic melanocytes among the keratinocytes. Optimal responses were obtained when melanocytes were seeded into plates 2 days before the keratinocytes; under these conditions, the melanocytes had time to form cell colonies, and when the keratinocytes were subsequently seeded, they survived well in the spaces around these melanocytes and grew over them. Two days later was found to be the optimal time to begin treating the co-cultures with melanogenic compounds, and media were replenished 2 and 3 days later. By day 8 (harvest day), the keratinocytes had grown almost to confluence in most areas of the wells and the proliferating melanocytes seemed to be growing under them; by phase-contrast counting the keratinocyte-to-melanocyte ratio at the end of the experiment had decreased from 10:1 to 5:1. The doubling time of keratinocytes (60–80 h) in classical culture media is rather slow compared with melanocytes (40–56 h).
Our previous study had shown that tyrosinase activity and melanin content in melanocytes could be stimulated by MSH in a dose-dependent manner with a two- to threefold increase over untreated controls (2, 18). A similar twofold stimulation of tyrosinase activity in melanocytes treated with 100 nM MSH for 4 days was verified in this study, as was a 50% increase in melanin production (Fig. 2), but the effects were significantly stronger when the melanocytes were co-cultured with keratinocytes (each about threefold). Such findings indicate that keratinocytes play an important synergistic role in the regulation of melanogenesis by MSH. Because melanocytes and keratinocytes are known to express the MSH receptor (MC1R), responses of keratinocytes to that hormone are not unexpected (25, 26). A number of studies have demonstrated that keratinocytes and keratinocyte-derived factors regulate the proliferation and differentiation of primary mouse epidermal melanocytes in culture (27–32). Keratinocytes have been reported to produce and secrete basic fibroblast growth factor (bFGF), endothelin-1, and other factors that can stimulate the proliferation and melanogenesis of melanocytes in vivo (4, 23, 24, 33). Further, keratinocytes from irradiated mice stimulated the proliferation and differentiation of neonatal and adult non-irradiated melanocytes more than did those from non-irradiated mice (34). These findings led us to consider the possibility that cytokines released by keratinocytes might be involved in regulating the MSH-induced melanogenesis. Morphologic alterations of melanocytes exposed to MSH in co-culture were viewed by phase contrast to show all cells and by bright-field optics to show melanin pigment (Fig. 3). Although MSH had little or no effect on rates of cell proliferation of melanocytes and/or keratinocytes (not shown), the percentage of pigmented and dendritic melanocytes was markedly increased in co-cultures treated with MSH, compared with untreated controls.
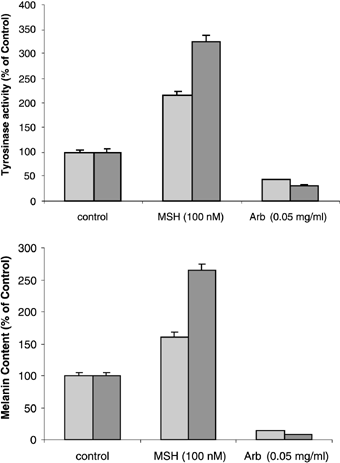
Analysis of tyrosinase activities (top) and melanin contents (bottom) in cultures of melanocytes with or without keratinocytes, as detailed in the Methods. Cultures were treated for 4 days with 100 nM MSH, with 0.2 mg/ml arbutin (Arb) or with medium only as a control. Tyrosinase activity was measured by radiometric assay and are expressed as the percentage of untreated controls (controls were 282 and 438 pmol/μg protein/h for melanocytes alone and in co-culture, respectively). Melanin content was estimated by lysing the cells with 1 N NaOH and measuring the absorbance at 405 nM, then extrapolating against a standard curve generated with synthetic melanin (controls were 152 and 248 μg melanin/mg protein for melanocytes alone and in co-culture, respectively). Each value shown represents the mean of quadruplicate assays; the error bars show the standard error of the mean (SEM). MC, melanocytes only; Co, co-culture of melanocytes and keratinocytes. This experiment was repeated twice with similar results.
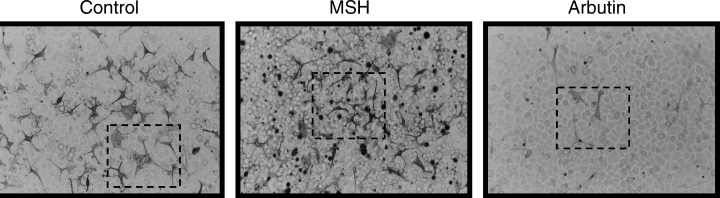
Phenotypic appearance of melanocytes co-cultured with keratinocytes. Representative morphologies of melanocyte:keratinocyte co-cultures exposed for 4 days to 100 nM MSH, 0.2 mg/ml arbutin (Arb) or medium only are viewed by bright-field microscopy (×200).
The rate-limiting step of melanin synthesis is regulated by tyrosinase, and the most logical regulatory point to control pigment formation is by inhibiting or stimulating tyrosinase activity. In this study, we also examined the effects of arbutin, which is the α-d-glucopyranoside derivative of hydroquinone. We measured effects on tyrosinase activity, melanin content, and cell proliferation of melanocytes in the presence or absence of co-cultured keratinocytes. It should be noted that in co-cultures, melanin may be distributed in keratinocytes as well as in melanocytes. The inhibitory effects of arbutin on melanocytes alone were similar to those found in our earlier report, but effects on melanocytes co-cultured with keratinocytes were much more dramatic (Fig. 2), again supporting the idea that keratinocytes play an active role in modulating melanocyte function in situ. The dramatic phenotypic changes (loss of dendrites and aberrant morphology) observed in melanocytes and keratinocytes treated with arbutin were also readily evident (Fig. 3).
Numerous pigmented melanoma cells or skin equivalent models have been described for evaluating the efficacy of melanogenic compounds (8, 35–37), but each has significant shortcomings when considered as a physiologically relevant model. We have now designed an attractive melanocyte–keratinocyte co-culture system which uses immortalized murine cell lines in a more physiologically relevant context. This system provides a reasonably easy, reproducible, and economically feasible methodology to test the efficacy of putative melanogenic regulatory compounds, and can be applied to generate co-culture model composed of melanocytes mutant at specific loci which should prove valuable in characterizing the function(s) of pigment genes whose effects depend, at least in part, on keratinocyte interactions. Although current evidence points to similar regulation of pigmentation in mouse and human melanocytes, it would ultimately be ideal to use immortalized human melanocytes and keratinocytes in this system as they become available. Our results further show the important synergistic contributions of keratinocytes to the regulation of the pigmentary functions of melanocytes and provide a model whereby novel factors that influence keratinocytes to modulate melanocyte function can be identified.




