Melanin has a Role in Ca2+ Homeostasis in Human Melanocytes
Abstract
We have examined whether melanin affects Ca2+ homeostasis in cultured normal human melanocytes. Intracellular Ca2+ concentrations ([Ca2+]i), were measured in four Caucasian and in three Negroid melanocyte cultures. Under resting conditions [Ca2+]i was around 100 nM in all cultures, but differences between cells within cultures were observed. All cultures responded to endothelin-1 (ET-1) with increases in [Ca2+]i and there were no differences between Caucasian and Negroid cultures. However, large differences in responses between cells within cultures were observed, indicating that melanocyte cultures are very heterogeneous. The addition of 2.5 mM CaCl2 to melanocytes kept in Ca2+-free medium resulted in rapid and transient increases in [Ca2+]i of up to 1500 nM. These increases were on average more than two times smaller in melanocyte cultures established from Negroid donors compared with Caucasian cultures. In addition, well melanized Caucasian melanocytes, cultured in the presence of 400 μM tyrosine and 10 mM NH4Cl, showed a reduced increase in cytoplasmic Ca2+ concentration upon the addition of extracellular Ca2+. The difference in maintaining Ca2+ homeostasis between poorly and well melanized melanocytes may be the result of the clearance of cytoplasmic Ca2+ into melanosomes and the greater capacity for this in the more pigmented melanocytes.
Abbreviations –
-
- bFGF
-
- basic fibroblast growth factor
-
- BPE
-
- bovine brain pituitary extract
-
- CT
-
- cholera toxin
-
- UG
-
- Ultroser G
-
- ET-1
-
- endothelin-1
-
- fura-2/AM
-
- fura-2 acetoxymethylester
-
- IBMX
-
- 3-isobutyl-1-methyl-xanthine
-
- TPA
-
- 12-O-tetradecanoyl-phorbol-13-acetate
-
- [Ca2+]i
-
- intracellular Ca2+ concentration
-
- [Ca2+]e
-
- extracellular Ca2+ concentration
Introduction
The calcium ion is of fundamental importance in cell regulation. It functions in a number of ways serving as a cofactor for many enzymes and also acting as an intracellular mediator. In this way, it regulates a wide variety of cellular responses, including secretion, movement and proliferation. In the skin, Ca2+ has an essential role in regulating epidermal proliferation and differentiation. Thus, low epidermal Ca2+ concentrations stimulate keratinocyte proliferation, whereas higher levels induce differentiation (1).
The role of Ca2+ in the melanocyte is, however, less clear. Evidence suggests that low extracellular Ca2+ concentrations ([Ca2+]e), increase the proliferation of cultured melanocytes, whereas high [Ca2+]e has no effect (2). It has also been reported that Ca2+ modulates melanogenic processes in the melanocyte (3). For instance, elevations in intracellular Ca2+ concentration ([Ca2+]i) have an inhibitory effect on the production of melanin (4). However, when accompanied by agents that increase cyclic adenosine monophosphate (cAMP), elevated [Ca2+]i stimulates melanogenesis (5). This is seen for instance in response to endothelin-1 (ET-1) which acts both as a potent melanocyte mitogen (6) and as a stimulator of melanogenesis (7). It has recently been suggested that Ca2+ regulates melanin production through its ability to act as a cofactor for phenylalanine hydroxylase (8). This enzyme catalyses the conversion of l-phenylalanine to l-tyrosine, the precursor of melanin.
It has been reported previously that melanin has Ca2+ binding properties (9–11). High concentrations of Ca2+ have been found in melanosomes of dermal (12) and retinal melanocytes (13). Furthermore, it has been demonstrated that melanosomes contain caffeine and IP3-sensitive calcium channels, suggesting that Ca2+ can be released from melanosomes (13). Ca2+ is considered to be of crucial importance in the functioning of pigment cells. This has been demonstrated especially for inner ear melanocytes, where melanin acts as a biological reservoir for Ca2+ and in this way is involved in the regulation of several Ca2+ dependent cellular processes such as melanosome movement, cell motility and melanogenesis (14). It would appear from the above studies that mechanisms involved in intracellular Ca2+ homeostasis in the melanocyte depend upon the presence of melanin. We would therefore hypothesize that melanin has a role in the maintenance of Ca2+ homeostasis in the melanocyte. In order to examine this hypothesis we have measured [Ca2+]i in human melanocytes obtained from skin types representing different levels of melanization. In addition, Caucasian melanocytes were cultured in presence of NH4Cl and elevated levels of tyrosine to increase melanin contents. [Ca2+]i was measured under resting conditions and following changes in [Ca2+]e. Furthermore, increases in [Ca2+]i in response to ET-1 were measured and compared in melanocyte cultures from different origin. The results reported in this study suggest that melanin is of importance in Ca2+ homeostasis in melanocytes.
Materials and methods
Cell Culture
Human epidermal melanocytes were isolated from foreskin of four Caucasian and of three Negroid donors and initially cultured in Ham's F10 nutrient medium (Invitrogen, Breda, the Netherlands) supplemented with 1% Ultroser G (UG) (Invitrogen, Breda, the Netherlands), 2% foetal calf serum (FCS) (Hyclone, Logan, UT, USA), 16 nM 12-O-tetradecanoyl-phorbol-13-acetate (TPA) (Sigma-Aldrich, Zwijndrecht, the Netherlands), 0.1 mM 3-isobutyl-1-methyl-xanthine (IBMX) (Sigma-Aldrich, Zwijndrecht, the Netherlands), 1 nM cholera toxin (CT) (Sigma-Aldrich, Zwijndrecht, the Netherlands) and penicillin/streptomycin (Invitrogen, Breda, the Netherlands) (15). After establishment, the cultures were changed to MCDB153 medium (Sigma-Aldrich, Zwijndrecht, the Netherlands) supplemented with 1% UG, 2% FCS, 2.5 ng ml−1 ET-1 (Sigma-Aldrich, Zwijndrecht, the Netherlands), 2.5 ng ml−1 basic fibroblast growth factor (bFGF) (Sigma-Aldrich, Zwijndrecht, the Netherlands), 6 μg ml−1 bovine brain pituitary extract (BPE) (Invitrogen, Breda, the Netherlands) and penicillin/streptomycin. Three days prior to the experiments the cells were seeded onto glass coverslips (φ 25 mm). Heavy melanized Caucasian melanocytes were obtained by addition of 400 μM tyrosine and 10 mM NH4Cl to the culture medium for 2 days. The cultures were kept in a humidified incubator with 5% CO2 at 37°C.
Measurement of [Ca2+]i by Fluorescence Microscopy
Fura-2 loading
Melanocytes were transferred from the culture medium to a N-2-hyroxyethylpiperazine-N′-2-ethansulfonic acid (HEPES) balanced salt solution (125 mM NaCl, 5 mM KCl, 1 mM MgSO4, 1 mM KH2PO4, 2.5 mM CaCl2, 10 mM NaHCO3, 20 mM HEPES, 5 mM glucose at pH 7.4) containing 5 μM Fura-2 acetoxymethylester (Fura-2/AM) (Molecular Probes, Leiden, the Netherlands) for a 45-min incubation at 37°C. After 45 min the glass coverslips were rinsed three times and mounted in a microscope culture dish (16). During the experiments the cells were kept in the described buffer.
Microscopic set-up
The microscopic set-up has been described in detail by Atsma et al. (17). In brief, the glass coverslip was mounted in a culture dish with a hole in the centre (16). The coverslip was placed over the hole and medium supplied to the dish. The dish was placed on a heating ring to maintain a constant temperature of 35°C (18). A Leitz Diavert inverted microscope equipped with a Nikon 20 times fluorite objective and a mercury light source (HBO-100, Osram, Berlin, Germany) was used for the measurements. Selective filters allowed excitation of the cells at subsequently 340 and 380 nm. Emission light was led through a 490-nm high-pass filter and the image recorded by a SIT digital camera (Hamamatsu C2400-08, Meyer, Houston, TX, USA). Every 28 s the cells were excited at 340 and 380 nm and the emission signal recorded. The images were analysed using image-processing software (TIM®, Difa, Breda, the Netherlands). The ratio image was created by dividing the 340 by the 380 nm image and multiplying each pixel value by 32. A template was used to distinguish between cellular and background pixels, and subsequently the background pixel values were set to zero. Statistical routines that excluded pixel values equal to zero were used. Conversion of ratio values to [Ca2+]i was carried out by using the formula of Grynkiewicz et al. (19).
Experimental procedures
Per microscopic field 4–8 melanocytes were selected for each measurement and [Ca2+]i recorded for periods up to 30 min The effect of extracellular Ca2+ concentration on [Ca2+]i was examined by changing the melanocytes to Ca2+ free buffer after fura-2 loading and by the subsequent addition of 2.5 mM CaCl2. Increases of [Ca2+]i in individual melanocytes in response to ET-1 were recorded and responses compared with neighbouring cells.
Calibration of the Fura-2 fluorescence
Every experiment was terminated with a calibration of the fura-2 fluorescence signal. Addition of 10 μM ionomycin to the cells produced the maximal 340/380 ratio value, whereas the minimum 340/380 ratio value was obtained by the addition of 20 mM ethyleneglycol-bis (beta-aminoethylether)-N,N′-tetraacetic acid (EGTA). The maximal and minimum ratio values, as well as β (F380 in the absence of Ca2+/F380 in the presence of Ca2+), were included in the formula of Grynkiewicz et al. to calculate [Ca2+]i (19).
Measurement of [Ca2+]i by Spectrofluorometry
Melanocytes were removed from culture flasks by incubation in a 0.7-mM ethylenediaminetetraacetic acid (EDTA) solution and loaded with 5 μM Fura-2/AM in 2 ml medium without supplements for 1 h at 37°C in the dark. The cells were then centrifuged, taken up in a buffer containing 138 mM NaCl, 6 mM KCl, 1.1 mM CaCl2·2H2O, 1 mM MgSO4·7H2O, 0.1 mM EGTA, 1 mM NaH2PO4·H2O, 5.5 mM glucose, 20 mM HEPES and 0.1% BSA, and transferred to a cuvette. The cuvette was placed in a spectrofluorometer (Photon Technology International, Lawrenceville, USA) operated by Felix software (Photon Technology International). The cells were excited intermittently between 340 and 380 nm every second and emissions recorded at 510 nm. Agonists were added under continuously stirring. At the end of each measurement a calibration of the measurements took place. The maximum and minimal [Ca2+]i were obtained by the addition of 0.2% Triton X-100 and 20 mM EGTA, respectively. [Ca2+]i was calculated using the equation of Grynkiewicz (19).
Results
Basal [Ca2+]i in Human Melanocytes
Fluorescence microscopy was used to measure [Ca2+]i in individual melanocytes. The basal [Ca2+]i for melanocytes of Caucasian or of Negroid origin maintained in medium containing 2.5 mM Ca2+ was around 100 nM. Within cultures, however, melanocytes showed individual differences in [Ca2+]i. Furthermore, a proportion of the melanocytes showed spontaneous changes in [Ca2+]i, which varied from slow increases to recurring fluctuations with amplitudes between 50 and 100 nM of 3–4 min duration. The fluctuations were often synchronized in cells within close proximity (Fig. 1). In one of the tested cultures melanocytes with [Ca2+]i of up to 2500 nM were observed. These particular melanocytes showed apoptotic bodies (Fig. 2).
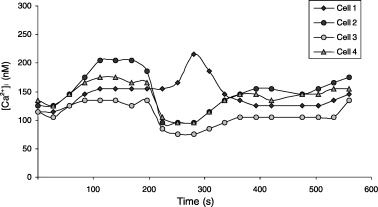
Spontaneous changes in [Ca2+]i in four melanocytes in close proximity.
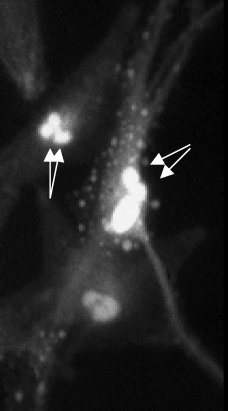
Two melanocytes with [Ca2+]i up to 2500 nM showing apoptotic bodies (arrows).
Effects of Melanin on Ca2+ Homeostasis
Experiments were carried out to examine the effect of changing extracellular Ca2+ concentrations on intracellular Ca2+ homeostasis in four Caucasian and in three Negroid cultures. In the absence of extracellular Ca2+ all melanocyte cultures showed a steady [Ca2+]i of around 80 mM with no spontaneous fluctuations. Upon the addition of 2.5 mM CaCl2, there was an immediate increase in [Ca2+]i in all tested cultures (Fig. 3). These increases reached a maximum within 1 min, after which [Ca2+]i decreased over a 3-min period and a new steady-state level of around 200 nM [Ca2+]i was reached. There were large differences in the increases in [Ca2+]i between the melanocyte cultures. Thus in one culture [Ca2+]i rose to 300 nM, whereas in others increases up to 1500 nM were observed. The magnitude of the increases in [Ca2+]i correlated with the level of melanization of the melanocyte cultures. The four cultures of Caucasian origin showed an average peak [Ca2+]i of 1146 ± 360 nM, for the three Negroid cultures that was 536 ± 326 nM. The results were tested for significance with a student t-test and a P-value of 0.07 was obtained.
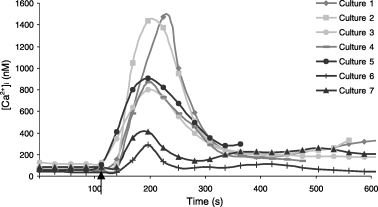
Addition of 2.5 mM CaCl2 to four Caucasian melanocyte cultures (1–4) and three Negroid cultures (5–7) kept in Ca2+ free buffer (arrow). For every culture the average [Ca2+]i for six to eight cells is shown.
Comparable experiments with those described above have been carried out in two Caucasian melanocyte cultures in which melanin production was stimulated in half of the melanocytes by culturing in presence of 400 μM l-tyrosine and 10 mM NH4Cl for 2 days. As a consequence, melanin levels increased 3.1 times. A spectrofluorometric method was used to measure [Ca2+]i in absence and upon addition of 2.5 mM CaCl2. In contrast to the above experiments, the addition of 2.5 mM CaCl2 did not result in an immediate and transient increase in [Ca2+]i. This is possibly due to different experimental conditions. However, upon the addition of CaCl2, slow and sustained increases in [Ca2+]i were observed. The average percentage increase for the control melanocytes was 171 (±18) and for the heavily pigmented cells 128 (±5) (Fig. 4).
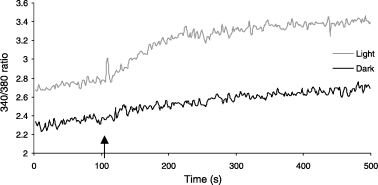
Addition of 2.5 mM CaCl2 (arrow) to a Caucasian melanocyte culture under normal conditions (light) and cultured for 2 days with 400 μM l-tyrosine and 10 mM NH4Cl (dark) kept in Ca2+ free buffer.
Response to Endothelin-1
We then examined and compared the responses of Caucasian and Negroid melanocytes to ET-1 and in addition tested the effect of increased melanin contents in two Caucasian cultures. In response to 40 nM ET-1, a concentration know to increase [Ca2+]i (20), all melanocyte cultures showed rapid and transient elevations of [Ca2+]i of up to 1300 nM after which a new, elevated, steady-state [Ca2+]i was reached (Fig. 5). The magnitudes of the responses varied considerably between individual cells within the same culture. As shown in Fig. 5, one cell responded to ET-1 with an elevation of [Ca2+]i to 400 nM whereas a neighbouring cell showed three times this response. In the absence of extracellular Ca2+, the response to ET-1 was inhibited.
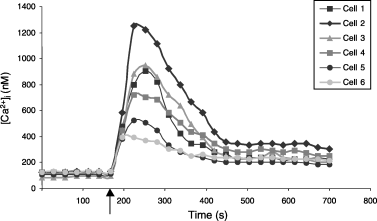
Response to 40 nM ET-1 in six melanocytes from a Caucasian culture. The melanocytes were deprived of ET-1 for on average 1 h before the response. ET-1 was added at 170 s as indicated by the arrow.
There was no significant difference in the response to ET-1 between Caucasian and Negroid melanocyte cultures. The average increase in [Ca2+]i for the four Caucasian cultures was 219% and for the three Negroid cultures this was 311%, but within each group the differences in responses were large (Table 1) (T-test P-value 0.51). A reduced ET-1 response was found in the cultures with experimentally increased melanin contents. Thus the control cultures increased [Ca2+]i with 254% whereas this was 148% for the more pigmented cultures (Table 1).
| Culture | % Increase [Ca2+]i |
|---|---|
| Caucasian 1 | 169 |
| Caucasian 2 | 199 |
| Caucasian 3 | 269 |
| Caucasian 4 | 238 |
| Caucasian 3 dark | 163 |
| Caucasian 4 dark | 134 |
| Negroid 1 | 200 |
| Negroid 2 | 191 |
| Negroid 3 | 543 |
- Measurements were taken in duplicate, and are expressed as percentage increase of pre-stimulation [Ca2+]i.
Discussion
In the present study, we have used fluorescent microscopy to determine [Ca2+]i in human melanocytes. Using this approach, it was possible to measure [Ca2+]i not only in different cultures but also in individual melanocytes within the same culture. Under resting conditions [Ca2+]i in the melanocyte cultures was around 100 nM. This is comparable with concentrations found in other cell types. A number of melanocytes in a culture obtained from a skin type I individual showed extremely high levels of Ca2+ of up to 2500 nM. These melanocytes also showed fragmentation of the cytoplasm. Our observations are therefore consistent with the view that high Ca2+ concentrations are associated with apoptosis (21).
An interesting observation in this study was the occurrence of spontaneous fluctuations in [Ca2+]i in many of the melanocytes, irrespective of their origin. These fluctuations were often synchronized in closely associated cells with amplitudes of 50–100 nM Ca2+. In the absence of extracellular Ca2+ these fluctuations disappeared, indicating a dependence upon extracellular Ca2+. The significance of the fluctuations is unknown. Similar fluctuations in [Ca2+]i have been reported in immature neurones (22, 23). It is believed that these fluctuations in [Ca2+]i are involved in processes such as migration and the formation of interneuronal connections. Local elevations of [Ca2+]i may also stimulate the outgrowth of filopodia in neurones (24). Considering the common origin of melanocytes and neurones, fluctuations in [Ca2+]i could have a similar significance in melanocytes and could be involved in their migration and interaction with keratinocytes.
A characteristic of melanocytes is their ability to produce melanin. In view of the Ca2+-binding properties of melanin (14) and the proposed role of melanin in Ca2+ homeostasis (25), we have considered the possibility that Ca2+ homeostasis in the melanocyte may depend upon melanosomal melanin contents. To examine this, we disturbed Ca2+ homeostasis in melanocytes with varying degrees of melanization and studied the restoration of a steady [Ca2+]i. Following the addition of 2.5 mM Ca2+ to melanocytes kept in Ca2+ free buffer, all cultures showed an immediate increase in cytoplasmic Ca2+ concentration which had returned to a new resting level by 3 min. The increases in cytoplasmic Ca2+ were most pronounced in melanocytes from less-pigmented skin (skin types I–III) and was noticeably attenuated in melanocytes from darker skin types. One has to take into account that the measured [Ca2+]i are cytoplasmic Ca2+ concentrations. Fura-2 is taken up in the cytosol and is not transported into the organelles. Therefore, the increases in [Ca2+]i upon the addition of CaCl2 represent increases in cytoplasmic Ca2+ concentration. The removal of cytoplasmic Ca2+ depends on the transport of Ca2+ either outside the cell or into the organelles. Melanosomes are one of the organelles that store Ca2+ and it has been shown that these melanocyte specific organelles contain the highest concentrations of Ca2+ in the melanocyte (13). The difference in melanosomal melanin contents between the Caucasian and Negroid melanocytes may account for the differences in the removal of Ca2+ observed in this study. Thus, it is possible that the smaller increases in cytoplasmic Ca2+ in more melanized melanocytes are due to the rapid mopping up of Ca2+ into the melanosomes. Poorly melanized melanosomes would be expected to show a reduced uptake of Ca2+ and consequently, as observed, cytoplasmic Ca2+ concentrations in melanocytes from less-pigmented skin would be higher.
The spectrofluorometric measurements of [Ca2+]i in Caucasian melanocytes and in melanocytes with induced melanin production showed not the immediate and transient increases in [Ca2+]i upon the addition of 2.5 mM CaCl2 as observed with fluorescence microscopy. The most important difference between the two methods is that the cells are in suspension and attached, respectively, and it is likely that this affects the responses. However, slow increases in [Ca2+]i were observed and these were larger in the less-pigmented melanocytes than in the darker cells. These results support the above hypothesis.
Experiments to measure responses to ET-1 were carried out to study the effect of melanosomal melanin contents on the release of intracellular Ca2+. In these experiments ET-1 produced increases in [Ca2+]i in all cultures. Large differences in responses between melanocytes within cultures were observed, indicating that melanocyte cultures are very heterogeneous. However, no difference in the average ET-1 response between Caucasian and Negroid cultures was found. A more extended study is needed to confirm these results. A reduction in the response to ET-1 was observed in the heavy melanized Caucasian melanocytes. There are at least two different explanations for this. First, ET-1 receptor levels may be reduced because of the high melanin contents. A second possibility is that the release of Ca2+ from the melanosomes is impaired by the increased melanosomal melanin contents.
The present results indicate that melanin has an important role in Ca2+ homeostasis in melanocytes. Melanin could also be involved in maintaining Ca2+ homeostasis in the epidermis. It is perhaps significant that the lowest concentrations of Ca2+ are found in the basal layer of the epidermis where melanin production occurs. Is it possible that melanocytes through their ability to produce melanin have a role in maintaining Ca2+ homeostasis in the epidermis and in this way regulate keratinocyte proliferation and differentiation?




