Tyrosinase Gene Correction Using Fluorescent Oligonucleotides
Abstract
Gene therapy and production of mutated cell lines or animal models should be improved significantly once efficient controlled gene targeting strategies are developed. We used short single-stranded oligodeoxynucleotides (ODN), in some cases coupled to the fluorescent dye fluorescein isothiocyanate (FITC), to correct an endogenic natural point mutation in melanocytes in culture. The addition of the FITC molecule to the 5′ extremity of the ODN did not interfere with the efficiency of the reversion of the mutation and did not have any deleterious side-effects. The use of fluorescent ODN could lead to great improvement in the technique. In particular, it may facilitate sorting of the transfected cells in the treated population, and thereby significantly increase the percentage of corrected cells.
Abbreviations –
-
- ODN
-
- oligodeoxynucleotides
-
- PFA
-
- paraformaldehyde
-
- RDO
-
- RNA–DNA chimeric oligonucleotides
-
- TE
-
- Tris-EDTA
-
- TFO
-
- triplex-forming oligonucleotide
-
- TPA
-
- phorbol 12-myristate 13-acetate
Introduction
The number of genes demonstrated to be involved in melanocyte development and transformation is continuously increasing (1, 2). Melanocytes are found in the skin of mammals and produce the melanin pigment. Melanin absorbs UV light and thereby protects the DNA of skin cells against its damaging effects. It is produced in a specialized organelle, the melanosome. The key enzyme in pigmentation is tyrosinase. Tyrosinase oxidizes tyrosine to produce dopaquinone, which spontaneously homopolymerizes to give melanin. In the absence of functional tyrosinase no pigment is produced, resulting in the easily recognizable albino phenotype. In mice, the most common mutation of the tyrosinase gene is the G (r) C transversion at nucleotide position 390 which causes the replacement of cysteine 85 (involved in a disulfide bridge) by a serine (3).
Point mutations or frame shift mutations are frequently associated with human genetic diseases and cancers (4). The genetic correction of these mutations may potentially help to cure these patients. Several types of nucleic acid derivative molecules have been used as gene ‘correctors’ with different degrees of success, in vitro and in vivo. They include small fragments of DNA, modified oligodeoxynucleotide (ODN), RNA–DNA chimeric oligonucleotides (RDO), and short DNA fragments bound or directed by a triplex-forming oligonucleotide (TFO) for mutagenesis (5–12). However, there are several major problems to overcome before this approach can become clinically useful. The main limitations are the accessibility of the cell to the gene ‘correctors’, their internalization into cells and into nuclei, and their efficiency of gene targeting. These different problems are, without doubt, part of the reason why the reproducibility of this approach is poor.
Here, we describe the targeted reversion of the point mutation (C85 to S85) in the tyrosinase gene using regular and fluorescent ODN. The attachment of the fluorescent compound to the ODN has two major advantages: (i) cells transfected with ODN can be easily distinguished from the non-transfected cells, and (ii) the exact cellular localization of the ODN can be followed. The presence of the fluorescent group did not interfere with the efficiency of the ODN. Such fluorescent ODN may make a substantial contribution in facilitating the enrichment in transfected cells for both reversing mutations in somatic cells and producing mutations in murine embryonic stem cells.
Materials and methods
Oligonucleotides
An ODN consisting of a tyrosinase gene sequence (Tyr-ODN, Fig. 1) protected with four methylated bases at the 5′ extremity to resist the action of nucleases was synthesized. When indicated, the modified ODNs were coupled with a fluorescein isothiocyanate (FITC) moiety (FITC-Tyr-ODN), and were purified by Eurogentec (Seraing, Belgium) and by MWG-Biotech (Ebersberg, Germany), respectively. After synthesis, the ODNs were precipitated and resuspended in sterile TE at a concentration of 3.5 mg/ml (215 μM).
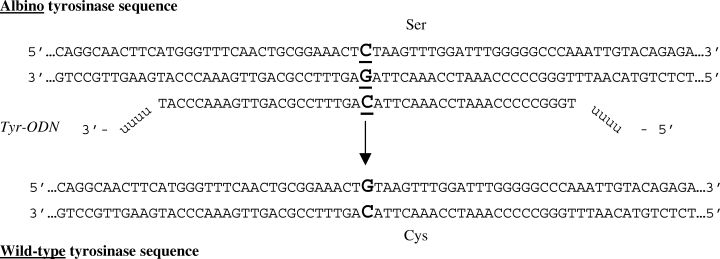
Sequence of the Tyr-ODN and the targeted sequences in the tyrosinase gene. The targeted nucleotide C site is in bold and underlined (nucleotide 390). The mutated tyrosinase TCT codon encodes a serine residue (amino acid 85), and the wild-type TGT codon a cysteine residue. The Tyr-ODN contains 45 deoxynucleotides identical to the wild-type tyrosinase (capital letters) with four 2′-O-methyl uracil at each extremity (U). The targeted cysteine codon is in the center of the Tyr-ODN.
Cell Culture and Transfection
Pigmented (melan-a cells) and non-pigmented (melan-c cells) melanocytes were grown in RPMI 1640 medium containing 10% heat inactivated fetal bovine serum, 200 nM TPA, 0.1 mM β-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM glutamine in the presence of 10% CO2. TPA is a phorbol ester required for the growth of melanocytes (13, 14).
Two transfection protocols were used for melan-c cells. (i) Melan-c cells were seeded at a density of 106 cells per 90-mm dish. Twenty-four hours later (day 0), 15 μg of Tyr-ODN was incubated for 20 min at room temperature with 90 μl of FuGENE 6 transfection reagent (Roche, Meylan, France) in a final volume of 600 μl of RPMI 1640 medium. This mixture, containing the neoformed complex was added to the cells in 3.4 ml of complete culture medium. (ii) Melan-c cells were seeded at a density of 5 × 104 cells per well in six-well plates. Forty-eight hours later (day 0), various amounts of Tyr-ODN (3.5–16 μg), or FITC-Tyr-ODN (molar equivalents), were incubated for 30 min at room temperature with 2–17.5 μl SuperFect reagent (Qiagen, Courtaboeuf, France), in RPMI in a final volume of 100 μl. The mixture containing the neoformed complex was added to the cells in 0.9 ml of complete RPMI culture medium. After 6 h of incubation with SuperFect or 24 h with FuGENE 6, cells were washed once with 1× phosphate-buffered saline (PBS), fed with complete culture medium and incubated further. The cells were monitored daily under the microscope. Melanin appears from day 5 in corrected melanocytes. On day 6, the cells were harvested with trypsin and split at one in three. From day 8, when the cells reached 50% confluence, TPA-free medium was used.
Molecular Analysis of Reverted Cells
Pigmented cells were recovered using cloning cylinders and were grown to enrich the pigmented cell population to 5% in a medium containing TPA. Genomic DNA was isolated from frozen cells by boiling and by treatment with proteinase K (15). Genomic DNA from 7 × 103 to 2 × 104 cells was used as the template for the PCR amplification of a tyrosinase gene fragment of 341 bp including the albino mutation (nucleotide position 390). For the PCR 5′-TCC GAA TTC AAA GGG GTG GAT GAC CG-3′ was used as the upstream primer (primer 1) and 5′-GAC ACA TAG TAA TGC ATC C-3′ as the downstream primer (primer 1′) (3). The PCR products were then diluted 1 in 106 and used as the template for a second round of PCR using two different set of primers: one specific for the wild-type tyrosinase gene (5′-GGT TTC AAC TGC GGA AAC TG-3′, primer 2); and the other for the albino tyrosinase gene (5′-GGT TTC AAC TGC GGA AAC TC-3′, primer 2′). Primers 2 and 2′ were usually associated with primer 1 in order to amplify the 263 bp PCR product (Fig. 3A).
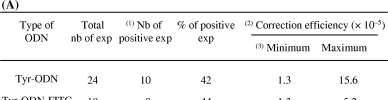
Comparison of correction events using Tyr-ODN and Tyr-ODN-FITC. Seventy-five thousand albino melanocytes were transfected with 6 μg/ml SuperFect and 440 nM fluorescent and non-fluorescent modified oligonucleotides, Tyr-ODN-FITC and Tyr-ODN, respectively. This experiment was repeated either 24 or 18 times as a function of the type of ODN. (A) Frequency of correction events. 1) An experiment (exp) was declared positive when at least one correction event occurred per transfection. 2) The correction efficiency is equal to the total number (nb) of correction events divided by the number of transfected cells. 3) The minimum correction efficiency does not take into account the negative experiments. (B) The repartition of correction was monitored and plotted as a function of relative number of experiments.
Fluorescent Oligonucleotide Uptake Measurement
Equal aliquots of cells were seeded on coverslips and transfected with a final concentration of 440 nM of FITC-Tyr-ODN (7.4 μg) and 2 μl of SuperFect reagent. Cells were washed with 1× PBS and fixed for 10 min at room temperature using 3.7% PFA 10, 30, 60, 180, 300, and 360 min after the beginning of the transfection. PFA-fixed cells were permeabilized by incubation in 0.2% Triton X-100 for 5 min at room temperature, and incubated in a 0.5-μg/ml 6′-diamidino-2-phenylindole (DAPI) solution. The cells were rapidly washed three times and mounted in Immu-mount (Shandon, Munich, Germany). The localization within cells of the fluorescence, corresponding to the ODN, was determined using a DMIRB fluorescence microscope (LEICA, Munich, Germany).
Results
Design of the Corrective Molecule
To correct the albino phenotype of mutant melan-c melanocyte cells to the wild-type pigmented phenotype, we designed a modified ODN designated as Tyr-ODN. Tyr-ODN can be used to change the C at nucleotide position 390 in the mutant tyrosinase gene to a G (Fig. 1). This transversion corrects the amino acid 85 from a serine to a cysteine, restoring the activity of the tyrosinase protein. The sequence of the Tyr-ODN is complementary to the region of the tyrosinase wild-type gene in the vicinity of nucleotide 390. Tyr-ODN is 45 nucleotides long, a length to be optimal for efficient correction in a similar system, and corresponds to the non-coding strand sequence which has been shown to give a better correction efficiency than the coding strand sequence (11). To protect the molecule from exonuclease degradation, four 2′-O-methyl uracil nucleotides were added at each extremity. In order to monitor the ODN during the transfection process, we also used a fluorescent version of Tyr-ODN, consisting of a Tyr-ODN molecule coupled at its 5′ extremity to a fluorescent FITC molecule.
Phenotype Correction
The delivery of a small fragment of DNA to its genomic target is one of the limiting steps of recombination-based gene correction. We tested several transfection protocols to optimize the amount of Tyr-ODN available for gene targeting without damaging the cells. Various amounts of Tyr-ODN were complexed with various concentrations of two different transfection reagents (FuGENE 6 and SuperFect).
FuGENE 6 is routinely used for albino and wild-type murine melanocytes in our laboratory (Fig. 2A,B). In transient transfections with various expression vectors, a ratio of FuGENE 6:DNA (v:w) of 6:1 leads to a good exogenous protein production. We therefore used a FuGENE 6:DNA (v:w) ratio of 6:1. Several transfection experiments, allowing the detection of a correction efficiency as low as 10−6, were performed independently using 1.5 × 106 melan-c cells. No corrected pigmented cells were observed using 15 μg of Tyr-ODN. It was not possible to use larger amounts of Tyr-ODN and maintain the same FuGENE:DNA ratio possible because of FuGENE 6 cytotoxicity.
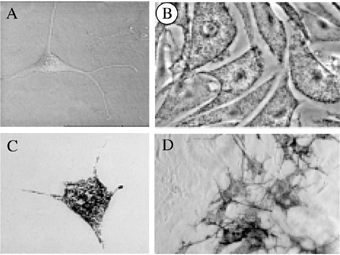
Wild-type, albino and corrected albino melanocytes. Phase contrast photomicrographs of typical melanocytes from (A) albino melan-c cell line, (B) wild-type melan-a cell line, (C, D) Tyr-ODN corrected and uncorrected melan-c cells showing either a single cell or a small colony of corrected melan-c prior cloning. Scale bar for A–C: 20 μm and for D: 6 μm.
Consequently, we tested another cationic transfection reagent, the SuperFect. We used several concentrations of this reagent (6–52 μg/ml), complexed with various amounts of Tyr-ODN (220–1000 nM). Surprisingly, melan-c cells appeared to be highly sensitive to SuperFect. The cytotoxicity associated with the SuperFect was observed at concentrations as low as 30 μg/ml and above: following transfection with more than 30 μg/ml SuperFect, the cells did not divide and died within 10 d. At concentrations of SuperFect below 30 μg/ml, the cells were healthy and pigmented cells were observed among non-pigmented cells in every tested condition (Fig. 2D). Similar experiments were performed in the absence of Tyr-ODN and spontaneous correction was never observed. The melanosome distribution in the corrected melanocytes was similar to that in the wild-type (Fig. 2C). The highest rate of correction obtained was 0.02% of the treated mutated cells. However, the correction efficiency was not reproducible: different results were obtained in different experiments with the same conditions (Fig. 3). About 40% of the experiments were positive using Tyr-ODN. This is in total agreement with recent findings, as Yoon and colleagues have reported that 60% of their experiments were positive (16). For instance, 24 independent experiments were performed with 75 000 cells, 440 nM Tyr-ODN and 6 μg/ml SuperFect. One experiment gave rise to 12 correction events, three experiments to two, six experiments to one, and 14 experiments to zero, leading to a mean overall correction efficiency of 0.002%. Increasing the amount of Tyr-ODN from 440 nM to 1 μM did not improve the correction efficiency. Similar amount of experiments were performed with the Tyr-ODN and the FITC-conjugated Tyr-ODN (=Tyr-ODN-FITC) in the same experimental conditions. The efficiency and the statistical distribution of correction following transfection of melan-c cells with Tyr-ODN-FITC was similar to that obtained with Tyr-ODN (Fig. 3).
Molecular Analysis of Reverted Cells
The corrected pigmented cells were partially purified and analyzed to confirm their correction at the molecular level. Their genotype was determined by nested PCR analysis (Fig. 4). Primers 1 and 1′ amplified a tyrosinase DNA fragment of 341 bp including the albino mutation (nucleotide 390). This fragment was used as the template for a second round of PCR using two different upstream primers allowing detection of either albino (primer 2′) or wild-type (primer 2) tyrosinase genes (Fig. 4A). The specificity of the amplification was tested using DNA extracted from melan-a and melan-c populations. Several dilutions of wild-type melan-a DNA in mutant melan-c DNA showed that the nested amplification procedure allowed the detection of one part mutant to up to 103 wild-type. In this respect, the sensitivity of this method is quite efficient. We confirmed that the cells with the corrected pigmented phenotype carry one wild-type sequence, whereas the parental albino cells carry the mutation (Fig. 4B). Moreover, pure populations of reverted melanocytes were isolated and expanded. The genotype of these cells was determined as heterozygous (data not shown). As an additional control, we determined the genotype of treated cells without reversion. As expected, no recombination was detected (data not shown). Thus, Tyr-ODN and Tyr-ODN-FITC had indeed corrected the albino mutation.
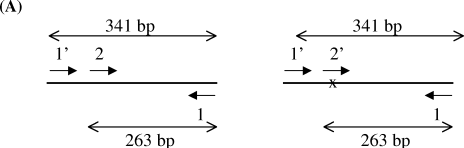
Molecular analysis of reverted cells. Genotype of corrected pigmented cells was determined by nested PCR analysis. The principle of the nested PCR is shown in panel A: the first round of PCR (primers 1 and 1′) led to the production of a 341-pb fragment. A second round of PCR uses this fragment as a template and two different upstream primers (2 and 2′) to distinguish between wild-type (left of the panel) and mutant sequences (right of the panel, underline with a cross). Both couple of primers, 1–2 and 1–2′, give rise to 263 bp PCR fragment. Panel B shows the separation on a 2% agarose gel of the various PCR products. The analysis is performed from DNA isolated from wild-type melan-a cells (lines a), from mutant melan-c cells (lines c) and from DNA isolated from the dishes in which the correction occurred (lines c1 and c2). The asterisks correspond to primers self amplification.
Uptake
One of the major limiting steps in gene correction experiments is the uptake of the correcting molecule into the nucleus of the cell. To investigate the uptake of ODN molecules into melan-c cell nuclei, we used Tyr-ODN-FITC that can be followed by fluorescence microscopy. Transfected melan-c cells were fixed at various times after the beginning of transfection. During the first 180 min of incubation with the fluorescent correcting molecules, no FITC signal was observed in melan-c cells (Fig. 5A–A″). After 180 min, an FITC signal was observed in the cytoplasm of about 20% of the cells. After 300 and 360 min, the FITC signal was present in the cytoplasm of about 40% of the cells and in the nucleus of about 1%, where it appeared as dots (Fig. 5B–C″). The precise localization of the diffuse and doted fluorescence in the nucleus was confirmed by confocal microscopy (data not shown). These results indicate that the Tyr-ODN-FITC reached the nucleus 5–6 h after the beginning of transfection, and only in 1% of the cells.
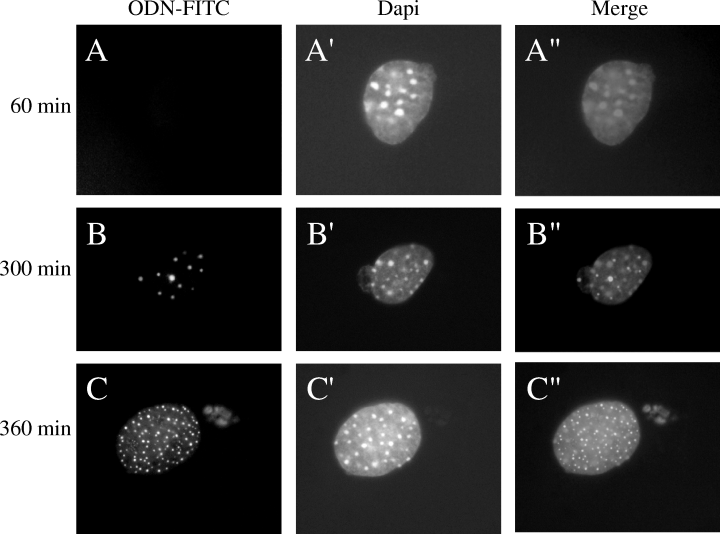
Uptake of the Tyr-ODN-FITC in melan-c cells. Cells were transfected with 440 nM Tyr-ODN-FITC in the presence of 6 μg/ml SuperFect. The cells were fixed and labeled with DAPI 60 (A–A″), 300 (B–B″) and 360 min (C–C″) after the beginning of transfection. Cells were observed under the fluorescence microscope. Fluorescence corresponding to Tyr-ODN is shown in A, B, C and to DAPI in A′, B′, C′. Superimposed images are shown in A″, B″ and C″. Scale bar = 3 μm.
Discussion
We show that ODN molecules are able to correct the C85 (G390) point mutation of the murine tyrosinase gene in the albino melanocyte melan-c cell line. The use of a fluorescent or non-fluorescent ODN (FITC-Tyr-ODN and Tyr-ODN) to target nucleotide 390 of the tyrosinase gene, led to a correction frequency of up to 2 × 10−4, with a mean correction efficiency of about 2 × 10−5. There was no evidence that the coupled FITC molecule interferes with the correction reaction or was cytotoxic. However, we cannot exclude potential mutagenic side-effects of the FITC molecule caused by light activation or free radicals, for instance. Nevertheless, as first approximation, FITC-Tyr-ODN was equally effective in correction as was the non-fluorescent Tyr-ODN. Using an ODN molecule to correct a point mutation in an integrated copy of LacZ gene in CHO cells, Igoucheva et al. reported a 2 log higher efficiency (0.1%) (11). The difference can also be explained by the type of cells and potentially in the transfection efficiency and/or delivery used. However, the difference may be a consequence of the characteristics of the primary sequence involved or the general structure of the gene: LacZ is a bacterial gene and its chromatin structure was not investigated. The chromatin structure of the endogenous tyrosinase gene has been already described and does not show any abnormality in melanocytes in culture (17).
The reaction of chromosome correction of the mutant sequence involves two important DNA processes. The formation of a complex with the upcoming ODN at the homologous position on the chromosome and the correction of the mismatch created by the difference between the mutant sequence on the chromosome and the wild-type sequence on the ODN. This last reaction requires long complex of proteins involved mismatch repair. We have chosen to use the non-coding sequence of the tyrosinase gene as the ODN sequence. This involves a C:C mismatch between the chromosomal target and the Tyr-ODN or the FITC-Tyr-ODN molecules. Although C:C mismatches have been reported to be poorly repaired in cells derived from triblastic organisms according to Holmes et al. (18), nevertheless we obtained gene correction at a detectable rate. Other systems of gene correction have been reported (5–7, 10–12). It is possible that the correction of another mutation, creating a different mismatch, could improve the correction efficiency.
Igoucheva et al. reported that ODN and chimeric RDO lead to the same gene correction efficiency (11). RDO molecules have been used in the melan-c cell line system to correct the C85S mutation and gave rise to a gene correction efficiency of 0.01–15% (19). Using exactly the same RDO molecule, and the same purification and transfection protocols, we have been unable to obtain any pigmented corrected cells. A similar negative result has been reported elsewhere by another group (20). These contradictory results are clearly problematic. We currently have no clear explanation, although the quality of the water and of the main reagents used may be involved: a contaminant present at a low concentration may help or inhibit the gene correction.
To investigate the efficiency of our transfection protocols, we used a fluorescent FITC-Tyr-ODN molecule. Observation by fluorescent microscopy revealed that 40% of the transfected cells were fluorescent. The distribution of the fluorescence was either diffuse and cytoplasmic (98%) or intense and nuclear (2%). The nuclear signals appeared as dots, revealing accumulation of Tyr-ODN-FITC in specific locations of the nuclei. The nature of these aggregates are not yet known and should be investigated further. For correction, the Tyr-ODN molecules must enter the nucleus to recombine with the mutated locus, and therefore treated cells with no nuclear fluorescence are unlikely to be corrected. Indeed, the corrected cells should arise from those showing nuclear fluorescence. Consequently, the physical separation of nuclear fluorescent cells, by a FACS sorter for instance, would enrich the cell population in potentially corrected cells by a factor of 100. This enrichment would lead to a frequency of corrected cells reaching 2 × 10−2, in the best situation. We are currently establishing the experimental conditions to separate the two types of cells by FACS. According to our preliminary results, the separation of the fluorescent cells seems possible. However, a large amount of technical work has to be performed to separate and to let the corrected cells grow efficiently. The presence of FITC on Tyr-ODN may have other advantages. For instance, FITC may affect the hydrophobicity of the Tyr-ODN molecules and may improve their access to the nucleus. It may, in addition to the 2′-O-methyl uracil at both extremities, also protect the ODN from nucleases.
In conclusion, the correction of endogenous but mutated genes, such as tyrosinase, can be achieved in cell culture using fluorescent ODN. The presence of an inert fluorescent dye on the ODN may help in the development of this type of therapeutic approach.
Acknowledgements– We are grateful to K. Yoon for helpful discussion and V. Hearing for advice. We thank CESFO for providing various supplies. This work was supported by a grant from Institut Curie, PIC IGRT. EB is a fellow of ARC.




